Abstract
Genome editing techniques, especially the CRISPR/Cas technology, increase the possibilities and the speed of altering genetic material in organisms. So-called genome editing is increasingly being used to achieve agriculturally relevant novel traits and/or genetic combinations in both plants and animals, although predominantly as proof of concept studies, with commercial growing or rearing so far limited to the U.S. and Canada. However, there are numerous reports of unintended effects such as off-target effects, unintended on-target effects and other unintended consequences arising from genome editing, summarised under the term genomic irregularities. Despite this, the searching for genomic irregularities is far from routine in these studies and protocols vary widely, particularly for off-target effects, leading to differences in the efficacy of detection of off-target effects. Here, we describe the range of specific unintended effects associated with genome editing. We examine the considerable possibilities to change the genome of plants and animals with SDN-1 and SDN-2 genome editing (i.e. without the insertion of genes conferring the novel trait) and show that genome editing techniques are able to produce a broad spectrum of novel traits that, thus far, were not possible to be obtained using conventional breeding techniques. We consider that the current EU risk assessment guidance for GMOs requires revision and broadening to capture all potential genomic irregularities arising from genome editing and suggest additional tools to assist the risk assessment of genome-edited plants and animals for the environment and food/animal feed in the EU.
Similar content being viewed by others
Background
Genetically modified organisms (GMOs), predominantly plants, have been commercially grown in some countries, notably the Americas, since the mid-1990s [1]. Almost all current commercial GMOs have been developed using what is termed here as ‘first generation’ genetic engineering technology. That is, these GMOs contain recombinant DNA where a DNA cassette containing a functional gene or genes, relating to a novel trait, is inserted at random into the genome of the recipient organism [2]. Examples include GM herbicide-tolerant ‘Roundup Ready’ soy and GM insect-resistant Bt maize [1]. Within the last decade, agriculturally orientated applications of newer, second-generation genetic engineering technologies have been developed, in particular so-called genome editing technologies [3, 4]. There is some controversy over the terminology used to describe these techniques. Genome editing is often called “gene editing”, but this would not include the alterations of multiple genes or regulatory genomic elements like enhancers or noncoding RNAs, as is possible with these techniques. Objections have been raised to the term “editing”, likening genome editing to a precise and predictable text editor, when in fact there is limited knowledge of the consequences of such interventions [5, 6]. Genome editing has also been termed “genome engineering”, implying greater intervention to the genome than simply “editing” [7], and are referred to “new genomic techniques” by the European Commission [8]. In this paper, we use the terminology of genome editing to encompass techniques such as oligonucleotide-directed mutagenesis (ODM), zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), meganucleases and clustered regularly interspaced short palindromic repeats/CRISPR-associated (CRISPR/Cas) techniques [9,10,11,12] with CRISPR/Cas becoming the most widely used genome editing technology [13].
All genetic engineering technologies, whether first or second generation, aim to directly modify genomes. That is, to change the genetic material (usually not only genomic DNA, but also potentially RNA and the epigenome) of an organism directly, without mating, by introducing either genetic material or material that enacts a change to genetic material into the cell. The material introduced into the cell is produced, or at least handled, in the laboratory by humans, i.e. in vitro techniques. This concept of direct modification of genomic material underlies the concept and definition of both a GMO in the EU (EU Directive No 2001/18/EC, [14]) and a living modified organism in the Cartagena Protocol on Biosafety [15].
Concerns regarding the potential negative impacts of GMOs to the environment from cultivation, and to animal and human consumers, have led to the requirement of a risk assessment for GMOs prior to cultivation and marketing in the EU under EU Directive No 2001/18/EC [14] and many other regions and countries around the world. One fundamental concern regarding GMOs is that direct modification of genetic material by genetic engineering technologies can unintentionally interfere with the well-orchestrated expression of genes or with the complex biochemical pathways operating within an organism. For example, genomic irregularities caused by recombinant DNA have given rise to unintended RNA variants [16] or altered secondary metabolites [17]. Hence, the biological and biochemical characteristics of the GMO might be changed in a way that impacts consumers and/or the environment. In addition, the novel trait conferred by the genetic engineering, e.g. herbicide tolerance in plants, is also of concern as this can have consequences for agricultural systems, the environment and often for food and animal feed safety [18, 19]. Further, in the EU, a system of traceability and labelling is necessary to allow for segregation of GM foods from non-GM foods to enable consumer choice and monitoring of any adverse effects in the human population post-marketing of GMOs.
In the EU, genome-edited organisms are required to undergo both environmental and food and feed risk assessments, as is required of first-generation GMOs [20]. Risk assessment guidelines for GM plants and animals have been developed by the European Food Safety Authority (EFSA) for the environment [21, 22] and food and feed [23,24,25] within the framework of European regulations. However, genome editing techniques are substantially different to first-generation genetic engineering techniques. Therefore, risk assessment guidelines will have to be examined, and potentially revised to ensure they capture unintended effects caused by the genome editing process. EFSA has issued an opinion on the risk assessment for the genome editing of plants where genes are inserted using site directed nuclease-3 (SDN-3) techniques [26] and has received a mandate from the Commission to produce an opinion on whether these risks are applicable to genome-edited plants not carrying novel genes, i.e. using site-directed nuclease-1 (SDN-1) and site-directed nuclease-2 techniques (SDN-2). The scientific opinion is expected by the end of 2020 [27]. There is, as yet, no mandate for EFSA to devise guidelines specifically for the risk assessment of genome-edited animals.
Here, we give an overview of genome editing techniques and describe the specific unintended effects related to their application in plants and animals. We give examples of market-orientated applications of genome editing in agriculture and provide evidence that genome editing can give rise to organisms with traits that differ significantly from existing traits developed by conventional breeding and first-generation GMOs. Finally, we examine considerations for the risk assessment of GMOs developed using genome editing in the EU.
Technical characterisation of new genetic engineering techniques
Genome editing techniques
Genome editing is the collective term for numerous new genetic engineering techniques. Most (e.g. ZFN, TALEN, CRISPR/Cas9) comprise site-directed nucleases (SDNs), which induce double-strand breaks (DSBs) of the DNA at specific, predefined target sites. This subsequently activates the cell’s own repair mechanisms and alterations of the DNA sequence can occur. Other techniques, that are based on the CRISPR/Cas system, include those that induce a break in only one DNA strand to increase specificity and those that can induce changes in RNA or the epigenome [28, 29]. ODM does not use SDNs, but is directed by short synthetic oligonucleotides which are introduced into plant cells where they mediate directed sequence changes at specific, predefined genomic loci and are supposed to be degraded by cellular processes [12, 30]. Genome editing can be applied to plants, animals and microorganisms [9] and also humans, e.g. for therapeutic use, although human applications are outside the scope of this review and not considered GMOs by the EU legislation under EU Directive No 2001/18/EC [14].
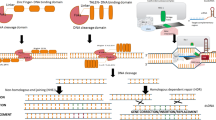
ZFNs and TALENs are protein-based systems that use engineered proteins to both recognise the DNA target sequence and induce a DNA DSB by a nuclease domain (e.g. FokI) at a predefined site in the genome of a target organism. These techniques have been largely outcompeted by the CRISPR/Cas system in recent years [13]. Nevertheless, some products developed by these earlier techniques are permitted to be cultivated and sold, at least in the U.S. [31], e.g. soybeans with altered fatty acid content developed by the company Calyxt [32], and it is possible that more products from these earlier techniques could enter the market in the future. The focus of this review is on CRISPR/Cas systems, of which only brief technical details are given, but are discussed in-depth elsewhere [33,34,35]. In short, CRISPR/Cas allows the targeting of an endonuclease (e.g. Cas9 from Streptococcus pyogenes) to specific genomic regions using a guide RNA (gRNA) [10, 11]. The gRNA is designed according to the genomic locus/loci that are to be altered. Cas9 interacts with the gRNA and upon recognition of the target sequence introduces a DNA DSB at that part of the genome [36]. DNA DSBs subsequently activate the cell’s non-homologous end joining (NHEJ) repair and homology-directed repair (HDR) mechanisms [37,38,39,40]. The NHEJ pathway is known to be error prone and frequently results in base substitutions, insertions or deletions (indels) at the DNA break sites [41]. These alterations can generate frameshift mutations or disrupt important functional domains, which can, for example, disrupt the functioning of target genes [42]. Kinetics and fidelity studies show that, as the cell attempts repair of the DNA double-strand break to its original structure, the application of the nuclease will typically result in a cell or an organisms in which the target site is altered [43]. For this reason, NHEJ repair is pursued for gene knockout applications. The HDR pathway utilises exogenous DNA donor templates to introduce nucleotide substitutions and DNA insertions at the target sites [44, 45]. Genome editing applications using SDNs can be used to either introduce small-sized, undirected changes (SDN-1) or directed sequence changes (SDN-2 and SDN-3) at specific, predefined genomic loci [46]. SDN-3 approaches involve the insertion of transgenic constructs at specific, predefined locations (including gene-stacking) [47]. Changes at multiple locations of the genome are possible using multiplexing approaches, which target several genes at once, or repeated applications using multiple gRNAs [34, 48, 49].
The most commonly used CRISPR/Cas endonuclease is Cas9 but others, e.g. CRISPR/Cpf1 (or Cas12a), have been used as well [48, 50, 51]. A catalytically inactive Cas9 variant (dead Cas9 or dCas9) has been developed and fused to different functional domains for various applications [52] such as base editing [53], editing of epigenetic modifications [54, 55], or transcriptional silencing [52, 56].
In base editing, dCas9 is coupled to enzymes that subsequently lead to the irreversible conversion of a specific DNA base into another without requiring DNA DSBs at the target sequence [53, 57]. Base editing has been the subject of several proof of concept studies in plants [58, 59] and animals [60, 61]. So far, base editing can generate only the four transition mutations (C-T, G-A, A-G and T-C) [53, 57], but it is still not possible to perform the eight transversion mutations (C-A, C-G, G-C, G-T, A-C, A-T, T-A and T-G) because pyrimidines and purines have totally different molecular structures.
Dead Cas9 can also be coupled to epigenetic modifiers such as DNA methyltransferases or histone acetylases to introduce changes in the epigenome of a target cell [29]. The epigenome is shaped through biochemical modifications of the DNA sequence itself (e.g. DNA methylation or demethylation) or associated histones (e.g. acetylation, methylation or phosphorylation) [54, 62, 63]. The epigenome regulates in a well-orchestrated manner the gene expression in all tissues and is indispensable for normal development and function of an organism [64, 65].
