Abstract
Oestrogens and 1α,25(OH)2-vitamin D3 (1,25-D3) are steroids that can provide effects by binding to their receptors localised in the cytoplasm and in the nucleus or the plasma membrane respectively inducing genomic and non-genomic effects. As confirmed notably by invalidation of the genes, coding for their receptors as tested with mice with in vivo and in vitro treatments, oestrogens and 1,25-D3 are regulators of spermatogenesis. Moreover, some functions of ejaculated spermatozoa as viability, DNA integrity, motility, capacitation, acrosome reaction and fertilizing ability are targets for these hormones. The studies conducted on their mechanisms of action, even though not completely elicited, have allowed the demonstration of putative interactions between their signalling pathways that are worth examining more closely. The present review focuses on the elements regulated by oestrogens and 1,25-D3 in the testis and spermatozoa as well as the interactions between the signalling pathways of both hormones.
Résumé
L’œstradiol et la 1α,25(OH)2-vitamin D3 (1,25-D3 ou calcitriol) sont respectivement la forme la plus active des œstrogènes et la forme hormonalement active de la vitamine D. Ces stéroïdes peuvent exercer leurs effets biologiques après fixation à des récepteurs localisés dans le cytoplasme et le noyau (récepteurs dit nucléaires) ou par fixation à des récepteurs localisés à la membrane plasmique (récepteurs membranaires) à l’origine d’effets appelés génomiques et non génomiques respectivement. Bien que les œstrogènes aient longtemps été considérés comme uniquement des hormones féminines, de nombreux travaux ont permis de montrer leur importance dans le bon déroulement de la spermatogenèse et la qualité des gamètes. De même, la 1,25-D3 est capable de réguler les fonctions testiculaires suggérant son importance dans la fertilité. Les études réalisées sur leurs mécanismes d’action, bien qu’ils ne soient pas complètement élucidés, ont permis de mettre en évidence des interactions entre les voies de signalisation de ces deux hormones. Cette revue est centrée sur les évènements régulés par les œstrogènes et la 1,25-D3 dans les testicules et les spermatozoïdes et les interactions entre leurs voies de signalisation.
Similar content being viewed by others
Background
Spermatogenesis is a complex biological process under the control of interplay of autocrine, paracrine and endocrine factors. In addition to gonadotropins and androgens, it is now well known that oestrogens could play a significant role in the regulation of the events of mammalian spermatogenesis but the mechanisms of oestrogens effects are not well established (for review [1]). Moreover, some data showed 1α,25(OH)2-vitamin D3 (1,25-D3) implications in some events of spermatogenesis where 1,25-D3 could, as well as steroids, act at genomic (gene expression regulation) and at non-genomic levels (initiated at the plasma membrane) (for review [2]). To this aim, their classical receptors classified as nuclear receptors could also be localised in the cytoplasm or in the plasmatic membrane. So, some interactions between the signalling pathways of oestrogens and 1,25-D3 could be identified; this suggests the existence of common roles in spermatogenesis and spermatozoa functions. This review is focused on:
-
(i)
oestrogen and 1,25-D3 receptors, their localization and signalling pathways in testicular cells and spermatozoa.
-
(ii)
oestrogen and 1,25-D3 effects and their roles in the events of spermatogenesis and maturation of spermatozoa.
-
(iii)
the signalling pathways interactions of both hormones.
Considering this data (mainly tested on both rodent and human species), it does ultimately provide insight into unresolved issues and future investigations.
Oestrogen
Oestrogen receptors in testis and spermatozoa
Classically, oestrogens produce genomic effects after their binding to the nuclear receptors ERalpha or ESR1 and ERbeta or ESR2. However, they can also come and attach to receptors localised on the plasma membrane and activate some signalling pathways (for review [3]). In mammalian, both forms ESR1 and ESR2 are retrieved in somatic and germ cells in testis and on spermatozoa. Although some data values contradict each other the full length receptors ESR1 and ESR2 can be both found in Leydig, Sertoli and germ cells from spermatogonia to spermatids in human and rodents species (Fig. 1a; for review [1, 4, 5]). Recently, in situ hybridisation and immunohistochemistry experiments on human testis confirmed ESR1 and ESR2 expression in germ cells, ESR1 expression in interstitial cells and ESR2 expression in Sertoli cells [6]. In addition, some variants have been found. On human beings, six ESR2 mRNA variants, in addition to the wild type receptor, are present in testis and their different localisation suggests that they undertake different roles in spermatogenesis [7]. In addition to the wild type ESR1 mRNA, human germ cells express some transcripts deleted from exon 1 whereas the human spermatozoa exclusively contain the truncated form [8]. Consistently with these results, the presence of a shorter isoform of ESR1 of 46 kDa could be observed on human spermatozoa [8] instead of the expected size receptor of 66/67 kDa detected by Aquila et al. [9] and Rago et al. [10]. Later on, these two forms of 66 and 45 kDa were both detected by Solakidi et al. [11] (Fig. 1b). Moreover, a 29 kDa protein was identified by western blotting in human sperm membranes [12]. Saunders and collaborators described the expression of two proteins ESR2 in human adult testis as such: ERβ1 (wild type) and a variant isoform of ERβ(hERβcx/2) formed by alternative splicing [13]. The two proteins ERβ1 and ERβ2 were retrieved in germ cells, which are in keeping with the two proteins of 60 kDa and 50 kDa observed in human immature germ cells [8] and ejaculated immature spermatozoa [10]. Whereas no ESR2 protein was detected in human spermatozoa in the earlier phases [8], a 64 kDa protein was finally detected later on [11, 14]. ESR1 and ESR2 were observed on the tail or/and in the mid-piece with an additional localization for ESR1 to the equatorial segment [11, 14] (Fig. 1b). Although the corresponding proteins could not have been observed until now, nevertheless, we have also identified in rat testis some mRNA variants of ESR1 and ESR2. Concerning ESR1, we have shown the presence of the full-length form and of one isoform with exon 4 deleted. For ESR2, besides the wild type, three isoforms were observed: one with exon 3 deleted and another with an insertion of 54 nucleotides, and the last one with both modifications [15]. In addition to ESR1 and ESR2, a transmembrane receptor coupled to a protein G: GPER (GPR30: G protein coupled receptor 30) is able to bind to estradiol and to mediate its effects through a non-genomic pathway (for review [16]). This protein can be found in the mouse spermatogonial cell line GC-1 and in the mouse spermatocyte-derived cell line GC-2 [17, 18] but also in the rat primary germ cells PS (pachytene spermatocyte) and RS (round spermatid) [4, 5], and in immature rat Sertoli cells [19]. In human testis, GPER was also described in Sertoli cells and germ cells (spermatogonia and spermatocytes) and exclusively overexpressed in seminomas, the most frequent testicular germ cell cancer [20]. But, then, GPER was predominantly described in peritubular cells [21] (Fig. 1a). These results are in part different from data on GPER gene expression, which was observed weakly in Sertoli cells and higher in interstitial cells [6]. The GPER protein of 42 KDa was retrieved on human spermatozoa, in the mid-piece (Fig. 1b) but its localization seems to be species dependent [22]. Therefore, the three forms of oestrogen receptors are expressed by testicular cells and spermatozoa.
Roles, effects and signalling pathways of oestrogens in testis and spermatozoa
KO and gene overexpression: mice models and men mutations
Some evidence on the role of oestrogens in male fertility came from the data obtained in the mouse knockout (KO) model. Especially, while male mice deficient in aromatase (Cyp19KO) were initially fertile, they developed progressive infertility with time. Disruptions of spermatogenesis were observed between 4.5 months and 1 year, despite no decreases in gonadotropins or androgens. Spermatogenesis was primarily stopped at early spermiogenic stages as characterized by an increase in apoptosis and the appearance of multinucleated cells, leading to a significant reduction in round and elongated spermatids without reduction changes in Sertoli cells and earlier germ cells. In addition, the presence of Leydig cells hyperplasia/hypertrophy could be clearly appreciated; presumably as a consequence of increased circulating luteinizing hormone [23]. The ERαKO mice were infertile [24] as the ERαβKO mice [25, 26]. Although spermatogenesis was normal up to 10 weeks, mice presented disrupted spermatogenesis and later degeneration of seminiferous tubules afterwards; this was due to the inability to reabsorb luminal fluids [24]. The same phenotype was revealed during the experiment on mice which were deleted from exon 3 of the Esr1 gene lacking the DNA binding domain and null for ERα (Ex3αERKO mice) [27]. Joseph and collaborators have observed that sperm recovered from the epididymis of ERαKO mice revealed abnormal coiled flagellum and increased the incidence rate of spontaneous acrosome reactions [28, 29]. To determine the importance of non-classical action of oestrogen receptor, a mouse model expressing exclusively an ERα mutant (2 aa mutation in the DNA binding domain) was produced [30]. It allowed us to demonstrate that non-ERE-dependent oestrogen pathways are sufficient to rescue the defective spermatogenesis observed in ERKO mice and play a prominent role in ERα action in the testis, including pathways that regulate water resorption and androgen biosynthesis. Moreover, oestrogen non responsive ERalpha knock-in (ENERKI) mice model (mutation in the ligand-binding domain) permitted to enlighten that oestrogen dependent and independent oestrogen receptor alpha signalling separately regulate male fertility, with an essential role for oestrogen independent ER signalling to concentrate epididymal sperm via regulation of efferent ductule fluid reabsorption and a necessity of oestrogen-dependent ER signalling for germ cell viability [31]. The first model of ERβKO mice was fertile [32] whereas the ERβKO mice developed by Antal later in which exon 3 was deleted through Cre/LoxP-mediated excision and which was devoid of any transcript downstream exon 3 were sterile. However, these mice presented no histopathological abnormalities [33]. Otto and collaborators suggest that GPR30 does not mediate oestrogenic responses in reproductive organs in mice as GPR30KO mice presented no difference in their litters as compared to wild type mice [34], however there was no description of the spermatogenic process.
Over-expressions of the Cyp19 gene encoding for aromatase enzyme were conducted and brought to light that transgenic male mice expressing human P450 aromatase (AROM+) were infertile (for review [35]).
Only eight cases of invalidation of aromatase have been reported in human beings (for review [36]),[37]. Among them, some patients presented an impaired reproductive function characterised by a decrease of motility and the number of spermatozoa (for review [36]). Only one case of congenital deficiency in oestrogens was described in human beings, due to a resistance to oestrogens consecutive to a punctual mutation of ERα gene, which revealed a higher sperm density than normal but a reduction in viability [38]. In human beings, polymorphisms of oestrogen related genes would mainly regulate sperm concentration and motility but not sperm morphology [39]. Guardacci and collaborators have observed an inverse correlation between higher TA, repeated in the promoter region of the ERα gene and the total sperm number in a group of infertile men, suggesting a possible negative influence on human spermatogenesis [40]. The long TA repeats would enhance oestrogen action [41]. The significance of the ERα gene in spermatogenesis and semen quality was supported by the data of Lazaros and collaborators who observed ERα polymorphisms associated to sperm motility and concentration [42]. Whereas the single nucleotide polymorphisms (SNPs) and ERβ gene mutations don’t seem to be a common cause of spermatogenesis failure in Indian men [43], Aschim and collaborators [44] concluded that some SNPs in ERβ could modulate human spermatogenesis. They observed a frequency of the heterozygous RsaI AG-genotype three times higher in infertile men compared to controls [44]. However, no significant associations were found between ERβ (1082G → A and 1730A → G) polymorphisms and sperm concentration or motility [42].
In vivo treatments
Studies conducted in rodents and primates have shown that spermatogenesis is partly under oestrogens control at different levels. In the immature bank vole, exposure to a low dose of estradiol induced acceleration of the onset of spermatogenesis, which is blocked by the injection of anti-oestrogen ICI 182,780. On the other hand, when males were treated with a high dose of estradiol or ICI 182,780, disruption of testicular structure, tubular atrophy and increased apoptosis of germ cells were observed [45]. Moreover, an improvement of the recrudescence of spermatogenesis in estradiol treated rodents was observed [46, 47]. The induction of spermatogenesis by estradiol in hypogonadal (hpg) mice involved an ERα dependent neuroendocrine mechanism increasing circulating FSH and premeiotic spermatogonia and meiotic spermatocytes [48]. Neonatal oestrogen administration to rats induced an increase in the number of spermatogonia at day 16 of life [49]. However, experiments conducted with adult rats showed that estradiol effects are dose and age dependent. While estradiol reduced sperm motility even at a low dose, doses below 10 μg/kg/day appeared to maintain whilst higher doses reversibly disrupted spermatogenesis [50]. Chronic estradiol benzoate treated adult rats showed a decrease of pachytene spermatocytes and round spermatids due to high testicular oxidative stress, altered serum hormonal levels (LH, FSH and testosterone) and low intra-testicular testosterone [51]. The exposure of the adult male rat to a high phytoestrogen diet confirmed spermatogenesis disruption by increasing germ cell apoptosis [52] (Fig. 2). These effects on spermatogenesis, apoptosis and hormone levels were maintained in adults after neonatal exposure [53]. Moreover, some works enlightened the possible implication of oestrogens in the regulation of differentiation (spermiogenesis) and thus the quality of the gamete and finally the success of all following steps (epididymal transit, capacitation, acrosomic reaction) until fertilization of the oocyte. In fact, treatment of adult monkeys with an aromatase inhibitor suggests that oestrogens are important for spermatid differentiation [54, 55] (Fig. 2). In rodents, the first sign of effects (deformation of acrosomal granule and nucleus) of neonatal estradiol administration was detected in the steps 2-3 spermatids [56]. Studies conducted in rats by D’Souza group suggest that the elongation process of spermatids from steps 8 to 19 is androgen dependent whereas differentiation of round spermatids from steps 1 to 6 is oestrogen relevant. This can be substantiated by the fact that 20 μg/kg/day of 17β-estradiol administered for 20 days induced prolonged deficiency of testosterone causing an absence of step 9–19 spermatids seen and the lack of apoptosis seen in steps 1–6 round spermatids [57]. In all estradiol treated groups, they observed an elevation of elongated spermatids failure to undergo spermiation demonstrating an inhibiting effect of exogenous 17β-estradiol on spermiation [58] that could be due to altered vimentin phosphorylation and reorganization [59]. However, this group observed that 20 and 100 μg/kg/day of estradiol have a different effect on the number of rat elongating and round spermatids whereas both induced a significant decrease in 2n cells (somatic and germ cells) and 4n cells (pachytene spermatocytes) in accordance with cell apoptosis effect of exogenous estradiol in testis [60]. The observation of abnormal acrosome development in the ArKO mouse suggests that acrosome biogenesis could be an oestrogen dependent process [23] (Fig. 2). This hypothesis is supported by high levels of aromatase in the Golgi complex of the developing spermatid [61] as well as the presence of oestrogen receptors in rat spermatids [5, 15]. The chromatin condensation occurring during spermiogenesis is also regulated by oestrogen (for review [62]). In fact, the levels of testicular and sperm proteins implicated in this step (TP1, TP2, P1 and CREMτ) decreased after estradiol treatment [63]. Cacciola and collaborators [64] have shown that low 17β-estradiol levels in CNR1 knockout mice play an important role in regulating chromatin remodelling of spermatids by interfering with chromatin reorganization. Moreover, oestrogens, by promoting histone displacement and chromatin condensation rescue, were able to efficiently reduce the greater nuclear length observed in Cnr1-/- sperm, a morphological parameter related to chromatin quality [65].
Knowing that effects of estradiol could implicate different oestrogen receptors subtypes, some studies were conducted with some oestrogen receptor subtype specific ligands [66, 67]. The over-activation of ERα or ERβ subtype had detrimental effects on the fertility parameters knowing that the two ERs had overlapping and distinct roles. The activation of ERβ would be notably involved in spermiation process and apoptosis whereas the activation of ERα would be involved in spermiogenesis regulation (Fig. 2).
Pathak and collaborators [68] had observed hypo-methylation at the Igf2-H19 ICR in the spermatozoa of tamoxifen-treated rats; this was also found in the methylation patterns of this loci in the embryos (Fig. 2). However, further studies from the same group did not indicate any changes in the methylation status of other imprinted genes (DMR) in spermatozoa [69].
In conclusion, in vivo exposures (anti-aromatase, anti-oestrogens or oestrogens) demonstrate that it is probably the balance between oestrogens and androgens which is crucial for the ongoing of spermatogenesis and quality of spermatozoa. In fact, whereas overexposure to estradiol or to SERM like tamoxifen can cause deleterious effects on the male reproductive tract and fertility, estradiol can attenuate the age related decline in spermatogenesis [70, 71].
Seasonal breeders
Some natural models of spermatogenesis arrest are the seasonal breeders and in several species the synthesis and somewhat the role of oestrogens have been explored. In the male black bear (Ursus americanus), the presence of aromatase has been reported at the beginning of testicular recrudescence in Sertoli cells and then in round and elongating spermatids in June, in mating season [72]. In Siberian Hamster, oestrogens are able to induce initiation of spermatogenesis, independently of FSH in photo-regressed adult male [47]. In roe deer (Capreolus capreolus) a study showed that oestrogens could be implicated in sperm production and in spermatozoa maturation by a regulated expression of ERα [73]. In the wild male ground squirrel (Citellus dauricus Brandt) a positive immunoreactivity for aromatase has been evidenced in Leydig and Sertoli cells and all types of spermatogenic cells only during the breeding season and was absent in the non-breeding season. Authors suggest that oestrogens could play an important role in spermatogenesis and testicular recrudescence and regression process [74]. In stallion, although no arrest of spermatogenesis is observed, the semen oestrogens/androgens ratio and ESR expression on spermatozoa in breeding season is higher to the non-breeding season [75, 76].