Further Cas variants are at the proof of concept stage. Recently, ‘prime’ editing was described in human cells, [66] rice and wheat [67,68,69]. This development of CRISPR/Cas technology allows the introduction of targeted insertions, deletions as well as all possible base-to-base conversions changes in DNA. Essentially, prime editing uses a modified Cas9 that introduces a single-strand break at the target site of the genome and is connected to a reverse transcriptase. A determined prime editing RNA (pegRNA) both specifies the target site of the DNA and encodes the desired template which is converted to DNA by the reverse transcriptase. Prime editing is intended to increase the efficiency to generate targeted DNA edits compared to DNA DSB-mediated HDR and to decrease off-target effects in comparison to the classical CRISPR/Cas9 system. Off-target effects are intended to be reduced as prime editing only nicks one DNA strand thereby not activating the error-prone NHEJ.
LwaCas13a from Leptotrichia wadei has been described and adopted for editing of RNA [70, 71]. Cas13a is structurally different to Cas9 and can be used to cleave specific mRNAs. Analogous to CRISPR/Cas9 approaches, Cas13 is recruited to a target mRNA using a crRNA (CRISPR RNA), leading to the binding and cutting thereof [70]. Cas13a has been used for a targeted knockdown of endogenous transcripts in rice protoplasts with comparable levels of knockdown as RNA interference [70]. Finally, an enzymatically inactive form of Cas13 (dead Cas13, dCas13) has also been developed for RNA editing via a linked adenosine deaminase acting on RNA 2 (ADAR2) [72], which enables the editing of bases at the target sequence of the respective mRNA without altering the underlying DNA sequences. At present, the extent to which these newer forms of genome editing give rise to genomic irregularities is not known as the relevant research has yet to be conducted.
First-generation genetic engineering techniques used for genome editing of plants
First-generation genetic engineering techniques are still commonly used to introduce CRISPR/Cas components into plant cells. The components can be delivered in form of DNA, RNA or ribonucleoproteins (RNPs) via Agrobacterium-mediated DNA transformation, particle bombardment or by protoplast transfection into the recipient cells. If plasmids are used to deliver the CRISPR/Cas reagents into the cells for stable expression, either Agrobacterium tumefaciens or particle bombardment is used and transformed cells are selected using marker genes such as antibiotic resistance. The transgenes containing the CRISPR/Cas components are inserted at random sites of the genome, potentially inducing genomic irregularities upon integration, and can be removed subsequently by segregation using conventional breeding.
Transient gene expression of CRISPR/Cas can be achieved without transgene integration by introducing a plasmid that encodes the CRISPR/Cas components in plant cells without selectable marker genes. One aim of this delivery technique is to reduce genomic irregularities created by the insertion of transgenes. Dupont Pioneer’s genome-edited waxy maize is an example of this approach [73]. However, there is potential for the introduced plasmid or template DNA (or fragments thereof) to unintentionally integrate into the genome of the host [73,74,75,76,77].
DNA-free CRISPR/Cas delivery has been developed using pre-assembled RNPs in plants mostly by particle bombardment or protoplast transfection [78,79,80]. The RNPs can cleave the target region immediately upon delivery in the nucleus and are then degraded quickly, so fewer off-target effects are to be expected [33]. Nevertheless, increasing specificity using RNPs thereby restricting its mode-of-action to a confined time also leads to a reduction in on-target cleavage. Thus, a balance between on-target cleavage efficiency and off-target effects has to be considered [81]. Proof of concept examples of CRISPR/Cas genome editing using RNPs include apple, grape, maize and wheat [74, 75, 79].
Regeneration of whole plants from protoplasts is still challenging for most agronomically important crops. Protoplasts are single cells enzymatically isolated from plant tissue, which can form a new plant. A major challenge still is the isolation of intact protoplasts from tissue material [82]. Another challenge occurring during regeneration of whole plants from protoplasts is genomic instability (i.e. chromosomal and segmental instability), e.g. in potatoes [83].
Genetic engineering techniques for genome editing in farm animals
The methodology for genetic engineering of farm animals is very different from plants because plants can regenerate from somatic cells, whereas animals can only develop from germline cells. The use of embryos raises ethical and welfare issues especially in vertebrates, as hundreds of genetic transformations, each requiring an embryo, are made in a typical genetic engineering (including genome editing) experiment [84, 85]. Transformed embryos with the desired modification and no apparent undesirable modifications are selected for impregnation, but further embryos are lost during impregnation and pregnancy [84, 85]. Thus, a considerably high number of embryos are needed for the genetical engineering of animals, which is ethically questionable. The methods of DNA introduction and regeneration in vertebrates are the same for both first-generation genetic engineering and genome editing. The two principal methods to generate genetically engineered animals are somatic cell nuclear transfer (cloning) and cytoplasmic injection (pronuclear injection or microinjection) [86, 87]. With cloning, primary cells from an adult animal (e.g. fibroblasts) are grown in cell culture and transfected (e.g. viral transfection or electroporation) with CRISPR/Cas components. After selection of the desired DNA alterations, the genome-edited somatic cell is fused with an enucleated egg cell to create a viable, genome-edited embryo. In contrast, microinjection involves direct injection of the genome editing complex into the cytoplasm of a zygote. However, microinjection has reportedly low editing efficiencies and commonly results in mosaicism (a mixture of edited and unedited alleles) [88]. Because of the difficulties involved with the microinjection, cloning methods are still widely used in the genome editing process for animals [86, 87]. Cloning commonly leads to birth defects, abortions and early postnatal death [86]. Therefore, particularly for vertebrate animals, genome editing experiments carry ethical and welfare issues.
Unintended effects associated with genome editing
Although genome editing techniques are often described as being precise in terms of intended changes to genetic material [89,90,91,92], genomic irregularities including unintended off-target effects (OTEs), on-target effects and chromosomal rearrangements have been reported [93, 94]. Unintended effects can occur with all genome editing techniques, but CRISPR/Cas systems are the most frequently genome editing tool used in studies. Thus, our focus is primarily on unintended effects associated with this technique.
Off-target effects of CRISPR/Cas applications
Off-target effects are the cleavage of, and subsequent change to, DNA at genomic sites other than the target site. Off-target effects are one of the major concerns regarding unintended effects generated by CRISPR/Cas systems [3, 84, 95]. Off-target effects have been demonstrated in several crop plants, including rice, soy, maize and barley [93, 95,96,97,98,99] and in farm animals such as pigs [100], as well as in rats and mice [101, 102]. Off-target effects occur at DNA sequences with even 3–5 base pair mismatches in the protospacer adjacent motif (PAM)-distal part of the gRNA, as there is a degree of tolerance for mismatches between the target DNA and the guide RNA [95, 103,104,105]. Some types of gRNA have a high degree of specificity, whilst some are more promiscuous [95]. Thus, a reliably accurate design of gRNAs, based on pre-existing genomic data, can minimise, but not necessarily eliminate, the possibility of off-target effects. In addition, the nature of the genome editing components (i.e. whether RNPs or plasmids are used), the target organism (meaning the complexity of its genome), the duration of exposure to the nuclease, pharmacokinetics of the delivered components, the type of Cas variant used and the amount of nuclease applied can all affect the specificity and the number of off-target events [103, 106,107,108]. Should off-target effects occur in protein coding genes, loss-of-function mutations or alterations of protein functions could result. Similarly, unintended alterations in non-coding DNA sequences, promoters, introns, terminators or insulators could alter gene expression. Therefore, the detection of off-target effects is an essential step in determining the safety of a genome-edited organism for the environment, food and feed.
Attempts are being made to make the CRISPR/Cas system less prone to off-target effects, e.g. it appears the CRISPR–Cpf1 system has a higher specificity than CRISPR/Cas9, which also increases the possibilities to target more genes [109, 110]. The composition of nucleotides around the PAM-sequence, the GC content of the gRNA and chromatin structure of the target sequence also have an influence in off-target activity [111, 112]. Interestingly, it has been suggested that the outcome of the plant repair process is also influenced by the local sequence properties at the target site [113, 114].
Whilst ODM may involve changes to only small number of DNA bases, there is the possibility of off- and on-target effects. Although there are, as yet, no published data examining the frequency of unintended effects with ODM [4, 13, 93], this does not mean that they do not occur. The possibility of oligonucleotide integration cannot be excluded [4].
Off-target effects associated with base and epigenetic editing
Base editors induce lower levels of unintended insertions and deletions when compared to SDN applications that induce DSBs [53, 115], but they have the potential to change all target nucleotides within an editing window of five base pairs [53]. Recent findings show an increased occurrence of off-target mutations using cytosine base editors (CBEs) compared to adenine base editors (ABEs) in rice and mouse embryos [116, 117]. Surprisingly, the off-target mutations, induced by CBEs, occurred predominantly in actively transcribed genic regions that were not depicted by in silico prediction tools [117].
Base editors can also generate transcriptome-wide off-target editing of RNA in addition to DNA editing [118]. These effects were found both in CBEs and ABEs and occurred independently of both the guide RNA used and off-target DNA editing. This demonstrates that off-target effects induced by base editors are multi-dimensional and illustrate the importance of a detailed assessment of off-target effects, not only of DNA, but also RNA in such organisms, especially if the DNA encoding the base editors is integrated into the genome. These off-target effects can result in missense (i.e. substitution of a different amino acid in the resulting protein) or nonsense mutations (i.e. generating a truncated protein by generating a stop codon) potentially generating an altered protein composition or generation of splice variants [118]. In an attempt to reduce unwanted effects and improve possible future applications, these systems are being further revised [119, 120].
Epigenome editing can induce unspecific genome-wide changes in the epigenome [121], which could lead to an altered gene expression in these cells. It also appears that, so far, the specificity of these dCas9-epigenetic modifiers cannot be reliably predicted [122].
Unintended on-target effects associated with CRISPR/Cas applications
In addition to off-target effects, unintended alterations either at, or in close proximity to, the target site, have been observed [94, 123,124,125]. We use the term ‘unintended on-target effects’ to describe these unintended alterations in the vicinity of the target site although these molecular changes can also occur further away, even distant from the target site. Large chromosomal deletions, insertions and inversions have been detected after applying CRISPR/Cas9 in mouse embryonic stem cells and differentiated human cells [94]. Such complex chromosomal rearrangements can be identified using long-range PCR or long-read next-generation sequencing platforms, such as the Pacific BioSciences or Oxford Nanopore Technology. However, these techniques are rarely used for routine genotyping of CRISPR/Cas-induced mutations in plants, so these on-target effects are likely to have remained undetected in many studies [126]. Small insertions or deletions at the target site can cause disruption of the alternative splicing mechanism, resulting in exon skipping by disruption of exon splicing enhancers [123, 127]. This misreading of DNA has the potential to produce aberrant proteins, confirmed by the detection of an aberrant protein resulting from the application of CRISPR/Cas9 to a human cell culture [127]. Alternative splicing occurs in all multicellular animals and plants, but to a greater extent in animals than plants [128]. This suggests that any disruption to alternative splicing could have a greater effect in animals compared to plants. Downstream effects from alternative splicing may remain undetected unless transcriptomic or proteomic techniques are applied to identify aberrantly generated mRNAs and proteins. In addition, large deletions induced by a single gRNA were found to delete whole exons causing exon skipping in cell lines [124, 129].
Both HDR and NHEJ-mediated repair have been shown to cause multiple unwanted head-to-tail insertions of DNA donor templates at the target site during the generation of conditional knockout mice models [130]. Conventionally applied PCR analysis failed to identify these insertions. Thus, it is essential to validate the integrity of the target DNA regions after applying CRISPR/Cas9 using a combination of suitably sensitive analytical techniques such as qPCR, digital droplet PCR and southern blotting [130].
The multifunctionality of genes, particularly in animals by virtue of the greater extent of alternative splicing, can result in unintended consequences from genome editing. A gene that is rendered dysfunctional (knocked out) by an intended small deletion, or even base edit, may have products that perform an essential function elsewhere in the cell [131]. This could cause errors in cell metabolism, including protein production. Understanding the implications of some of the consequences of genome editing will require further research, especially as many of the studies examining on-target effects use cell cultures, including human and animal cells. For example, applying CRISPR/Cas9 in human pluripotent stem cells and retinal pigment epithelial cells was shown to result in the accumulation of mutations in the p53 gene. These cells were exposed to a selection against functional p53 [132, 133] which could lead to a subsequent increased accumulation of mutations. Further research is needed to determine how relevant this effect might be to market-orientated agricultural applications of CRISPR/Cas, particularly CRISPR/Cas9-induced mutations in plants SOG1 which has similar functions as p53 in animals [134].
Unintended integration of CRISPR/Cas components or plasmid DNA
When CRISPR/Cas components are delivered as plasmids into the cells, both unintended additional integration events of this DNA or its fragments have been reported in both plants and animals, and where the plasmid was intended to enact genome editing without any DNA integration, unintentionally integrated [74, 76, 130, 135]. This can refer to the CRISPR/Cas components as well as the plasmid backbone. For example, in one study, the DNA template encoding CRISPR/Cas9 was not only detected at the target location in soybeans as intended, but also at other multiple, apparently random, genomic locations [135]. In another study, CRISPR/Cas sequences were found at multiple genomic sites showing microhomology at the transgene integration sites indicating that the integration of CRISPR/Cas sequences might not be completely random [136].
Unintentional integration of CRISPR/Cas-encoding DNA fragments in the genome of other plants has also been reported [74]. Unintended plasmid integration into the genome was discovered by whole genome sequencing (WGS) [76] in livestock when TALENs were used to insert an allele of the POLLED gene in bovine embryonic fibroblast to ultimately generate hornless dairy cattle [137]. However, the developers initially did not detect the unintended plasmid integration in their own analysis, possibly because either the plasmid backbone was not included in the sequence alignment, elevated noise at the target locus, limited signal of the sequencing data, and/or PCR conditions insensitive to detect the integrations [76, 77].
Unintended effects induced by applying first-generation genetic engineering techniques
Whilst the actual genome editing allows modifying the DNA at a target site, this claimed precision may not hold true for the delivery and integration of its tools. The use of first-generation genetic engineering techniques to integrate DNA encoding the CRISPR/Cas components results in insertion at a random location in the genome, often with multiple and flawed (e.g. partial) copies [138, 139]. Random integration of the transfer DNA (T-DNA) from Agrobacterium-mediated plant transformations (and fragments thereof) could have unwanted consequences for the resultant GMO, such as the disruption of genes important for plant growth or development. Alternatively, T-DNA can integrate into regions of the genome that are poorly or unstably expressed when cultivated in the field. For example, integration of T-DNA fragments can cause the formation of unintended mRNA variants [16]. The findings of Jupe et al. [141] highlight the need to search for irregularities in both the genome and epigenome, particularly in regions flanking integration sites, of GMOs that were transformed by Agrobacterium tumefaciens. Plants transformed by direct delivery methods such as particle bombardment show genomic DNA rearrangements as well as rearrangements of the transgenic loci [140].
The insertion of genetic material can give rise to genomic irregularities, including large genomic rearrangements, deletions, insertions, genome-wide mutations and epigenetic alterations in the vicinity of the integration site [139, 141,142,143,144].
Once inserted CRISPR/Cas components have enacted a change in the organism’s genomic material (usually DNA), their transgenes can be removed from plants by backcrossing with parental lines. As a result, in theory at least, the resultant organisms from SDN-1 and SDN-2 applications of genome editing do not contain any inserted genes, but it is important to verify the complete absence of inserted genes including vector backbone sequences [13, 145].
Potential applications of genome editing in agriculture
Genome-edited crops that contain novel genomic combinations
Many plant species have complex genomes exhibiting considerable diversity in both size and structure [146]. Challenges to plant breeding include polyploidy, a large number of orthologous genes, heterozygosity, repetitive DNA and linkage drag. Major agricultural relevant crops like rapeseed, wheat, potato, cotton, apple and sugarcane are polyploid, i.e. combine more than two paired sets of chromosomes, which either originate from the same (autopolyploids) or related species (allopolyploids) [147]. For example, oilseed rape (Brassica napus) is allotetraploid as it consists of two different diploid sets of chromosomes, one from B. oleracea and one from B. rapa [148]. In addition, plant genomes often contain highly repetitive genomic regions due to transposable elements exhibiting large genome sizes. For example, the genome of allohexaploid wheat consists of approximately 14.5 × 109 bases, composed of three closely related sub-genomes, each of which contains a set of homologous genes [149].
The complexity of plant genomes poses a serious challenge for generating genetic alterations that require the targeting of multiple genes by traditional breeding and mutagenesis techniques that use chemicals or radiation to introduce mutations in plants [150]. Strategies to overcome limits of conventional breeding have been developed using genome editing. Genome editing techniques such as CRISPR/Cas enable complex alterations of genomes in a way that, until now, was not possible [151]. Multiplexing approaches, which combine multiple gRNAs, allow the targeting and alteration of multiple alleles, all members of a gene family or different functional genes [13, 152, 153]. Multiplexing genome editing applications have been used to change many major crop plants [154,155,156]. In Camelina sativa, for example, which is an allohexaploid plant, a complete knockout of all alleles of FAD2 (fatty acid desaturase 2) was achieved using CRISPR/Cas9 [157]. These changes would be extremely difficult, if not impossible to achieve using traditional mutagenesis or via spontaneously occurring mutations in nature. The camelina genome contains three subgenomes in two copies, thus each gene exists in six copies [158, 159]. To knock out all alleles of FAD2 by traditional mutagenesis, three complementary mutations in the FAD2 gene would have to be induced in each genetic locus in separate plants and subsequently each mutation made homozygous. Those mutant plants would then have to be crossed with each other in order to obtain a single individual plant that contains all mutations. Simultaneous generation of a homozygous triple mutation of FAD2 causing an effective gene knockout using chemical or physical mutagenesis is extremely unlikely, as is the occurrence of such a camelina plant due to spontaneously emerging mutations.
Genome editing techniques can overcome limitations of the genetic linkage between different traits sometimes present in conventional breeding of plants [151, 160]. If a desired gene is linked to a gene with adverse effects on, e.g. yield or fruit shape, genome editing can be used to break this linkage drag by knocking out the undesirable gene. This removes the need for excessive backcrossing to break the linkage. Separation of linked genes is challenging and cumbersome using classical breeding methods, but is assisted by biotechnologies such as marker-assisted selection and genomic selection [161].
The limitations of traditional mutagenesis have led to a lack of regulatory experience with plants bearing more complex (multiple) novel traits (e.g. alteration of metabolic pathways), as these have not been generated by traditional mutagenesis or recombinant DNA approaches but can now easily be induced by genome editing. Table 1 gives examples of genome-edited crops that contain novel genetic combinations that would be difficult to generate by traditional mutagenesis or first-generation genetic engineering techniques.
Market-orientated genome-edited crops
In an extensive review of genome editing applications and detection of off-target effects in plants, Modrzejewski et al. systemically examined publications between 1996 and May 2018 [93]. They found the majority of genome editing studies were published since 2013, demonstrating the recent rapid rise in the use of genome editing technologies in producing GM plants. Most plant genome editing publications examined used CRISPR/Cas techniques, with a much smaller contribution from the other genome editing technologies TALENs, ZFNs and ODM. In most of the reviewed studies, crops were altered by SDN-1 approaches rather than SDN-2. They induced point mutations or indels without providing a DNA donor template as the efficiency of homology-directed repair is still low [93]. In their review [93], Modrzejewski et al. considered 99 different applications in 28 different plants to be market-orientated (in contrast to basic research). They classified genome editing applications as market-orientated when studies met the criteria: (1) genome editing was applied in an agricultural crop; (2) a trait was addressed that may be of interest for commercialisation, and (3) the targeted trait was expressed in the edited plant when grown [93]. Rice had the most market-orientated applications, followed by tomato, maize, potato, wheat and several other crops. Rice is relatively easy to regenerate from cell culture, facilitating genome editing and contributing to the high number of genome editing applications in rice. Rice is also a highly important crop globally, particularly in China where a substantial number of research papers on genome-edited crops originate [162]. However, it is worth noting that, despite research and field trials of GM rice, there is no commercially grown GM rice in China or anywhere in the world [163].
Market-orientated traits in genome-edited plants are diverse but the most common are those relating to agronomic value, followed by compositional changes (see Table 2) [93]. Searching for off-target effects can take the form of biased or unbiased searching. In biased searching, in silico tools are used to predict possible off-target sites based on the sequence of the gRNA. These predicted sites are subsequently examined for off-target changes after the application of CRISPR/Cas using classical PCR analysis. In unbiased searching, WGS approaches are used to identify off-target effects genome-wide and not limited to only predicted sites. The number of predicted off-target effects was found to be highly variable across studies performing bias detection methods in plants, from zero to over 4000 [93]. As discussed in Modrzejewski et al. [93], the number of predicted off-target sites depends on various factors: differing sizes of genomes and number of chromosome sets between plants; different prediction and analytical tools used; the number of hypothetical mismatches tolerated between the target sequence and potential off-target site and the design or selection of gRNAs to minimise off-target effects. So far, the vast majority of plant studies using genome editing applications are looking for off-target effects in a biased manner by investigating solely at in silico predicted sites of the genome, whilst a scant minority of these studies are using unbiased WGS approaches to identify off-target effects [93].