In vitro experiments
The experiences conducted in vitro permitted to precise the events regulated by oestrogens (Fig. 2) and their mechanisms of action. Whereas endogenous oestrogens inhibited male germ cell line development in mice during perinatal life [77], estradiol stimulated the proliferation of rat gonocytes [78, 79]. There is evidence of the direct role of oestrogens in preventing germ cell apoptosis. The protective effects of estradiol observed in cultures of human adult seminiferous tubules develop very quickly, indicating a non-genomic action of oestrogens [80]. However, estradiol was reported to induce in vitro apoptosis of rat spermatogenetic cells via the mitochondrial pathway; these elements tend to demonstrate the direct action of oestrogen in the absence of testicular somatic cells and without the interference of the hypothalamo-hypophyseal axis [81]. More recently, we were able to observe that estradiol activates the EGFR/ERK signalling pathway that modulates the expression of genes involved in the balance between cell proliferation and apoptosis within PS and RS purified by rats’testes [4, 5]. Oestrogens’ effects seem to depend on the cellular environment and estradiol concentration, as we have seen in cultures of adult rat seminiferous tubules, estradiol at 10-9 M expresses a different regulation of cyclins A1 and B1 [82]. In cultured immature rat Sertoli cells, 17β-estradiol induces the translocation of oestrogen receptors ESR1 and ESR2 to the cell membrane, as well as MAPK3/1 phosphorylation and their proliferation [83] whereas the binding of estradiol to GPER mediates MAPK3/1 activation through G protein beta gamma subunits that promote SRC-mediated metalloprotease dependent release of EGFR ligands which regulate gene expression involved in apoptosis [19, 84]. The anti-apoptotic effect of 17β-estradiol in immature Sertoli cells was confirmed by Simoes et al. [85]. 17β-estradiol and G1 (agonist of GPER) also induce PI3K/AKT signalling pathway activation and CREB phosphorylation in immature Sertoli cells [84]. A recent publication from the same lab suggests that ESR1 and ESR2 activation by estradiol is respectively involved in proliferation and in Sertoli cells differentiation respectively. In fact, from 15-day-old rats E2 modulates Sertoli cell proliferation through ESR1/NF-kB-mediated increase of CCND1, and cell cycle exit and differentiation through ESR2/CREB-mediated increase of CDKN1B, GATA-1 and DMRT1 [86]. Kumar and collaborators used seminiferous tubule culture to demonstrate that the genes involved in actin remodelling (Arpc1b, Evl and Picalm), could also play a role in spermiation and are oestrogen-regulated [87].
Spermatozoa
In addition to spermatogenesis events regulated by oestrogens, these hormones could also regulate some functions of spermatozoa (Table 1). Knowing that spermatozoa are transcriptionally inactive cells [88], the effects of oestrogen are non-genomic. Upon estradiol exposure, Aquila and collaborators observed an enhancement in the phosphorylation of the proteins in the PI3K/Akt pathway, some of them involved in cell survival signals [9]. However, Bennetts and collaborators observed no effect of 17β-estradiol on human spermatozoa viability whereas catechol oestrogens were able to induce a significant loss of cell viability [89]. In the same way, estradiol was described inducing or not DNA damage [89, 90]. 17β-estradiol also stimulated golden hamster spermatozoa motility [91] whereas it reduced the average path velocity (VAP) and the straight-line velocity (VSL) of stallion sperm [92]. In human spermatozoa, only catechol oestrogens (no estradiol) were reported to modify their motility [89] although it was previously said to regulate them positively [93–95]. The activation of ERK1/2 by estradiol could be involved in the positive effects of estradiol [95]. It was recently demonstrated that LRH-1, a transcription factor located in human sperm head, could also be implicated in oestrogen signalling pathway of motility, knowing that oestrogen receptors ESR1 and ESR2 are mainly described on flagellum [9, 11, 14] and without hypothesis of molecular mechanisms [96]. In vivo mice exposure to increased 17β-estradiol concentrations caused premature sperm capacitation in the epididymis [97]. This effect was confirmed by an increase of protein tyrosine phosphorylation level after in vitro incubations of epididymal spermatozoa with 17β-estradiol; but the number of sperm that underwent the acrosome reaction was lower in this group [98].
Oestrogens and/or xenooestrogens also stimulated in vitro mouse, human, boar and bovine sperm capacitation [95, 98–102], human, mouse, boar and bovine sperm acrosome reaction [95, 99, 102, 103] and the fertilizing ability of mouse sperm suspensions [99]. These effects were reported to be time and species dependent [102] (Table 1) but not involving classical oestrogen receptors [99]. However, it has been observed that oestrogens can inhibit some events induced by progesterone. In fact, estradiol inhibited Ca2+ influx and acrosomic reaction induced by progesterone in human spermatozoa (for review [3]) and hyper-activation enhanced by progesterone and melatonin in hamster spermatozoa [104, 105]. In human, 17β-estradiol also regulated cholesterol efflux, protein tyrosine phosphorylation, motility, and acrosin activity of sperm but the impact of E2 treatments were reduced or absent in the case of varicocele [14]. Finally, estradiol was also able to regulate lipid and glucose metabolism of sperm [14].
1α,25(OH)2Vitamin D3 (1,25-D3)
Vitamin D3 receptors
The vitamin D receptor (VDR), originally identified as a chromatin-associated protein [106] binds 1,25-D3 with high affinity and specificity, and is associated to 1,25-D3 classical effects. On the other hand, the main assumption is that rapid responses to this steroid hormone first occur in the plasma membrane through a membrane-associated receptor [107]. Through crystallography studies, the group of Norman [108–110] showed that besides the genomic binding pocket (VDRnuc), VDR also has an alternative pocket (VDRmem) that could be associated with non-genomic responses initiated in the plasma membrane.
Another membrane-associated receptor for 1,25-D3 has also been described. PDIA3 (protein disulfide isomerase family A, member 3) was first isolated from the basal membrane of intestinal epithelial cells of chicks [107]. It was demonstrated that this receptor could bind to 1,25-D3 with high affinity, being first nominated 1,25-MARRS (1,25-D3 membrane associated rapid response to steroids). This protein also received other denominations, such as ERp60, ERp57 and GRp58. Later on, it was demonstrated that they were one and the same protein, each having an identical structure when compared to others [111]. Like the classical 1,25-D3 receptor VDR, PDIA3 is also present in the caveolae at the plasma membrane [112, 113].
Vitamin D and all its metabolites, including the steroid hormone 1,25-D3, are conformationly flexible (for review [2]). The conformational flexibility (6-s-trans and 6-s-cis) determines the signal transduction pathway that has to be activated and, consequently, the biological response produced by its activation (respectively genomic or non-genomic respectively) [109, 114]. Studies about the structure-function of 1,25-D3 showed that the 6-s-cis analogue vitamin, 1α,25-dihydroxylumisterol3 (JN) can efficiently induce transcaltachia in intestinal epithelium and also stimulate Ca2+ uptake in osteosarcoma cell line via VDRmem. This analogue, however, failed to activate the genomic action, and it has shown a very low capacity to bind to VDRnuc [115, 116]. On the other hand, another analogue vitamin, the 1β,25-dihydroxyvitamin D3 (HL) has been proved to block rapid responses induced by 1,25-D3 or JN and is recognized as a specific antagonist of the non-genomic action [117, 118]. Similarly, the 1,25-D3 genomic response can be blocked by co-incubation with the analogue vitamin (23S)-25-dehydro-1α(OH)-vitamin D3-26,23-lactone (MK), which antagonistic action is caused by the inhibition of heterodimer formation between VDR and RXR, and of VDR interaction with co-activator, steroid receptor co-activator 1 (SRC-1) [119].
The presence of VDR in several tissues and cells of the male reproductive system has been demonstrated by several studies; this could explain the importance of this hormone in reproductive tissues. Animal studies have shown VDR expression in both nucleus and cytoplasm in primary cultures of immature rat Sertoli cells and in immature mice Sertoli cell line TM4 [120–122], seminiferous tubules and the caput epididymis [123, 124]. A staining of Leydig cells was also observed for human and mouse [125, 126]. VDR is expressed in germ cells of both rodents and humans: spermatogonia, spermatocytes and round and elongated spermatids [121, 125, 126] as well as in sperm [127, 128] but Sertoli cells are still considered to be the main 1,25-D3 target in the adult testis [2] (Fig. 1a). Previous studies have shown that, after injection of [3H]-1,25(OH)2-vitamin D3 (soltriol), the nuclear labelling is found in Sertoli cells, and is the highest one proportionally to the seminiferous tubules in which residual bodies in the apical part are distinctly stained [129]. ERp57, before being described as a vitamin D receptor, was investigated in human testis and spermatozoa. ERp57 was located in spermatocytes to spermatozoa and Leydig cells; a faint labelling was also observed in Sertoli cells [130] (Fig. 1). In the rat testis, PDIA3 is also present in Leydig and germ cells (from spermatogonia to spermatozoa). PDIA3 protein was observed on the entire membrane of rat spermatozoa and in the acrosome [131].