Genome-edited farm animals
Currently, there are no commercial GM farm animals anywhere in the world, and the only GM animal approved for food use is limited to a GM salmon in Canada and the U.S. [179]. One pharmaceutical produced by a GM goat was approved for medicinal use in the EU but has since been withdrawn from the EU market [180, 181]. The production of GM animals was limited by difficulties with first-generation genetic modification techniques for animals [84, 179]. In contrast, genome editing is reported to give more predictable results in animals [84,85,86] and the number of market-oriented studies of genome-edited farm animals is increasing. Although these are mostly proof of concept studies, they indicate that there may be applications in the near future to raise and market genome-edited farm animals as food.
An examination of the published scientific literature from 2014 to 2019, identified using in Google Scholar and Web of Science the search terms “genome-editing” or “gene-editing” with “animal” and “farm”, revealed that a wide range of traits have been engineered in farm animals using various genome editing techniques. Although not a comprehensive search, the examination provided examples of genome-edited farm animals that fall into two basic categories: those for increased productivity (Table 3) and those for increased efficiency of production (Table 4), meaning adaptation of the farm animals to husbandry conditions and altered quality of the animal product. The majority (14) of genome-edited animals in Tables 3 and 4 were produced using SDN-1, whilst one used SDN-2 and five used SDN-3. However, there are multiple other studies of SDN-1 genome editing applications for both enhanced muscle growth and increased wool length (both productivity), raising the proportion of studies using SDN-1.
A systemic literature survey reviewing off-target and other unintended effects in genome-edited farm animals is lacking. We screened the publications from Tables 3 and 4 for their analysis of off-target effects. No analysis for off-target effects was performed in eight of the 19 studies, whilst eight used biased detection methods (in silico prediction tools and conventional PCR) and one used WGS. Two reported altered cytokine levels or metabolism (Tables 3 and 4). Studies searching for off-target effects in genome-edited animals follow a similar pattern to genome-edited plants, predominantly using biased detection methods with very few using unbiased WGS approaches. None of the studies conducted thorough analysis for unintended on-target effects, although some searched for unintended integration of the plasmid. The hornless cattle were subsequently found to have unintended integration of the template plasmid containing the plasmid backbone and a second copy of the template in the genome of the cow [76, 77].
Risk assessment for organisms developed through genome editing techniques
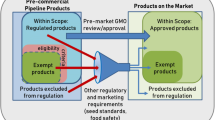
In the EU, genome-edited organisms are required to undergo both environmental and food and feed risk assessments, as is required of first-generation GMOs [20]. As yet, there are currently very few commercial applications of genome editing and these are confined to agricultural applications and largely confined to the U.S., where the regulatory approach differs from that of Europe [182]. The regulatory approach in the U.S. relies largely on whether the inserted components, or the organisms they are derived from are plant pests, e.g. a known toxin or invasive species [183]. This approach has led the United States Department of Agriculture (USDA) to not require an environmental risk assessment for genome-edited plants that “could otherwise have been developed through traditional breeding techniques as long as they are not plant pests or developed using plant pests” [31]. Nevertheless, many crops that are altered by genome editing applications contain genetic combinations (resulting in novel traits) that have not been developed through traditional breeding techniques so far, even though they do not contain inserted genes. Although genome-edited plants intended as food are likely to undergo a voluntary food safety assessment prior to being placed on the U.S. market [184], the lack of an environmental risk assessment for genome-edited crops in the U.S. has been met with concerns from scientists and other stakeholders, many of whom consider oversight is necessary [182, 185].
To the end of 2019, the USDA Animal and Plant Health Inspection Service (APHIS) lists 28 letters (27 genome-edited plants and one genome-edited mushroom) from companies and research institutions enquiring whether their genome-edited products fall under the biotechnology regulations. All are deemed to not meet the definition of a regulated article under the U.S. biotechnology regulations. This means unintended effects, including genomic irregularities are neither considered (including their presence or absence) nor assessed. Furthermore, in many of the letters of inquiry filed on the USDA-APHIS list, no information about the altered genes contained in these genome-edited organisms are provided as it is considered confidential business information [31]. So far, all the genome-edited organisms’ applications deemed non-regulated have used first-generation genetic engineering techniques (e.g. transformation with Agrobacterium tumefaciens or particle bombardment) to randomly insert DNA containing the CRISPR/Cas components into the recipient’s genome.
There is currently discussion whether genome-edited organisms could be exempted from the EU GMO regulation and whether it should be revised to focus on the product, rather than the process used to generate them [186]. One argument for the exemption of certain genome-edited organisms includes considerations of whether the changes induced by SDN-1 (or even SDN-2) are similar to those that might arise spontaneously and naturally or through conventional breeding or traditional mutagenesis (i.e. do not contain inserted genes). The basis for this line of argument is that GMOs developed by traditional mutagenesis are exempted from the EU GMO regulations based on their “history of safe use” [187]. However, genome editing is a relatively new genetic engineering technique and has no history of use, or indeed “history of safe use”. Unintended effects such as unintended integration of DNA caused by the genome editing process have only been observed relatively recently, and more types of unintended effects may be discovered as research progresses. A second argument considers whether the precision of genome editing renders it less prone to unexpected effects than conventional breeding or traditional mutagenesis [188,189,190]. However, we have shown here that genome editing can cause specific unintended effects and can be used to generate novel genetic combinations that cannot readily be achieved using conventional breeding or mutagenesis techniques. In a more general sense, genome editing utilises SDNs and oligonucleotides, which can be classified as biological mutagens [191]. In contrast to chemical or physical mutagens used in traditional mutagenesis, these agents can interact in a targeted way with the biological mechanisms in the cell, on the level of the genome and/or epigenome. Hence, the basis of the two types of techniques is fundamentally different and not comparable.
As the example of genome editing in sugarcane shows (see Table 1) [192], the power of even the least intrusive genome editing application (SDN-1) can readily surpass the extent of changes feasible with traditional mutagenesis techniques or spontaneous mutations, especially if repeatedly applied. Other examples described here demonstrate the range of genomic irregularities, both off-target and on-target that have been found in genome-edited plants and animals from SDN-1 and SDN-2 applications. Similarly, even with SDN-1, unintended consequences of genome editing, such as exon skipping can arise. Therefore, it is not possible to make a general presumption that genome-edited organisms created by SDN-1 and SDN-2 applications do not pose additional risks compared to conventional breeding processes based on the fact that no novel genes remain (or were not inserted) in the genome. Genomic irregularities, caused by the genome editing process, have potential implications for food, feed and environmental safety.
The reported unintended effects, including off-target effects and on-target effects, in the published literature suggest that a robust risk assessment is necessary for genome-edited organisms to identify unintended alterations arising from the application of genome editing and how these relate to food, feed and environmental safety. We therefore consider, in keeping with others [13, 111], that not only is risk assessment necessary, but that broadening of the EU GM risk assessment is also necessary to ensure that new types of genomic irregularities and associated hazards are captured within the risk assessment process.
Risk assessment guidelines for products of first-generation genetic engineering technology have been developed by EFSA for the environment [21, 22] and for food and feed [23, 24]. Depending on the specific genome editing application, many of the concerns associated with first-generation GMOs also apply to organisms developed through new genetic engineering techniques. However, genome editing techniques can cause additional, specific unintended genomic irregularities in many cases, implying that current guidelines and much of the experience gained from risk assessment of first-generation GMOs cannot be directly extrapolated to the risk assessment of genome editing organisms. Therefore, the risk assessment guidelines, both for the environment and food/feed, will require revision and expansion to ensure they capture all hazards associated with genome-edited organisms [111]. The risk assessment guidelines will also have to undergo regular review and revision as genome editing techniques and their new applications (e.g. base editing, prime editing, RNA editing, epigenome modification) develop and as knowledge of the risks (e.g. of unintended effects) is gained.
In general, the risk assessment procedure falls short of identifying and quantifying all risks to the environment, animals and humans because of incomplete knowledge of the organismal effects of genetic modification (intended or unintended), the receiving environment (e.g. ecology of the agricultural environment) and interactions between the GMO and the receiving environment. In addition, EFSA’s approach to the risk assessment has been criticised as reductionist in terms of its conceptual understanding of biology, genetics and ecology, primarily because of the assumption made that each genetic change acts independently of any and all other genes and changes [185]. It is evident that the scientific methodology used in the risk assessment will need further consideration and the role of the precautionary principle strengthened. All GMOs, including genome-edited GMOs, can have adverse impacts on the environment and food/feed safety but data are limited, and scientific uncertainty remains high.
There are two broad categories of hazards relating to the risk assessment of GMOs. These are:
-
1)
Those related to the genetic engineering process and
-
2)
Those related to the trait.
Both categories require additional elements to be considered to include hazards specifically associated with genome-edited organisms.
Risk assessment related to the genome editing process
All genome editing applications, SDN-1, SDN-2 and SDN-3, can lead to genomic irregularities at the molecular level, including alterations at off-target sites and at/around the target site [76, 93, 125, 127]. Additional ways in which unintended effects could arise may yet be discovered. The consequences of unintended effects for the risk assessment cannot be evaluated in a general sense as they are likely to be highly dependent on the actual unintended effect itself. Similar to genomic irregularities in GMOs produced by first-generation genetic engineering technologies, unintended effects in genome-edited crops could lead to a variety of unexpected effects. For example, the functioning of a particular (non-target) gene may be compromised if its component DNA has been cleaved by the nuclease. This could lead to changes in the organisms’ biochemistry, including its metabolic and protein profile which, in turn, could affect its toxicity and allergenicity. As this could impact food, feed and environmental safety, any genome-edited organism would need to be screened genome-wide for genetic irregularities. Such effects that are detected would need to be evaluated for their potential consequences prior to any deliberate release to the environment (including field trials) and placing on the market as food or feed.