Spermatozoa are highly compartmentalized cells, and all studies investigating VDR expression in mature human spermatozoa showed VDR expression in the post-acrosomal part of the head, mid-piece, and in the neck region (Fig. 1b) [125, 127, 128]. Subcellular VDR expression may therefore depend on optimal spermatogenesis and maturation, because incomplete formation of the subcellular compartments may lead to an unusual localisation and, potentially, an unusual function of the receptor [132, 133].
Roles, effects and signalling pathways of 1,25-D3 in testis and spermatozoa
KO mice models and in vivo treatments
There is accumulating evidence that 1,25-D3 and VDR constitute an important part of the reproductive tissues. In fact, although the male vitamin D receptor of null mutant mice produced by Erben and collaborators were fertile [134]; the VDR knockout mice produced by the group of Kinuta [135] showed gonadal insufficiencies. Indeed, in the male, decreased sperm count and decreased motility with histological abnormality of the testis were observed [135]. This difference may be related to a different genetic background [134]. Early studies in vitamin D-deficient male rats have shown that, even if they are able to reproduce themselves, animals have a 45% reduction in successful mattings as well as a decreased overall fertility rate that is reduced by 73% when compared to controls [136]. The testes of vitamin D-deficient rats showed incomplete spermatogenesis and degenerative changes [137]. The fertility of vitamin D deficient male rats is restored by treatment with vitamin D and its active metabolite 1,25-dihydroxycholecalciferol but their effect is indirect as fertility is also restored by a diet supplemented with high levels of calcium [138]. 1,25-D3 also had some protective actions in testis of diabetic rats. Hamden and collaborators [139] have observed that the administration of 1,25-D3 3 weeks before and after diabetes induction prevented oxidative stress, toxicity and hypo-fertility in diabetic rat testes. Finally, it has been shown that vitamin D treatment up-regulates some testis-specific genes in cryptorchid mouse testis whereby 19 out of 2483 testis-specific genes showed upregulation by 1,25-D3 treatment [124].
In vitro treatments
As observed in osteoblasts notably by Enjuanes et al. [140], we observed that 1,25-D3 is able to stimulate the expression of Cyp19, the gene which encodes the enzyme P450 aromatase, in immature rat Sertoli cells [121]. Menagaz et al. [141] reported that 1,25-D3 plays an important role in testis through genomic effects that can be triggered by protein kinase A, as well as by rapid responses (amino acid accumulation) involving Ca2+/K+ channels on the plasma membrane and stimulating exocytosis via Cl−channel activation in the Sertoli cell line TM4 [120]. Other studies demonstrated that 1,25-D3 triggers plasma membrane-initiated actions by modulating calcium uptake and by altering gamma-glutamyltranspeptidase (GGTP) activity in immature rat testis. GGTP is involved in the synthesis of specific proteins known to be secreted by Sertoli cells [142]. Also, Rosso et al. [143] reported that 1,25-D3 activates p38 MAPK and reorganizes microtubules, involving Ca2+, PKC and ERK1/2 as upstream regulators, and that extracellular Ca2+ have a central role to rapidly start hormone-induced gene transcription and/or the secretory activity of Sertoli cells.
Spermatozoa
In a study investigating human sperm at the molecular level, 1,25-D3 had an effect on cholesterol efflux, protein phosphorylation, and increased sperm survival [128]. Thus, 1,25-D3 might play an important role in the extra testicular maturation of sperm by influencing capacitation and might modulate sperm survival. Also, Aquila et al. [144] demonstrated that 1,25-D3 through VDR increased intracellular Ca2+ levels, motility, and acrosin activity, and reduced triglyceride content in sperm, revealing an effect of 1,25-D3 in the acquisition of fertilizing ability in human sperm. Moreover, 1,25-D3 increased sperm motility and induced acrosome reaction [145]. 1,25-D3 effect on motility was dependent on the characteristics of samples as 1,25-D3 significantly increased spermatozoa motility in young men but was unable to do so in spermatozoa from sub-fertile men [133].
Interactions between oestrogens and 1,25-D3 signalling pathways
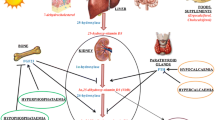
As reported before, oestrogens and 1,25-D3 could regulate spermatogenesis or spermatozoa’s maturation, suggesting possible interactions between their signalling pathways. This idea is, in fact, supported by some already published data (Fig. 3). First, both hormones’ respective receptors could be expressed by the same cells (testicular cells and spermatozoa) (Fig. 1). Then, VDR ablation was responsible for oestrogen deficiency and abnormal spermatogenesis observed in VDRKO mice whereas oestrogen supplementation protected the testis of histological abnormality [135]. Moreover, a significantly lower ERβ expression was found in testis of Vdr+/- and Vdr-/- Leuven strain mice without changes in the histology of the testis whereas epididymal expression of ERα and the oestrogen target gene Aqp9 were higher [146]. 1,25-D3 reduced significantly the levels of the steroid receptors (ERα, PRA and PRB) in human uterine leiomyoma (HuLM) cells, regulated positively the levels of its receptor (VDR) and inhibited oestrogen induced proliferation of cultured (HuLM) cells suggesting that 1,25-D3 could function as anti-oestrogenic/progesteronic agent [147].
Interactions between genomic and nongenomic action of 1,25-D3 and oestrogens.1,25-D3 can bind VDR localized at the plasma membrane or intracellular VDR and could regulate Cyp19 and ERα gene expression, aromatase activity and estradiol production. Estradiol can regulate VDR gene expression by ESR2 localized at the plasma membrane and Cyp19 gene expression. E2: estradiol, 1,25-D3: 1α,25 dihydroxyvitamin D3, ESR1/2: estrogen receptor 1 and 2, GPER: G protein coupled estrogen receptor, VDRmem: vitamin D receptor localized at the plasma membrane, VDRnuc: nuclear vitamin D receptor, VDRE: vitamin D responsive element, ERE: oestrogen responsive element
1,25-D3 could also regulate seric steroid levels and notably estradiol levels. In fact, 1,25-D3 positively regulated Cyp19 expression in immature rat Sertoli cells [121] as previously observed in human osteoblasts [140, 148]. Estradiol production of rat costochondral chondrocytes [149] and aromatase activity of human mesenchymal stem cells [150] and prostate cancer cells [151] are stimulated by vitamin D. However, 1,25-D3 effect on oestrogen metabolism is tissue specific. Admittedly it had a negative effect on aromatase expression and estradiol production in MCF-7 cells, whereas it had no effect on estradiol production in LnCap and NCI-H295R. Nevertheless, it induced a significant increase of estradiol production in NCI-H295R in presence of additional androstenedione [152]. 1,25-D3 also had a positive effect in estradiol production in osteosarcoma and ovarian cancer cells, but resulted a negative effect in oestrogen receptor-positive breast cancer (BCa) cells and adipocytes [153]. Aromatase down regulation by 1,25-D3 in BCa cells was due to a direct repression of aromatase transcription via promoter II through the vitamin D-response elements identified in this promoter and an indirect suppression by reducing the levels of prostaglandins [153]. It is interesting to note that Cyp19 expression in male gonad is in part driven by the promoter PII (for review [154]). The analogue vitamin D EB1089 is also able to decrease Cyp19 gene expression and aromatase activity and to inhibit the aromatase dependent cell growth of breast cancer cells [155]. In rat granulosa cells, 1,25-D3 reduced testosterone induced aromatase expression but improved 17β-estradiol production by a calcium dependent pathway [156]. In human activated macrophages, 1,25-D3 may downregulate the pro-inflammatory cytokine production by significantly decreasing the aromatase activity, especially in presence of an estrogenic milieu [157]. In HEK-293, ERK1/2 phosphorylation and up regulation of VDR protein expression induced by estradiol required the association of ERβ to plasma membrane caveolae components [158]. 1,25-D3 is able to restore the expression of a functional ERα in ER negative breast cancer cells probably by a transcriptional VDR dependent regulation, as the ER promoter contains several putative vitamin D response elements [159]. 1,25-D3 also regulates androgen production, the aromatase substrate, in a tissue specific manner [152, 160, 161]. It was interesting to note a testosterone level increase in a group of men who received vitamin D supplementation [160]. 1,25-D3 had a protective effect on alloxan-induced damage in reproductive system by enhancing the testosterone and 17β-estradiol levels consequently protecting from oxidative stress, cellular toxicity and maintaining the number and motility of spermatozoids [139]. Matsuda and collaborators have also demonstrated that the effects of oestrogen on mouse vaginal development are influenced by 1,25-D3 [162]. Finally, it was observed that in a reducing environment, estradiol competed for binding to 1,25-D3-MARRS receptor/PDIA3/ERp57 and was able to stimulated calcium uptake in isolated enterocytes [163].
Therefore, in testicular cells and in spermatozoa, vitamin D could modulate oestrogen non-genomic effects but also their genomic effects and reciprocally.
Conclusions
To conclude, we assume that common testicular cells and spermatozoa express oestrogen receptors (ESR) and 1,25-D3 receptors (VDR); moreover, in vitro and in vivo studies suggest that oestrogens and 1,25-D3 play roles in spermatogenesis and spermatozoa functions. However, the mechanisms involved in the regulation of the events under 1,25-D3 and 17β-estradiol control; and their possible interactions could not be completely identified. Indeed, even if the recent review published by Blomberg Jensen [164] also indicated a relation between VDR and oestrogen in the male reproductive organs, there were few data about dialogue mechanisms between oestrogen and vitamin D signalling pathways. Some data obtained in numerous cell types suggest that calcium could play an intermediate role in non-genomic effects, notably in spermatozoa but also in other testicular cell types like germ cells and Leydig cells. In fact, these cells, in which calcium currents were measured express VDR and ESR [165, 166]. The physiological significance and the specific roles endorsed by these hormones and their receptors in human spermatozoa require further investigation. A better comprehension of the matter could give us information regarding the potential altering effects of environmental xenobiotics (and notably xenoestrogens) on male fertility [167]. It has been stated that serum 1,25-D3 at high (>50 ng ml-1) and low (<20 ng ml-1) levels can be negatively associated with semen parameters [168] and that seminal plasma estradiol levels have shown an increase in infertile men [169]; but without identifying any pathophysiologic features in relation with male fertility, the interest to carry out such studies in men becomes all the more relevant. Moreover, in 2012, Lerchbaum and Obermayer-Pietsch [170] as well as Boisen and collaborators in 2016 [171] indicated that other studies (notably high quality randomized controlled trials) would be necessary to evaluate the effect of vitamin D supplementation on the improvement of semen quality and subsequently on fertility.
Abbreviations
- 1,25-D3 :
-
1α,25(OH)2-vitamin D3
- E2 :
-
Estradiol
- ESR:
-
Estrogen receptor
- GPER:
-
G protein-coupled estrogen receptor
- PS:
-
Pachytene spermatocyte
- RS:
-
Round spermatid
- VDR:
-
Vitamin D receptor
- VDRmem:
-
VDR localized at the plasma membrane
- VDRnuc:
-
VDR localized in the nucleus
References
Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos T Roy Soc B. 2010;365(1546):1517–35.
Zanatta L, Zamoner A, Zanatta AP, Bouraïma-Lelong H, Delalande C, Bois C, et al. Nongenomic and genomic effects of 1α,25(OH)(2) vitamin D(3) in rat testis. Life Sci. 2011;89(15-16):515–23.
Luconi M, Forti G, Baldi E. Genomic and nongenomic effects of estrogens: molecular mechanisms of action and clinical implications for male reproduction. J Steroid Biochem Mol Biol. 2002;80(4-5):369–81.
Chimento A, Sirianni R, Delalande C, Silandre D, Bois C, Andò S, et al. 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Mol Cell Endocrinol. 2010;320(1-2):136–44.
Chimento A, Sirianni D, Zolea F, Bois C, Delalande C, Andò S, et al. Gper and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. Int J Androl. 2011;34(5 Pt 1):420–9.
Fietz D, Ratzenböck C, Hartmann K, Raabe O, Kliesch S, Weidner W, et al. Expression pattern of estrogen receptors α and β and G-protein-coupled estrogen receptor 1 in the human testis. Histochem Cell Biol. 2014;142(4):421–32.
Aschim EL, Saether T, Wiger R, Grotmol T, Haugen TB. Differential distribution of splice variants of estrogen receptor beta in human testicular cells suggests specific functions in spermatogenesis. J Steroid Biochem Mol Biol. 2004;92(1-2):97–106.
Lambard S, Galeraud-Denis I, Saunders PT, Carreau S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J Mol Endocrinol. 2004;32(1):279–89.
Aquila S, Sisci D, Gentile M, Middea E, Catalano S, Carpino A, et al. Estrogen receptor (ER)alpha and ER beta are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J Clin Endocrinol Metab. 2004;89(3):1443–51.
Rago V, Siciliano L, Aquila S, Carpino A. Detection of estrogen receptors ER-alpha and ER-beta in human ejaculated immature spermatozoa with excess residual cytoplasm. Reprod Biol Endocrinol. 2006;4(1):36.
Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum Reprod. 2005;20(12):3481–7.
Baldi E, Luconi M, Muratori M, Forti G. A novel functional estrogen receptor on human sperm membrane interferes with progesterone effects. Mol Cell Endocrinol. 2000;161(1-2):31–5.
Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, et al. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab. 2002;87(6):2706–15.
Guido C, Perrotta I, Panza S, Middea E, Avena P, Santoro M, et al. Human sperm physiology: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J Cell Physiol. 2011;226(12):3403–12.
Bois C, Delalande C, Nurmio M, Parvinen M, Zanatta L, Toppari J, et al. Age and cell related gene expression of aromatase and estrogen receptors in the rat testis. J Mol Endocrinol. 2010;45(3):147–59.
Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–26.
Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Andò S, et al. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149(10):5043–51.
Chimento A, Sirianni R, Casaburi I, Ruggiero C, Maggiolini M, Andò S, et al. 17β-Estradiol activates GPER- and ESR1-dependent pathways inducing apoptosis in GC-2 cells, a mouse spermatocyte-derived cell line. Mol Cell Endocrinol. 2012;355(1):49–59.
Lucas TF, Royer C, Siu ER, Lazari MF, Porto CS. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biol Reprod. 2010;83(2):307–17.
Chevalier N, Vega A, Bouskine A, Siddeek B, Michiels JF, Chevallier D, et al. GPR30, the non-classical membrane G protein related estrogen receptor, is overexpressed in human seminoma and promotes seminoma cell proliferation. PLoS One. 2012;7(4):e34672.
Sandner F, Welter H, Schwarzer JU, Köhn FM, Urbanski HF, Mayerhofer A. Expression of the oestrogen receptor GPER by testicular peritubular cells is linked to sexual maturation and male fertility. Andrology. 2014;2(5):695–701.
Rago V, Giordano F, Brunelli E, Zito D, Aquila S, Carpino A. Identification of G protein-coupled estrogen receptor in human and pig spermatozoa. J Anat. 2014;224(6):732–6.
Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci U S A. 1999;96(14):7986–91.
Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–805.
Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286(5448):2328–31.
Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–91.
Goulding EH, Hewitt SC, Nakamura N, Hamilton K, Korach KS, Eddy EM. Ex3αERKO male infertility phenotype recapitulates the αERKO male phenotype. J Endocrinol. 2010;207(3):281–8.
Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod. 2010;82(5):958–67.
Joseph A, Hess RA, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, et al. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod. 2010;82(5):948–57.
Weiss J, Bernhardt ML, Laronda MM, Hurley LA, Glidewell-Kenney C, Pillai S, et al. Estrogen actions in the male reproductive system involve estrogen response element-independent pathways. Endocrinology. 2008;149(12):6198–206.
Sinkevicius KW, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL. Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology. 2009;150(6):2898–905.
Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–82.
Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105(7):2433–8.
Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80(1):34–41.
Li X, Rahman N. Impact of androgen/estrogen ratio: lessons learned from the aromatase over-expression mice. Gen Comp Endocrinol. 2008;159(1):1–9.
Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3(5):414–21.
Lanfranco F, Zirilli L, Baldi M, Pignatti E, Corneli G, Ghigo E, et al. A novel mutation in the human aromatase gene: Insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–35.
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–61. Erratum in: N Engl J Med. 1995; 332(2):131.
Lee IW, Kuo PH, Su MT, Kuan LC, Hsu CC, Kuo PL. Quantitative trait analysis suggests polymorphisms of estrogen-related genes regulate human sperm concentrations and motility. Hum Reprod. 2011;26(6):1585–96.
Guarducci E, Nuti F, Becherini L, Rotondi M, Balercia G, Forti G, et al. Estrogen receptor alpha promoter polymorphism: stronger estrogen action is coupled with lower sperm count. Hum Reprod. 2006;21(4):994–1001.
Becherini L, Gennari L, Masi L, Mansani R, Massart F, Morelli A, et al. Evidence of a linkage disequilibrium between polymorphisms in the human estrogen receptor alpha gene and their relationship to bone mass variation in postmenopausal Italian women. Hum Mol Genet. 2000;9(13):2043–50.
Lazaros LA, Xita NV, Kaponis AI, Zikopoulos KA, Plachouras NI, Georgiou IA. Estrogen receptor alpha and beta polymorphisms are associated with semen quality. J Androl. 2010;31(3):291–8.