For each genome-edited organism, the risks will need to be assessed individually, covering genomic irregularities affecting both off-target sites, alterations at/around the intended target site and unintended consequences, including those of gene multifunctionality. The reporting of on-target effects away from the target site indicates the need to broaden the definition of off-target effects beyond those created by gRNA recognition error. Off-target effects may not only be exclusively small, unintended alterations of the nucleotide sequence occurring, but can also include large rearrangements and deletions. Unintended effects arising from the application of first-generation genetic engineering technology (i.e. insertion of recombinant DNA) to introduce the CRISPR/Cas components will need to be considered as well. There is a need to search for irregularities in both the genome and epigenome of a first-generation GMO and genome-edited GMOs that employ first-generation genetic engineering techniques. However, as yet, EFSA does not require applicants to submit data on epigenetic alterations for GMOs.
Although according to the EU Directive 2001/18/EC and Regulation (EC) No. 1829/2003 information on both intended and unintended effects as a result of the genetic modifications are required, the requirements for molecular data are focussed on the DNA insert and flanking regions, pertinent to first-generation GMOs. These requirements may need to be revised to ensure that DNA data are not restricted to any particular region of the genome, but are instead genome-wide, e.g. a mandatory request for sequencing data on the whole genome. To date, a substantial majority of genome editing studies searching for off-target effects used biased in silico approaches to investigate the genome at predicted sites [93]. However, these approaches may miss genomic irregularities including unintended insertions and genomic rearrangements, which could be better identified by applying standardised protocols, e.g. whole genome sequencing combined with a robust bioinformatic analysis. However, some genetic variations identified by whole genome sequencing may not be able to be unambiguously assigned to either unintended alterations or naturally occurring variations. More research is needed to decide on a protocol for adequate and unbiased investigation of off-target effects and other genomic irregularities.
Particularly for genome-edited GMOs, it is already apparent that unintended irregularities can occur at several levels, not only at the genomic level, but also at the epigenetic and transcriptomic levels. Thus, a risk assessment requires information, not only of the whole genome and epigenome, but also of the transcriptome, proteome and metabolome to assess the consequences of unintended effects. However, data on neither the epigenome nor transcriptome are currently required in the risk assessment of GMOs. Several available techniques could assist assessment of the risks of genome-edited GMOs, and also improve the current risk assessment of GMOs created by the first-generation genetic engineering technology. These are collectively summarised as ‘omics approaches and include analyses of the DNA (genomics), the RNA profile (transcriptomics), proteins (proteomics) and metabolites (metabolomics) [193,194,195]. These techniques are either being, or could be, further developed to refine their capabilities to be used to analyse GMOs [196,197,198], and in the near future, submission of data from these techniques could be a requirement in support of an application to commercialise all genome-edited organism. Further developments and improvements of metabolomics methods are potentially useful to assist the traceability and labelling of genome-edited organisms [191]. Plants share their habitat with a variety of microbes that include bacteria, fungi, oomycetes and viruses [199,200,201]. The composition of a plant’s microbiota depends on complex multilateral interactions between the abiotic environment and its biotic inhabitants [201]. The rhizosphere, for example, is part of the soil that is influenced by secretions of a plant’s roots and can contain more than 30 000 prokaryotic species [202]. The genome of all microbes (microbiome) is considered as a plant’s second genome as it is much larger than that of the plant. Microbiomes are important for nutrient uptake like phosphorus and nitrogen of plants. In return, the microbiome is provided with carbon in root exudates [201]. Amongst others, the microbiome also modulates a plant’s immunity and prevents its colonisation by pathogens [203]. Thus, the microbiome plays an important role for functional traits of a plant such as crop yield and nutrient quality [204]. Genome-edited plants would also need to be analysed in regard to the composition of their microbiome using, for example, metabolomics approaches [205]. Comprehensive studies investigating community dynamics are necessary, as individual microbial species regulate the community structure and stability [205]. More research needs to be done further investigating the host–microbiome interaction and defining host–microbiome systems for crop plants with standardised microbial culture collections and reference genomes [206].
EFSA’s opinion on SDN-3 from 2012 [26] considers that genome editing can “minimize hazards” or that off-target changes would be “fewer than those occurring with most mutagenesis techniques” and be “of the same types as those produced by conventional breeding techniques”. However, as described above, a large number of publications since 2012 demonstrate that several types of genomic irregularities can often be generated using SDN-1, SDN-2 and SDN-3 applications. Many of these irregularities are specific to genome editing, and substantially different to those produced by conventional breeding. Genome editing techniques are fundamentally different to traditional mutagenesis, inferring that errors induced are not directly comparable. Therefore, this EFSA statement has little to no scientific basis and the comparison with conventional breeding and traditional mutagenesis requires re-visitation and revision in light of the new publications. Genomic irregularities generated by genome editing could have far reaching consequences, possibly with important consequences for environmental, food and feed safety. In addition to genomic irregularities, SDN-1 and SDN-2 applications also have the potential to generate changes of the genome that were not possible so far, creating biological characteristics which were not achieved by conventional breeding up to now. Thus, the risks of a genome-edited organism need to be fully investigated for further conclusion on its safety.
Risk assessment related to the trait
The risk assessment for genome-edited plants will have to consider a broader spectrum of new genetic combinations and novel traits compared to the rather few traits introduced by first-generation genetic engineering technology (predominantly herbicide and insecticide resistance and combinations thereof) [13, 111, 151]. For genome-edited farm animals, most traits and genetic combinations will be novel as, to date, there have been no applications to market GM farm animals for food use in the EU and existing EFSA risk assessment guidelines for animals largely focusing on insects and fish [22, 24]. The broad spectrum of possible traits is likely to provide new, and potentially complex, issues for risk assessment. Certain traits represent a special challenge for risk assessment. Traits such as altered nutrition can introduce a compound into an (agricultural) ecosystem where that compound does not normally or naturally exist. For example, a (first generation) GM camelina producing long-chain fatty acids which are novel to the terrestrial environment [207] was found to exert toxic effects on certain Lepidoptera [208] which could adversely affect the food web.
Risk assessment related to the trait of a genome-edited organism is, to some extent, similar to that which exists for GMOs developed using first-generation genetic engineering techniques [21, 23, 24]. That is, the trait will need to be assessed for its environmental safety (e.g. inter alia toxicity to non-target organisms, potential changes to invasiveness) and human and animal safety (e.g. inter alia allergenicity). First-generation GMOs predominantly consist of herbicide-tolerant and insect-resistant crops, and this is where the EU experience of assessing GMO traits lies. By contrast, the traits that can, at least theoretically, be conferred by genome editing are highly varied and the possibilities to alter the genome resulting in new genetic combinations are more numerous [13, 111, 151, 160]. We showed (Table 1) some examples of crops containing novel traits, that were naturally present in the plants and modified by SDN-1 applications (e.g. wheat modified by CRISPR/Cas to have a lower gluten content [168]). These altered traits pose challenges for risk assessment as they can have an impact on plant interactions with the biotic environment. Thus, there is a requirement for studies to assess the potential impacts of traits other than herbicide tolerance and insect resistance. Furthermore, the impact of the intended (multiple) changes of the genome requires evaluation regarding their potential interference in signalling and metabolic pathways. In some cases, it will be difficult or even not possible to identify appropriate non-GM comparators, a problem also identified with nutritionally enhanced GM crops developed through first-generation GM techniques.
Eckerstorfer et al. reviewed the novel traits of GM plants developed by new genetic engineering techniques [13]. They suggest to group these into three classes for the risk assessment: (1) those related to traits in conventionally bred plants; (2) those with traits similar to established first-generation GM plants and (3) those which have been established neither in conventional nor other biotechnological methods. Prior knowledge may be insufficient and available information limited for many of these traits. The authors suggested that, for each trait, it is important to consider, not only the modification itself, but also the impact of the modification and the novel trait on the physiology and phenology of the GM plant [13]. Furthermore, they highlight the importance of considering the specific characteristics of the technical approach and the existing knowledge on potential for unintended changes/off-target activity. This suggests that, whilst it is important to both detect and assess unexpected effects at the organism level, it may also be important to attribute these changes to either unintended genomic irregularities, or consequences of the novel trait.
Broadening the risk assessment
Currently, there are only a few publications discussing risk assessment of genome-edited organisms in detail [13, 111, 182]. This is remarkable considering the growing number of publications, as reviewed here, that describe the potential for the creation of genetic errors during the genome editing process. As we have shown here, there are specific risks associated with genome editing that could impact food/feed and environmental safety. Therefore, the current EU risk assessment of GMOs will require broadening to encompass the additional challenges posed by genome-edited plants and animals. This broadening of the risk assessment would be greatly facilitated by a well-funded, independent research programme to comprehensively examine the range of potential genetic errors created by genome editing processes and validate (or otherwise reject) any assumptions and premises regarding the potential risks of gene-edited organism for the environment and human and animal health.
Specific risks associated with the genome editing process and risks associated with the novel trait generated using genome editing, as well as risks associated with the use of older genetic engineering techniques in genome editing are summarised in Fig. 1. The additional types of unintended genomic irregularities require the current examination of DNA to be expanded to encompass examination of epigenetic changes and changes in the transcriptome, proteome and metabolome of the GMO (Fig. 1d). Such examinations will require further development of WGS and ‘omics approaches [191], and may be assisted by new analytical tools in the future. Such tools may also facilitate the detection and identification of genome-edited crops and animals, which in turn could assist in the traceability and labelling of GMOs to enable consumer choice [209], and protection of agricultural systems that exclude GMOs, e.g. organic agriculture [210]. For traceability, the key documentation required is molecular data on the altered DNA sequences (both the intended and unintended changes) made during the genome-editing process. As with first-generation GMOs, these data would assist independent monitoring for the presence/absence of these GMOs, e.g. in food [163]. In addition, should undesirable effects emerge documentation of altered genomic sequences will allow the originator to be traced, facilitating recall, if at all possible.
Elements of a risk assessment for genome-edited organisms. Risk assessment of genome-edited organisms requires consideration of risks associated with (a) the process of genome editing causing unintended changes of the genome, epigenome, transcriptome, metabolome and microbiome, (b) the use of first-generation genetic engineering techniques causing unintended effects such as rearrangements of the genome or epigenetic changes, and (c) the trait leading to unintended effects at the molecular, cellular, organismal and ecosystem levels. For a robust risk assessment standardised protocols (d) are needed as well as the implementation of comprehensive ‘omics studies for detection and prediction of unintended changes
Whilst suitable tools for the detection of genome-edited organisms may currently be considered difficult to develop or implement [211], it is notable that methods to detect the occurrence of genomic irregularities at, or near, the target site during the genome editing process have only recently been developed and applied [94, 125, 212]. Risk assessment related to traits will require additional knowledge of their consequences for the organism and the impacts when released into the environment, which would be aided by further research. This may be particularly necessary for traits where experience with either current GM plants or conventional plants are lacking and/or there is a lack of adequate comparators. In addition, genomic irregularities may be important in terms of gene x environment interactions and could be combinatorial and/or cumulative. This aspect could magnify uncertainties and unknowns in regard to environmental risk assessment of genome-edited organisms [213].
There is a complete lack of experience in the risk assessment related to any GM traits in farm animals as there have not yet been applications for the marketing of GM animals in the EU, developed by either first-generation or genome editing techniques. This too may require further research. Unlike the molecular characterisation, risk assessment related to the GM trait may necessitate evaluation as applications with new traits are made.
It is important that adequate standards for the risk assessment of genome-edited organism are set by the EU Commission by establishing a robust framework for EFSA [214, 215]. The EU Commission adopted Regulation (EC) No 503/2013 which sets the standards for assessing food and feed safety. A similar implementing regulation, using EU Directive 2001/18/EC as a legal basis, could set the standards for the risk assessment of genome-edited plants and animals without a change of EU GMO regulation. Such an implementing regulation would require EFSA to consider the specific risks associated with genome editing (as indicated in Fig. 1). The monitoring of GMOs after being placed on the market is a required part of risk management in the EU, either as a general surveillance for the detection of unanticipated adverse effects or as case-specific monitoring to detect direct and indirect effects that have been identified in the environmental risk assessment [14]. According to Council Decision 2002/811/EC, monitoring should not be regarded as research per se but as a means to evaluate or verify results and assumptions arising from previous research and evaluation of potential risk and research [216]. Thus, post-market monitoring can be used to survey for any, as yet unidentified, new or novel risks associated both with each individual genome-edited GMOs, and more generally, as a result of the genome editing process but monitoring cannot be considered as being a substitute to risk assessment.
There is growing awareness that science-based risk assessment for GMOs, including genome-edited organisms, is limited in scope and that the use (or non-use) of GMOs in agriculture also depends on societal values. Proposals to expand the scope of the regulation and governance of GMOs beyond science-based risk assessment include the recognition of the underlying values and assumptions shaping science and innovation, respect for ethical, societal and cultural values, ensuring the sustainability of agricultural systems and the consideration of a range of alternatives to food derived from GMOs [217, 218]. The complexity of issues in this expanded governance may benefit from the involvement of a broad range of people from different societal sectors [217].
Significant societal concern surrounds GMOs, including genome-edited animals, particularly farm animals as they are sentient [85, 179, 219]. Considerations of the welfare of GM animals are included in EFSA’s guidance [24]. However, what constitutes “better” welfare for GM animals is ill-defined as a trait aimed at improving animal welfare may, in practice, facilitate poor animal management in the first place. For example, disease resistance allows pigs to be kept in less hygienic or more crowded enclosures [179]. Animal welfare issues highlight the need for expanded governance as societal concerns may be critical in terms of consumer acceptance of products from GM animals [85, 219].
Conclusions
We have shown here that genome editing can cause genomic irregularities in the resultant GMOs, even if genes are not inserted, or inserted only transiently. Whilst molecular characterisation requirements in the current EU risk assessment guidelines for GMOs may capture many of these irregularities, some may be specific to genome editing, e.g. unintentional integration of plasmid components or large deletions at genomic locations distant to the target side and elude detection under current guidelines.
Therefore, the current EU risk assessment guidance for GMOs requires revision and expansion. Expansion of the molecular characterisation element of the risk assessment that enables analysis for off-target effects, unintended on-target effects and effects on genomic regulation is needed. Detection of any downstream effects and genomic irregularities will require development and standardisation of ‘omics technologies. Applications of such technologies would also improve the risk assessment of first-generation GMOs. The EU risk assessment for genome-edited organisms will also require consideration of a broader range of crop traits than first-generation GMOs. For some traits of genome-edited crops and animals there may be a complete lack of experience in assessing risks to the environment, food and animal feed. Further developments in technologies to assist detection of off-target effects and unintended on-target effects caused by genome editing are needed, as are developments in technologies to detect the resultant GMOs. However, the technological problems are not insurmountable, and techniques can be developed if there is political will to do so.
Availability of data and materials
Not applicable.
Abbreviations
- ABE:
-
Adenine base editors
- ADAR2:
-
Adenosine deaminases that act on ribonucleic acid 2
- ANPEP:
-
Amino peptidase N
- APHIS:
-
Animal and Plant Health Inspection Service
- Bt:
-
Bacillus thuringiensis
- Cas9:
-
Clustered regularly interspaced palindromic repeats-associated 9
- CBE:
-
Cytosine base editor
- Cpf1:
-
Clustered regularly interspaced palindromic repeats-associated endonuclease in Prevotella and Francisella 1
- CRISPR/Cas:
-
Clustered regularly interspaced palindromic repeats/Clustered regularly interspaced palindromic repeats-associated
- crRNA:
-
Clustered regularly interspaced palindromic repeats ribonucleic acid
- dCas9:
-
Dead Clustered regularly interspaced palindromic repeats-associated 9
- dCas13:
-
Dead Clustered regularly interspaced palindromic repeats-associated 13
- DNA:
-
Deoxyribonucleic acid
- DSB:
-
Double strand break
- EFSA:
-
European food safety authority
- EU:
-
European Union
- FAD2:
-
Fatty acid desaturase-2
- FGF-5:
-
Fibroblast growth factor-5
- GM:
-
Genetically modified
- GMO:
-
Genetically modified organism
- Gb:
-
Giga base pairs
- gRNA:
-
Guide ribonucleic acid
- HDR:
-
Homology-directed repair
- LwaCas13a:
-
Clustered regularly interspaced palindromic repeats-associated 13a from Leptotrichia wadei
- Mbp:
-
Mega base pairs
- mRNA:
-
Messenger ribonucleic acid
- MSTN:
-
Myostatin
- NHEJ:
-
Non-homologous end joining
- ODM:
-
Oligonucleotide-directed mutagenesis
- OTE:
-
Off-target effect
- OV:
-
Ovalbumin
- OVM:
-
Ovomucoid
- PAM:
-
Protospacer adjacent motif
- PCR:
-
Polymerase chain reaction
- pegRNA:
-
Prime editing ribonucleic acid
- RNA:
-
Ribonucleic acid
- PRRSV:
-
Porcine reproductive and respiratory syndrome virus
- RNP:
-
Ribonucleoprotein
- SDN-1:
-
Site-directed nuclease-1
- SDN-2:
-
Site-directed nuclease-2
- SDN-3:
-
Site-directed nuclease-3
- shRNA:
-
Small hairpin ribonucleic acid
- TALEN:
-
Transcription activator-like effector nuclease
- T-DNA:
-
Transfer deoxyribonucleic acid
- TGEV:
-
Transmissible gastroenteritis virus
- USDA:
-
United States Department of Agriculture
- ZNF:
-
Zinc finger nuclease
References
ISAAA (2018) Global status of commercialized biotech/GM crops: 2018. http://www.isaaa.org/resources/publications/briefs/54/default.asp. Accessed 26 Nov 2019
Shou H, Frame BR, Whitham SA, Wang K (2004) Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol Breeding 13:201–208. https://doi.org/10.1023/B:MOLB.0000018767.64586.53
Yin K, Gao C, Qiu JL (2017) Progress and prospects in plant genome editing. Nat Plants 3:17107. https://doi.org/10.1038/nplants.2017.107
Scientific Advisory Mechanism (2017) New techniques in agricultural biotechnology. https://ec.europa.eu/research/sam/pdf/topics/explanatory_note_new_techniques_agricultural_biotechnology.pdf. Accessed 26 Nov 2019
Gelinsky E, Hilbeck A (2018) European Court of Justice ruling regarding new genetic engineering methods scientifically justified: a commentary on the biased reporting about the recent ruling. Environ Sci Eur 30(1):52. https://doi.org/10.1186/s12302-018-0182-9
O’Keefe M, Perrault S, Halpern J, Ikemoto L, Yarborough M (2015) “Editing” genes: a case study about how language matters in bioethics. Am J Bioeth 15(12):3–10. https://doi.org/10.1080/15265161.2015.1103804
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339(6121):819–823. https://doi.org/10.1126/science.1231143
European Commission (2020) Ad hoc Stakeholder meeting on new genomic techniques. https://ec.europa.eu/food/sites/food/files/plant/docs/gmo_mod-bio_stake-cons_sum-rep-stakeholder.pdf. Accessed 29 Apr 2020
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32(4):347–355. https://doi.org/10.1038/nbt.2842
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821. https://doi.org/10.1126/science.1225829
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096. https://doi.org/10.1126/science.1258096
Lusser M, Parisi C, Plan D, Rodriguez-Cerezo E (2012) Deployment of new biotechnologies in plant breeding. Nat Biotechnol 30(3):231–239. https://doi.org/10.1038/nbt.2142
Eckerstorfer MF, Dolezel M, Heissenberger A, Miklau M, Reichenbecher W, Steinbrecher RA, Wassmann F (2019) An EU perspective on biosafety considerations for plants developed by genome editing and other new genetic modification techniques (nGMs). Front Bioeng Biotechnol 7:31. https://doi.org/10.3389/fbioe.2019.00031
European Commission (2001) Directive 2001/18/EC of The European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32001L0018&from=EN. Accessed Accessed 28 Apr 2020
CBD (2000) Cartagena protocol on biosafety to the convention on biological diversity. Secretariat of the Convention of Biological Diversity. http://bch.cbd.int/protocol/text/. Accessed 10 Jan 2020
Rang AL, Linke B, Jansen B (2005) Detection of RNA variants transcribed from the transgene in Roundup Ready soybean. Eur Food Res Technol 220:438–443. https://doi.org/10.1007/s00217-004-1064-5