Khattri A, Pandey RK, Gupta NJ, Chakravarty B, Deenadayal M, Singh L, et al. Estrogen receptor beta gene mutations in Indian infertile men. Mol Hum Reprod. 2009;15(8):513–20.
Aschim EL, Giwercman A, Ståhl O, Eberhard J, Cwikiel M, Nordenskjöld A, et al. The RsaI polymorphism in the estrogen receptor-beta gene is associated with male infertility. J Clin Endocrinol Metab. 2005;90(9):5343–8.
Gancarczyk M, Paziewska-Hejmej A, Carreau S, Tabarowski Z, Bilińska B. Dose- and photoperiod-dependent effects of 17beta-estradiol and the anti-estrogen ICI 182,780 on testicular structure, acceleration of spermatogenesis, and aromatase immunoexpression in immature bank voles. Acta Histochem. 2004;106(4):269–78.
Ebling FJ, Brooks AN, Cronin AS, Ford H, Kerr JB. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology. 2000;141(8):2861–9.
Pak TR, Lynch GR, Tsai PS. Estrogen accelerates gonadal recrudescence in photo-regressed male siberian hamsters. Endocrinology. 2002;143(10):4131–4.
Allan CM, Couse JF, Simanainen U, Spaliviero J, Jimenez M, Rodriguez K, et al. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-{alpha} mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology. 2010;151(6):2800–10.
Walczak-Jedrzejowska R, Slowikowska-Hilczer J, Marchlewska K, Kula K. Maturation, proliferation and apoptosis of seminal tubule cells at puberty after administration of estradiol, follicle stimulating hormone or both. Asian J Androl. 2008;10(4):585–92.
Gill-Sharma MK, Dsouza S, Padwal V, Balasinor N, Aleem M, Parte P, et al. Antifertility effects of estradiol in adult male rats. J Endocrinol Invest. 2001;24(8):598–607.
Chaki SP, Misro MM, Gautam DK, Kaushik M, Ghosh D, Chainy GB. Estradiol treatment induces testicular oxidative stress and germ cell apoptosis in rats. Apoptosis. 2006;11(8):1427–37.
Assinder S, Davis R, Fenwick M, Glover A. Adult-only exposure of male rats to a diet of high phytoestrogen content increases apoptosis of meiotic and post-meiotic germ cells. Reproduction. 2007;133(1):11–9.
Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, et al. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140(11):5364–73.
Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar S, Moudgal RN. Effect of estrogen deprivation on the reproductive physiology of male and female primates. J Steroid Biochem Mol Biol. 1997;61(3-6):157–66.
Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar S, Moudgal RN. Effect of long-term treatment with aromatase inhibitor on testicular function of adult male bonnet monkeys (M. radiata). Steroids. 1998;63(7-8):414–20.
Toyama Y, Yuasa S. Effects of neonatal administration of 17beta-estradiol, beta-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol. 2004;19(2):181–8.
D’Souza R, Gill-Sharma MK, Pathak S, Kedia N, Kumar R, Balasinor N. Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Mol Cell Endocrinol. 2005;241(1-2):41–8.
D’Souza R, Pathak S, Upadhyay R, Gaonkar R, D’Souza S, Sonawane S, et al. Disruption of tubulobulbar complex by high intratesticular estrogens leading to failed spermiation. Endocrinology. 2009;150(4):1861–9.
Upadhyay R, D’Souza R, Sonawane S, Gaonkar R, Pathak S, Jhadav A, et al. Altered phosphorylation and distribution status of vimentin in rat seminiferous epithelium following 17β-estradiol treatment. Histochem Cell Biol. 2011;136(5):543–55.
Balasinor NH, D’Souza R, Nanaware P, Idicula-Thomas S, Kedia-Mokashi N, He Z, et al. Effect of high intratesticular estrogen on global gene expression and testicular cell number in rats. Reprod Biol Endocrinol. 2010;8:72.
Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Millette CF, et al. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132(3):1396–401.
Cacciola G, Chioccarelli T, Fasano S, Pierantoni R, Cobellis G. Estrogens and spermiogenesis: new insights from type 1 cannabinoid receptor knockout mice. Int J Endocrinol. 2013;2013:501350.
Aleem M, Padwal V, Choudhari J, Balasinor N, Parte P, Gill-Sharma MK. Estradiol affects androgen-binding protein expression and fertilizing ability of spermatozoa in adult male rats. Mol Cell Endocrinol. 2006;253(1-2):1–13.
Cacciola G, Chioccarelli T, Altucci L, Ledent C, Mason JI, Fasano S, et al. Low 17beta-estradiol levels in CNR1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biol Reprod. 2013;88(6):152.
Cacciola G, Chioccarelli T, Altucci L, Viggiano A, Fasano S, Pierantoni R, et al. Nuclear size as estrogen-responsive chromatin quality parameter of mouse spermatozoa. Gen Comp Endocrinol. 2013;193:201–9.
Dumasia K, Kumar A, Kadam L, Balasinor NH. Effect of estrogen receptor-subtype-specific ligands on fertility in adult male rats. J Endocrinol. 2015;225(3):169–80.
Dumasia K, Kumar A, Deshpande S, Sonawane S, Balasinor NH. Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis. Mol Cell Endocrinol. 2016;428:89–100.
Pathak S, Kedia-Mokashi N, Saxena M, D’Souza R, Maitra A, Parte P, Gill-Sharma M, Balasinor N. Effect of tamoxifen treatment on global and insulin-like growth factor 2-H19 locus-specific DNA methylation in rat spermatozoa and its association with embryo loss. Fertil Steril. 2009;91(5 Suppl):2253–63.
Kedia-Mokashi NA, Kadam L, Ankolkar M, Dumasia K, Balasinor NH. Aberrant methylation of multiple imprinted genes in embryos of tamoxifen-treated male rats. Reproduction. 2013;146(2):155–68.
Hamden K, Silandre D, Delalande C, El Feki A, Carreau S. Protective effects of estrogens and caloric restriction during aging on various rat testis parameters. Asian J Androl. 2008;10(6):837–45.
Clarke M, Pearl CA. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst Biol Reprod Med. 2014;60(2):89–97.
Tsubota T, Howell-Skalla L, Nitta H, Osawa Y, Mason JI, Meiers PG, et al. Seasonal changes in spermatogenesis and testicular steroidogenesis in the male black bear Ursus americanus. J Reprod Fertil. 1997;109(1):21–7.
Schön J, Blottner S. Estrogens are involved in seasonal regulation of spermatogenesis and sperm maturation in roe deer (Capreolus capreolus). Gen Comp Endocrinol. 2008;159(2-3):257–63.
Zhang H, Sheng X, Hu X, Li X, Xu H, Zhang M, et al. Seasonal changes in spermatogenesis and immunolocalization of cytochrome P450 17alpha-hydroxylase/c17-20 lyase and cytochrome P450 aromatase in the wild male ground squirrel (Citellus dauricus Brandt). J Reprod Dev. 2010;56(3):297–302.
Lemazurier E, Moslemi S, Sourdaine P, Desjardins I, Plainfosse B, Seralini GE. Free and conjugated estrogens and androgens in stallion semen. Gen Comp Endocrinol. 2002;125(2):272–82.
Gautier C, Barrier-Battut I, Guénon I, Goux D, Delalande C, Bouraïma-Lelong H. Implication of the estrogen receptors GPER, ESR1, ESR2 in post-testicular maturations of equine spermatozoa. Gen Comp Endocrinol. 2016;233:100–8.
Delbès G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, et al. Estrogen receptor beta-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology. 2004;145(7):3395–403.
Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138(3):1289–98.
Wang Y, Thuillier R, Culty M. Prenatal estrogen exposure differentially affects estrogen receptor-associated proteins in rat testis gonocytes. Biol Reprod. 2004;71(5):1652–64.
Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab. 2000;85(5):2057–67.
Mishra DP, Shaha C. Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway: role of superoxide and nitric oxide. J Biol Chem. 2005;280(7):6181–96.
Bois C, Delalande C, Bouraïma-Lelong H, Durand P, Carreau S. 17β-Estradiol regulates cyclin A1 and cyclin B1 gene expression in adult rat seminiferous tubules. J Mol Endocrinol. 2012;48(2):89–97.
Lucas TF, Siu ER, Esteves CA, Monteiro HP, Oliveira CA, Porto CS, et al. 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod. 2008;78(1):101–14.
Royer C, Lucas TF, Lazari MF, Porto CS. 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol Reprod. 2012;86(4):108.
Simões VL, Alves MG, Martins AD, Dias TR, Rato L, Socorro S, et al. Regulation of apoptotic signaling pathways by 5α-dihydrotestosterone and 17β-estradiol in immature rat Sertoli cells. J Steroid Biochem Mol Biol. 2013;135:15–23.
Lucas TF, Lazari MF, Porto CS. Differential role of the estrogen receptors ESR1 and ESR2 on the regulation of proteins involved with proliferation and differentiation of Sertoli cells from 15-day-old rats. Mol Cell Endocrinol. 2014;382(1):84–96.
Kumar A, Dumasia K, Gaonkar R, Sonawane S, Kadam L, Balasinor NH. Estrogen and androgen regulate actin-remodeling and endocytosis-related genes during rat spermiation. Mol Cell Endocrinol. 2015;404:91–101.
Lalancette C, Miller D, Li Y, Krawetz SA. Paternal contributions: new functional insights for spermatozoal RNA. J Cell Biochem. 2008;104(5):1570–9.
Bennetts LE, De Iuliis GN, Nixon B, Kime M, Zelski K, McVicar CM, et al. Impact of estrogenic compounds on DNA integrity in human spermatozoa: evidence for cross-linking and redox cycling activities. Mutat Res. 2008;641(1-2):1–11.
Anderson D, Schmid TE, Baumgartner A, Cemeli-Carratala E, Brinkworth MH, Wood JM. Oestrogenic compounds and oxidative stress (in human sperm and lymphocytes in the Comet assay). Mutat Res. 2003;544(2-3):173–8.
Jin W, Arai KY, Watanabe G, Suzuki AK, Takahashi S, Taya K. The stimulatory role of estrogen on sperm motility in the male golden hamster (Mesocricetus auratus). J Androl. 2005;26(4):478–84.
Filannino A, Stout TA, Gadella BM, Sostaric E, Pizzi F, Colenbrander B, et al. Dose-response effects of estrogenic mycotoxins (zearalenone, alpha- and beta-zearalenol) on motility, hyperactivation and the acrosome reaction of stallion sperm. Reprod Biol Endocrinol. 2011;9:134.
Idaomar M, Guerin JF, Lornage J, Moncharmont P, Czyba JC. Effects of estradiol and its antagonist tamoxifen on motility and metabolism of human spermatozoa. Adv Contracept Deliv Syst. 1987;3(4):337–45.
Idaomar M, Guerin JF, Lornage J, Czyba JC. Stimulation of motility and energy metabolism of spermatozoa from asthenozoospermic patients by 17 beta-estradiol. Arch Androl. 1989;22(3):197–202.
Aquila S, Sisci D, Gentile M, Carpino A, Middea E, Catalano S, et al. Towards a physiological role for cytochrome P450 aromatase in ejaculated human sperm. Hum Reprod. 2003;18(8):1650–9.
Montanaro D, Santoro M, Carpino A, Perrotta I, De Amicis F, Sirianni R, et al. Human sperm liver receptor homolog-1 (LRH-1) acts as a downstream target of the estrogen signaling pathway. J Anat. 2015;227(4):541–9.
Ded L, Sebkova N, Cerna M, Elzeinova F, Dostalova P, Peknicova J, et al. In vivo exposure to 17β-estradiol triggers premature sperm capacitation in cauda epididymis. Reproduction. 2013;145(3):255–63.
Sebkova N, Cerna M, Ded L, Peknicova J, Dvorakova-Hortova K. The slower the better: how sperm capacitation and acrosome reaction is modified in the presence of estrogens. Reproduction. 2012;143(3):297–307.
Adeoya-Osiguwa SA, Markoulaki S, Pocock V, Milligan SR, Fraser LR. 17beta-Estradiol and environmental estrogens significantly affect mammalian sperm function. Hum Reprod. 2003;18(1):100–7.
Fraser LR, Beyret E, Milligan SR, Adeoya-Osiguwa SA. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum Reprod. 2006;21(5):1184–93.
Ded L, Dostalova P, Dorosh A, Dvorakova-Hortova K, Peknicova J. Effect of estrogens on boar sperm capacitation in vitro. Reprod Biol Endocrinol. 2010;8:87.
Ryu DY, Kim YJ, Lee JS, Rahman MS, Kwon WS, Yoon SJ, et al. Capacitation and acrosome reaction differences of bovine, mouse and porcine spermatozoa in responsiveness to estrogenic compounds. J Anim Sci Technol. 2014;56:26.
Mohamed e-SA, Park YJ, Song WH, Shin DH, You YA, Ryu BY, et al. Xenoestrogenic compounds promote capacitation and an acrosome reaction in porcine sperm. Theriogenology. 2011;75(6):1161–9.
Fujinoki M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by estrogen. Reproduction. 2010;140(3):453–64.
Fujinoki M, Takei GL. Estrogen suppresses melatonin-enhanced hyperactivation of hamster spermatozoa. J Reprod Dev. 2015;61(4):287–95.
Haussler MR, Norman AW. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969;62(1):155–62.
Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994;269(38):23750–6.
Menegaz D, Mizwicki MT, Barrientos-Duran A, Chen N, Henry HL, Norman AW. Vitamin D Receptor (VDR) Regulation of Voltage-Gated Chloride Channels by Ligands Preferring a VDR-Alternative Pocket (VDR-AP). Mol Endocrinol. 2011;25(8):1289–300.
Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2(75):re4.
Norman AW, Mizwicki MT, Norman DPG. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3(1):27–41.
Khanal RC, Nemere I. The ERp57/GRp58/1,25D3-MARRS receptor: multiple functional roles in diverse cell systems. Curr Med Chem. 2007;14(10):1087–93.
Buitrago C, Boland R. Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;121(1-2):169–75.
Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285(47):37041–50.
Norman AW, Henry HL, Bishop JE, Song XD, Bula C, Okamura WH. Different shapes of the steroid hormone 1alpha,25(OH)(2)-vitamin D(3) act as agonists for two different receptors in the vitamin D endocrine system to mediate genomic and rapid responses. Steroids. 2001;66(3-5):147–58.
Norman AW, Bishop JE, Collins ED, Seo EG, Satchell DP, Dormanen MC, et al. Differing shapes of 1α,25-dihydroxyvitamin D3 function as ligands for the D-binding protein, nuclear receptor and membrane receptor: a status report. J Steroid Biochem Mol Biol. 1996;56(1-6 Spec No):13–22.
Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, et al. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1alpha,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol Endocrinol. 1997;11(10):1518–31.
Norman AW, Nemere I, Muralidharan KR, Okamura WH. 1β,25-(OH)2-vitamin D3 is an antagonist of 1α,25-(OH)2-vitamin D3 stimulated transcalthachia (the rapid hormonal stimulation of intestinal calcium transport). Biochem Biophys Res Commun. 1992;189(3):1450–6.
Norman AW, Bouillon R, Farach-Carson MC, Bishop JE, Zhou LX, Nemere I, et al. Demonstration that 1β,25-dihydroxyvitamin D3 is an antagonist of the nongenomic but not genomic biological responses and biological profile of the three A-ring diastereomers of 1α,25-dihydroxyvitamin D3. J Biol Chem. 1993;268(27):20022–30.
Bula CM, Bishop JE, Ishizuka S, Norman AW. 25-Dehydro-1α-hydroxyvitamin D3-26,23S-lactone antagonizes the nuclear vitamin D receptor by mediating a unique noncovalent conformational change. Mol Endocrinol. 2000;14(11):1788–96.
Menegaz D, Barrientos-Duran A, Kline A, Silva FR, Norman AW, Mizwicki MT, Zanello LP. 1alpha,25(OH)2-Vitamin D3 stimulation of secretion via chloride channel activation in Sertoli cells. J Steroid Biochem Mol Biol. 2010;119(3-5):127–34.
Zanatta L, Bouraïma-Lelong H, Delalande C, Silva FR, Carreau S. Regulation of aromatase expression by 1α,25(OH)2 vitamin D3 in rat testicular cells. Reprod Fertil Dev. 2011;23(5):725–35.
Zanatta L, Zamoner A, Gonçalves R, Zanatta AP, Bouraïma-Lelong H, Carreau S, et al. 1α,25-Dihydroxyvitamin D(3) signaling pathways on calcium uptake in 30-day-old rat Sertoli cells. Biochemistry. 2011;50(47):10284–92.
Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D3 receptor in rat reproductive tissues. Histochem Cell Biol. 1996;105(1):7–15.
Hirai T, Tsujimura A, Ueda T, Fujita K, Matsuoka Y, Takao T, et al. Effect of 1,25-dihydroxyvitamin d on testicular morphology and gene expression in experimental cryptorchid mouse: testis specific cDNA microarray analysis and potential implication in male infertility. J Urol. 2009;181(3):1487–92.
Blomberg Jensen M, Nielsen JE, Jørgensen A, Rajpert-De Meyts E, Kristensen DM, Jørgensen N, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25(5):1303–11.
Mahmoudi AR, Zarnani AH, Jeddi-Tehrani M, Katouzian L, Tavakoli M, Soltanghoraei H, et al. Distribution of vitamin D receptor and 1α-hydroxylase in male mouse reproductive tract. Reprod Sci. 2013;20(4):426–36.
Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006;68(6):1345–9.
Aquila S, Guido C, Perrotta I, Tripepi S, Nastro A, Andò S. Human sperm anatomy: ultrastructural localization of 1alpha,25-dihydroxyvitamin D receptor and its possible role in the human male gamete. J Anat. 2008;213(5):555–64.
Schleicher G, Privette TH, Stumpf WE. Distribution of soltriol [1,25(OH)2-vitamin D3] binding sites in male sex organs of the mouse: an autoradiographic study. J Histochem Cytochem. 1989;37(7):1083–6.
Zhang J, Wu J, Huo R, Mao Y, Lu Y, Guo X, et al. ERp57 is a potential biomarker for human fertilization capability. Mol Hum Reprod. 2007;13(9):633–9.
Zhao XJ, Tang RZ, Wang ML, Guo WL, Liu J, Li L, et al. Distribution of PDIA3 transcript and protein in rat testis and sperm cells. Reprod Domest Anim. 2013;48(1):59–63.
Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Hum Reprod Update. 2006;12(3):253–67.
Blomberg Jensen M, Jørgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012;35(4):499–510.
Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, et al. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol. 2002;16(7):1524–37.
Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141(4):1317–24.
Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989;119(5):741–4.
Osmundsen BC, Huang HF, Anderson MB, Christakos S, Walters MR. Multiple sites of action of the vitamin D endocrine system: FSH stimulation of testis 1,25-dihydroxyvitamin D3 receptors. J Steroid Biochem. 1989;34(1-6):339–43.
Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992;122(6):1338–44.
Hamden K, Carreau S, Jamoussi K, Ayadi F, Garmazi F, Mezgenni N, et al. Inhibitory effects of 1alpha, 25dihydroxyvitamin D3 and Ajuga iva extract on oxidative stress, toxicity and hypo-fertility in diabetic rat testes. J Physiol Biochem. 2008;64(3):231–9.
Enjuanes A, Garcia-Giralt N, Supervia A, Nogués X, Mellibovsky L, Carbonell J, et al. Regulation of CYP19 gene expression in primary human osteoblasts: effects of vitamin D and other treatments. Eur J Endocrinol. 2003;148(5):519–26.
Menegaz D, Rosso A, Royer C, Leite LD, Santos AR, Silva FR. Role of 1alpha,25(OH)2 vitamin D3 on alpha-[1-(14)C]MeAIB accumulation in immature rat testis. Steroids. 2009;74(2):264–9.
Zanatta L, Zamoner A, Gonçalves R, Zanatta AP, Bouraïma-Lelong H, Bois C, et al. Effect of 1α,25-dihydroxyvitamin D(3) in plasma membrane targets in immature rat testis: ionic channels and g-glutamyltranspeptidase activity. Arch Biochem Biophys. 2011;515(1-2):46–53.
Rosso A, Pansera M, Zamoner A, Zanatta L, Bouraima-Lelong H, Carreau S, et al. 1α,25(OH)(2)-vitamin D(3) stimulates rapid plasma membrane calcium influx via MAPK activation in immature rat Sertoli cells. Biochimie. 2012;94(1):146–54.
Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, et al. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009;7:140.
Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26(6):1307–17.
Blomberg Jensen M, Lieben L, Nielsen JE, Willems A, Jørgensen A, Juul A, et al. Characterization of the testicular, epididymal and endocrine phenotypes in the Leuven Vdr-deficient mouse model: Targeting estrogen signaling. Mol Cell Endocrinol. 2013;377(1-2):93–102.
Al-Hendy A, Diamond MP, El-Sohemy A, Halder SK. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J Clin Endocrinol Metab. 2015;100(4):E572–82.
Enjuanes A, Garcia-Giralt N, Supervía A, Nogués X, Ruiz-Gaspà S, Bustamante M, et al. Functional analysis of the I.3, I.6, pII and I.4 promoters of CYP19 (aromatase) gene in human osteoblasts and their role in vitamin D and dexamethasone stimulation. Eur J Endocrinol. 2005;153(6):981–8.
Sylvia VL, Gay I, Hardin R, Dean DD, Boyan BD, Schwartz Z. Rat costochondral chondrocytes produce 17beta-estradiol and regulate its production by 1alpha,25(OH)(2)D(3). Bone. 2002;30(1):57–63.
Pino AM, Rodríguez JM, Ríos S, Astudillo P, Leiva L, Seitz G, et al. Aromatase activity of human mesenchymal stem cells is stimulated by early differentiation, vitamin D and leptin. J Endocrinol. 2006;191(3):715–25.
Lou YR, Murtola T, Tuohimaa P. Regulation of aromatase and 5alpha-reductase by 25-hydroxyvitamin D(3), 1alpha,25-dihydroxyvitamin D(3), dexamethasone and progesterone in prostate cancer cells. J Steroid Biochem Mol Biol. 2005;94(1-3):151–7.
Lundqvist J, Norlin M, Wikvall K. 1α,25-Dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen metabolism. Biochim Biophys Acta. 2011;1811(4):263–70.
Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151(1):32–42.
Carreau S, Bourguiba S, Lambard S, Silandre D, Delalande C. The promoter(s) of the aromatase gene in male testicular cells. Reprod Biol. 2004;4(1):23–34.
Lundqvist J, Hansen SK, Lykkesfeldt AE. Vitamin D analog EB1089 inhibits aromatase expression by dissociation of comodulator WSTF from the CYP19A1 promoter-a new regulatory pathway for aromatase. Biochim Biophys Acta. 2013;1833(1):40–7.
Lee CT, Wang JY, Chou KY, Hsu MI. 1,25-Dihydroxyvitamin D3 increases testosterone-induced 17beta-estradiol secretion and reverses testosterone-reduced connexin 43 in rat granulosa cells. Reprod Biol Endocrinol. 2014;12:90.
Villaggio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin Exp Rheumatol. 2012;30(6):934–8.
Gilad LA, Schwartz B. Association of estrogen receptor beta with plasma-membrane caveola components: implication in control of vitamin D receptor. J Mol Endocrinol. 2007;38(6):603–18.
Santos-Martínez N, Díaz L, Ordaz-Rosado D, García-Quiroz J, Barrera D, Avila E, et al. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: a potential new therapeutic approach. BMC Cancer. 2014;14:230.
Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43(3):223–5.
Blomberg JM. Vitamin D metabolism, sex hormones and male reproductive function. Reproduction. 2012;144(5):135–52. Erratum 144(5):647.
Matsuda M, Kurosaki K, Okamura N. Activated vitamin D3 and pro-activated vitamin D3 attenuate induction of permanent changes caused by neonatal estrogen exposure in the mouse vagina. J Reprod Dev. 2014;60(4):274–9.
Nemere I, Garbi N, Winger Q. The 1,25D3-MARRS receptor/PDIA3/ERp57 and lifespan. J Cell Biochem. 2015;116(3):380–5.
Blomberg JM. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10(3):175–86.
Arnoult C, Lemos JR, Florman HM. Voltage-dependent modulation of T-type calcium channels by protein tyrosine phosphorylation. EMBO J. 1997;16(7):1593–9.
Costa RR, Reis RI, Aguiar JF, Varanda WA. Luteinizing hormone (LH) acts through PKA and PKC to modulate T-type calcium currents and intracellular calcium transients in mice Leydig cells. Cell Calcium. 2011;49(3):191–9.
Toppari J. Is semen quality declining? Andrologia. 1996;28(6):307–8.
Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14(6):855–9.
Bujan L, Mieusset R, Audran F, Lumbroso S, Sultan C. Increased oestradiol level in seminal plasma in infertile men. Hum Reprod. 1993;8(1):74–7.
Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166(5):765–78.
Boisen IM, Bøllehuus Hansen L, Mortensen LJ, Lanske B, Juul A, Blomberg Jensen M. Possible influence of vitamin D on male reproduction. J Steroid Biochem Mol Biol. 2016. doi: 10.1016/j.jsbmb.2016.09.023.
Acknowledgements
We are grateful to Céline Quint of the “centre de traduction”, Université Caen Normandie for English revision.
Funding
This work was supported by the National Institute of Research in Agronomy (INRA) and by the French Ministry of Education and Research. Ana Paula Zanatta and Renata Gonçalves were benefited of PhD fellowship from CNPq-Science without borders Brazil (Process # 236866/2012-5 and 204568/2014-5), Camille Gautier from the MESR and Vanessa Brouard was funded by the Low Normandy Regional Council (CRBN).
Availability of data and materials
Not applicable.
Authors’ contributions
APZ and CD wrote the manuscript. VB, CG, HBL, RG and FRMBS critically revised the manuscript. All authors have approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zanatta, A.P., Brouard, V., Gautier, C. et al. Interactions between oestrogen and 1α,25(OH)2-vitamin D3 signalling and their roles in spermatogenesis and spermatozoa functions. Basic Clin. Androl. 27, 10 (2017). https://doi.org/10.1186/s12610-017-0053-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12610-017-0053-z