Aharoni A, Galili G (2011) Metabolic engineering of the plant primary-secondary metabolism interface. Curr Opin Biotechnol 22(2):239–244. https://doi.org/10.1016/j.copbio.2010.11.004
Beckie HJ, Busi R, Bagavathiannan MV, Martin SL (2019) Herbicide resistance gene flow in weeds: under-estimated and under-appreciated. Agric Ecosyst Environ. 283:106566. https://doi.org/10.1016/j.agee.2019.06.005
Annett R, Habibi HR, Hontela A (2014) Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J Appl Toxicol 34(5):458–479. https://doi.org/10.1002/jat.2997
European Court of Justice (2018) Judgement of the Court (Grand Chamber), 25 July 2018 in Case C-528/16. http://curia.europa.eu/juris/document/document.jsf?text=&docid=204387&pageIndex=0&doclang=en&mode=req&dir=&occ=first&part=1&cid=133112. Accessed 10 Feb 2020
European Food Safety Agency (2010) Guidance on the environmental risk assessment of genetically modified plants. EFSA J 8(11):1879. https://doi.org/10.2903/j.efsa.2010.1879
European Food Safety Agency (2013) Guidance on the environmental risk assessment of genetically modified animals. EFSA J 11(5):3200. https://doi.org/10.2903/j.efsa.2013.3200
European Food Safety Agency (2011) Guidance for risk assessment of food and feed from genetically modified plants. EFSA J 9(5):2150. https://doi.org/10.2903/j.efsa.2011.2150
European Food Safety Agency (2012) Guidance on the risk assessment of food and feed from genetically modified animals and on animal health and welfare aspects. EFSA J 10(1):2501. https://doi.org/10.2903/j.efsa.2012.2501
European Commission (2013) Commission Implementing Regulation (EU) No 503/2013 of 3 April 2013 on applications for authorisation of genetically modified food and feed in accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and amending Commission Regulations (EC) No 641/2004 and (EC) No 1981/2006. https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1565205598558&uri=CELEX:32013R0503. Accessed 7 Feb 2020
European Food Safety Agency (2012) Scientific opinion addressing the safety assessment of plants developed using zinc finger nuclease 3 and other site-directed nucleases with similar function. EFSA J 10(10):2943. https://doi.org/10.2903/j.efsa.2012.2943
European Food Safety Agency (2019) Network on risk assessment of GMOs Minutes of the 10th meeting. http://www.efsa.europa.eu/sites/default/files/event/190618-m.pdf. Accessed 31 Jan 2020
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154(6):1380–1389. https://doi.org/10.1016/j.cell.2013.08.021
Lee JE, Neumann M, Duro DI, Schmid M (2019) CRISPR-based tools for targeted transcriptional and epigenetic regulation in plants. PLoS ONE 14(9):e0222778. https://doi.org/10.1371/journal.pone.0222778
Sauer NJ, Narvaez-Vasquez J, Mozoruk J, Miller RB, Warburg ZJ, Woodward MJ, Mihiret YA, Lincoln TA, Segami RE, Sanders SL, Walker KA, Beetham PR, Schopke CR, Gocal GF (2016) Oligonucleotide-mediated genome editing provides precision and function to engineered nucleases and antibiotics in plants. Plant Physiol 170(4):1917–1928. https://doi.org/10.1104/pp.15.01696
USDA-APHIS (2018) Am I regulated under 7 CFR part 340. https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/am-i-regulated/regulated_article_letters_of_inquiry/regulated_article_letters_of_inquiry. Accessed 13 May 2020
Calyxt (2019) First commercial sale of Calyxt high oleic soybean oil on the U.S. market. https://calyxt.com/wp-content/uploads/2019/02/20190226_PR-Calyno-Commercialization.pdf. Accessed 21 Jan 2020
Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70:667–697. https://doi.org/10.1146/annurev-arplant-050718-100049
Wang H, La Russa M, Qi LS (2016) CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem 85:227–264. https://doi.org/10.1146/annurev-biochem-060815-014607
Jiang F, Doudna JA (2017) CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys 46:505–529. https://doi.org/10.1146/annurev-biophys-062215-010822
Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156(5):935–949. https://doi.org/10.1016/j.cell.2014.02.001
Rudin N, Sugarman E, Haber JE (1989) Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics 122(3):519–534
Plessis A, Perrin A, Haber JE, Dujon B (1992) Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics 130(3):451–460
Rouet P, Smih F, Jasin M (1994) Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci U S A 91(13):6064–6068. https://doi.org/10.1073/pnas.91.13.6064
Choulika A, Perrin A, Dujon B, Nicolas JF (1995) Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 15(4):1968–1973
Gorbunova VV, Levy AA (1999) How plants make ends meet: DNA double-strand break repair. Trends Plant Sci 4(7):263–269
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339(6121):823–826. https://doi.org/10.1126/science.1232033
Brinkman EK, Chen T, de Haas M, Holland HA, Akhtar W, van Steensel B (2018) Kinetics and fidelity of the repair of Cas9-induced double-strand DNA breaks. Mol Cell 70(5):801–813. https://doi.org/10.1016/j.molcel.2018.04.016
Svitashev S, Young JK, Schwartz C, Gao H, Falco SC, Cigan AM (2015) Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol 169(2):931–945. https://doi.org/10.1104/pp.15.00793
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu JL, Gao C (2013) Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 31(8):686–688. https://doi.org/10.1038/nbt.2650
Podevin N, Davies HV, Hartung F, Nogue F, Casacuberta JM (2013) Site-directed nucleases: a paradigm shift in predictable, knowledge-based plant breeding. Trends Biotechnol 31(6):375–383. https://doi.org/10.1016/j.tibtech.2013.03.004
Petolino JF, Kumar S (2016) Transgenic trait deployment using designed nucleases. Plant Biotechnol J 14(2):503–509. https://doi.org/10.1111/pbi.12457
Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, Winblad N, Choudhury SR, Abudayyeh OO, Gootenberg JS, Wu WY, Scott DA, Severinov K, van der Oost J, Zhang F (2017) Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 35(1):31–34. https://doi.org/10.1038/nbt.3737
Raitskin O, Patron NJ (2016) Multi-gene engineering in plants with RNA-guided Cas9 nuclease. Curr Opin Biotechnol 37:69–75. https://doi.org/10.1016/j.copbio.2015.11.008
Tang X, Lowder LG, Zhang T, Malzahn AA, Zheng X, Voytas DF, Zhong Z, Chen Y, Ren Q, Li Q, Kirkland ER, Zhang Y, Qi Y (2017) A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants 3:17103. https://doi.org/10.1038/nplants.2017.103
Kim H, Kim ST, Ryu J, Kang BC, Kim JS, Kim SG (2017) CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat Commun 8:14406. https://doi.org/10.1038/ncomms14406
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152(5):1173–1183. https://doi.org/10.1016/j.cell.2013.02.022
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533(7603):420–424. https://doi.org/10.1038/nature17946
McDonald JI, Celik H, Rois LE, Fishberger G, Fowler T, Rees R, Kramer A, Martens A, Edwards JR, Challen GA (2016) Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation. Biol Open 5(6):866–874. https://doi.org/10.1242/bio.019067
Huang YH, Su J, Lei Y, Brunetti L, Gundry MC, Zhang X, Jeong M, Li W, Goodell MA (2017) DNA epigenome editing using CRISPR-Cas SunTag-directed DNMT3A. Genome Biol 18(1):176. https://doi.org/10.1186/s13059-017-1306-z
Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8(11):2180–2196. https://doi.org/10.1038/nprot.2013.132
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR (2017) Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551(7681):464–471. https://doi.org/10.1038/nature24644
Lu Y, Zhu JK (2017) Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol Plant 10(3):523–525. https://doi.org/10.1016/j.molp.2016.11.013
Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, Qiu JL, Wang D, Gao C (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat Biotechnol 35(5):438–440. https://doi.org/10.1038/nbt.3811
Kim K, Ryu SM, Kim ST, Baek G, Kim D, Lim K, Chung E, Kim S, Kim JS (2017) Highly efficient RNA-guided base editing in mouse embryos. Nat Biotechnol 35(5):435–437. https://doi.org/10.1038/nbt.3816
Zhang Y, Qin W, Lu X, Xu J, Huang H, Bai H, Li S, Lin S (2017) Programmable base editing of zebrafish genome using a modified CRISPR-Cas9 system. Nat Commun 8(1):118. https://doi.org/10.1038/s41467-017-00175-6
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293(5532):1074–1080. https://doi.org/10.1126/science.1063127
Hilton IB, D’Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA (2015) Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33(5):510–517. https://doi.org/10.1038/nbt.3199
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447(7143):407–412. https://doi.org/10.1038/nature05915
Margueron R, Reinberg D (2010) Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet 11(4):285–296. https://doi.org/10.1038/nrg2752
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR (2019) Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576(7785):149–157. https://doi.org/10.1038/s41586-019-1711-4
Li H, Li J, Chen J, Yan L, Xia L (2020) Precise modifications of both exogenous and endogenous genes in rice by prime editing. Mol Plant. https://doi.org/10.1016/j.molp.2020.03.011
Xu W, Zhang C, Yang Y, Zhao S, Kang G, He X, Song J, Yang J (2020) Versatile nucleotides substitution in plant using an improved prime editing system. Mol Plant 13(5):675–678. https://doi.org/10.1016/j.molp.2020.03.012
Lin Q, Zong Y, Xue C, Wang S, Jin S, Zhu Z, Wang Y, Anzalone AV, Raguram A, Doman JL, Liu DR, Gao C (2020) Prime genome editing in rice and wheat. Nat Biotechnol 38(5):582–585. https://doi.org/10.1038/s41587-020-0455-x
Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, Cox DBT, Kellner MJ, Regev A, Lander ES, Voytas DF, Ting AY, Zhang F (2017) RNA targeting with CRISPR-Cas13. Nature 550(7675):280–284. https://doi.org/10.1038/nature24049
Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, Shmakov S, Makarova KS, Semenova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, Zhang F (2016) C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353(6299):aaf5573. https://doi.org/10.1126/science.aaf5573
Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, Zhang F (2017) RNA editing with CRISPR-Cas13. Science 358(6366):1019–1027. https://doi.org/10.1126/science.aaq0180
Dupont Pioneer (2015) Confirmation of regulatory status of waxy corn developed by CRISPR-Cas technology. Letter to USDA-APHIS, December 14. https://www.pioneer.com/CMRoot/Pioneer/About_Global/Non_Searchable/news_media/15-352-01_air_inquiry_cbidel.pdf. Accessed 6 Nov 2019
Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, Liu J, Zhang H, Liu C, Ran Y, Gao C (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8:14261. https://doi.org/10.1038/ncomms14261
Malnoy M, Viola R, Jung MH, Koo OJ, Kim S, Kim JS, Velasco R, Nagamangala Kanchiswamy C (2016) DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci 7:1904. https://doi.org/10.3389/fpls.2016.01904
Norris AL, Lee SS, Greenlees KJ, Tadesse DA, Miller MF, Lombardi HA (2020) Template plasmid integration in germline genome-edited cattle. Nat Biotechnol 38(2):163–164. https://doi.org/10.1038/s41587-019-0394-6
Young AE, Mansour TA, McNabb BR, Owen JR, Trott JF, Brown CT, Van Eenennaam AL (2019) Genomic and phenotypic analyses of six offspring of a genome-edited hornless bull. Nat Biotechnol 38(2):225-232. https://doi.org/10.1038/s41587-019-0266-0
Woo JW, Kim J, Kwon SI, Corvalan C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33(11):1162–1164. https://doi.org/10.1038/nbt.3389
Jung C, Capistrano-Gossmann G, Braatz J, Sashidhar N, Melzer S (2017) Recent developments in genome editing and applications in plant breeding. Plant Breeding 137:1–9. https://doi.org/10.1111/pbr.12526
Weeks DP (2017) Gene editing in polyploid crops: wheat, camelina, canola, potato, cotton, peanut, sugar cane, and citrus. Progress in molecular biology and translational science, vol 149. Elsevier, Amsterdam, pp 65–80. https://doi.org/10.1016/bs.pmbts.2017.05.002
Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH (2015) Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucleic Acids 4:e264. https://doi.org/10.1038/mtna.2015.37
Lin CS, Hsu CT, Yang LH, Lee LY, Fu JY, Cheng QW, Wu FH, Hsiao HC, Zhang Y, Zhang R, Chang WJ, Yu CT, Wang W, Liao LJ, Gelvin SB, Shih MC (2018) Application of protoplast technology to CRISPR/Cas9 mutagenesis: from single-cell mutation detection to mutant plant regeneration. Plant Biotechnol J 16(7):1295–1310. https://doi.org/10.1111/pbi.12870
Fossi M, Amundson K, Kuppu S, Britt A, Comai L (2019) Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant Physiol 180(1):78–86. https://doi.org/10.1104/pp.18.00906
West J, Gill WW (2016) Genome editing in large animals. J Equine Vet Sci 41:1–6. https://doi.org/10.1016/j.jevs.2016.03.008
Ishii T (2017) Genome-edited livestock: ethics and social acceptance. Animal Front 7:24–32. https://doi.org/10.2527/af.2017.0115
Tan W, Proudfoot C, Lillico SG, Whitelaw CB (2016) Gene targeting, genome editing: from Dolly to editors. Transgenic Res 25(3):273–287. https://doi.org/10.1007/s11248-016-9932-x
Yum SY, Youn KY, Choi WJ, Jang G (2018) Development of genome engineering technologies in cattle: from random to specific. J Anim Sci Biotechnol 9:16. https://doi.org/10.1186/s40104-018-0232-6
Lamas-Toranzo I, Galiano-Cogolludo B, Cornudella-Ardiaca F, Cobos-Figueroa J, Ousinde O, Bermejo-Alvarez P (2019) Strategies to reduce genetic mosaicism following CRISPR-mediated genome edition in bovine embryos. Sci Rep 9(1):14900. https://doi.org/10.1038/s41598-019-51366-8
Duensing N, Sprink T, Parrott WA, Fedorova M, Lema MA, Wolt JD, Bartsch D (2018) Novel features and considerations for ERA and regulation of crops produced by genome editing. Front Bioeng Biotechnol 6:79. https://doi.org/10.3389/fbioe.2018.00079
Sauer NJ, Mozoruk J, Miller RB, Warburg ZJ, Walker KA, Beetham PR, Schopke CR, Gocal GF (2016) Oligonucleotide-directed mutagenesis for precision gene editing. Plant Biotechnol J 14(2):496–502. https://doi.org/10.1111/pbi.12496
Voytas DF, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12(6):e1001877. https://doi.org/10.1371/journal.pbio.1001877
Hartung F, Schiemann J (2014) Precise plant breeding using new genome editing techniques: opportunities, safety and regulation in the EU. Plant J 78(5):742–752. https://doi.org/10.1111/tpj.12413
Modrzejewski D, Hartung F, Sprink T, Krause D, Kohl C, Wilhelm R (2019) What is the available evidence for the range of applications of genome-editing as a new tool for plant trait modification and the potential occurrence of associated off-target effects: a systematic map. Environ Evid 8:27. https://doi.org/10.1186/s13750-019-0171-5
Kosicki M, Tomberg K, Bradley A (2018) Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol 36(8):765–771. https://doi.org/10.1038/nbt.4192
Wolt JD, Wang K, Sashital D, Lawrence-Dill CJ (2016) Achieving plant CRISPR targeting that limits off-target effects. Plant Genome 9(3):1–8. https://doi.org/10.3835/plantgenome2016.05.0047
Zhu C, Bortesi L, Baysal C, Twyman RM, Fischer R, Capell T, Schillberg S, Christou P (2017) Characteristics of genome editing mutations in cereal crops. Trends Plant Sci 22(1):38–52. https://doi.org/10.1016/j.tplants.2016.08.009
Lawrenson T, Shorinola O, Stacey N, Li C, Ostergaard L, Patron N, Uauy C, Harwood W (2015) Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol 16:258. https://doi.org/10.1186/s13059-015-0826-7
Peterson BA, Haak DC, Nishimura MT, Teixeira PJ, James SR, Dangl JL, Nimchuk ZL (2016) Genome-wide assessment of efficiency and specificity in CRISPR/Cas9 mediated multiple site targeting in Arabidopsis. PLoS ONE 11(9):e0162169. https://doi.org/10.1371/journal.pone.0162169
Lee K, Zhang Y, Kleinstiver BP, Guo JA, Aryee MJ, Miller J, Malzahn A, Zarecor S, Lawrence-Dill CJ, Joung JK, Qi Y, Wang K (2019) Activities and specificities of CRISPR/Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant Biotechnol J 17(2):362–372. https://doi.org/10.1111/pbi.12982
Ryu J, Prather RS, Lee K (2018) Use of gene-editing technology to introduce targeted modifications in pigs. J Anim Sci Biotechnol 9:5. https://doi.org/10.1186/s40104-017-0228-7
Anderson KR, Haeussler M, Watanabe C, Janakiraman V, Lund J, Modrusan Z, Stinson J, Bei Q, Buechler A, Yu C, Thamminana SR, Tam L, Sowick MA, Alcantar T, O’Neil N, Li J, Ta L, Lima L, Roose-Girma M, Rairdan X, Durinck S, Warming S (2018) CRISPR off-target analysis in genetically engineered rats and mice. Nat Methods 15:512–514. https://doi.org/10.1038/s41592-018-0011-5
Shin HY, Wang C, Lee HK, Yoo KH, Zeng X, Kuhns T, Yang CM, Mohr T, Liu C, Hennighausen L (2017) CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat Commun 8:15464. https://doi.org/10.1038/ncomms15464
Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR (2013) High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31(9):839–843. https://doi.org/10.1038/nbt.2673
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31(9):822–826. https://doi.org/10.1038/nbt.2623
Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, Kim JS (2014) Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res 24(1):132–141. https://doi.org/10.1101/gr.162339.113
Tycko J, Myer VE, Hsu PD (2016) Methods for optimizing CRISPR-Cas9 genome editing specificity. Mol Cell 63(3):355–370. https://doi.org/10.1016/j.molcel.2016.07.004
Cameron P, Fuller CK, Donohoue PD, Jones BN, Thompson MS, Carter MM, Gradia S, Vidal B, Garner E, Slorach EM, Lau E, Banh LM, Lied AM, Edwards LS, Settle AH, Capurso D, Llaca V, Deschamps S, Cigan M, Young JK, May AP (2017) Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat Methods 14(6):600–606. https://doi.org/10.1038/nmeth.4284
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31(9):827–832. https://doi.org/10.1038/nbt.2647
Begemann MB, Gray BN, January E, Gordon GC, He Y, Liu H, Wu X, Brutnell TP, Mockler TC, Oufattole M (2017) Precise insertion and guided editing of higher plant genomes using Cpf1 CRISPR nucleases. Sci Rep 7(1):11606. https://doi.org/10.1038/s41598-017-11760-6
Mahfouz MM (2017) Genome editing: the efficient tool CRISPR-Cpf1. Nat Plants 3:17028. https://doi.org/10.1038/nplants.2017.28
Agapito-Tenfen SZ, Okoli AS, Bernstein MJ, Wikmark OG, Myhr AI (2018) Revisiting risk governance of GM plants: the need to consider new and emerging gene-editing techniques. Front Plant Sci 9:1874. https://doi.org/10.3389/fpls.2018.01874
Jamal M, Khan FA, Da L, Habib Z, Dai J, Cao G (2016) Keeping CRISPR/Cas on-target. Curr Issues Mol Biol 20:1–12. https://doi.org/10.21775/cimb.020.001
Vu GTH, Cao HX, Fauser F, Reiss B, Puchta H, Schubert I (2017) Endogenous sequence patterns predispose the repair modes of CRISPR/Cas9-induced DNA double-stranded breaks in Arabidopsis thaliana. Plant J 92(1):57–67. https://doi.org/10.1111/tpj.13634
Allen F, Crepaldi L, Alsinet C, Strong AJ, Kleshchevnikov V, De Angeli P, Palenikova P, Khodak A, Kiselev V, Kosicki M, Bassett AR, Harding H, Galanty Y, Munoz-Martinez F, Metzakopian E, Jackson SP, Parts L (2018) Predicting the mutations generated by repair of Cas9-induced double-strand breaks. Nat Biotechnol 37(1):64–72. https://doi.org/10.1038/nbt.4317
Rees HA, Komor AC, Yeh WH, Caetano-Lopes J, Warman M, Edge ASB, Liu DR (2017) Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat Commun 8:15790. https://doi.org/10.1038/ncomms15790
Zuo E, Sun Y, Wei W, Yuan T, Ying W, Sun H, Yuan L, Steinmetz LM, Li Y, Yang H (2019) Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 364(6437):289–292. https://doi.org/10.1126/science.aav9973
Jin S, Zong Y, Gao Q, Zhu Z, Wang Y, Qin P, Liang C, Wang D, Qiu JL, Zhang F, Gao C (2019) Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364(6437):292–295. https://doi.org/10.1126/science.aaw7166
Grunewald J, Zhou R, Garcia SP, Iyer S, Lareau CA, Aryee MJ, Joung JK (2019) Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 569(7756):433–437. https://doi.org/10.1038/s41586-019-1161-z
Gehrke JM, Cervantes O, Clement MK, Wu Y, Zeng J, Bauer DE, Pinello L, Joung JK (2018) An APOBEC3A-Cas9 base editor with minimized bystander and off-target activities. Nat Biotechnol 36(10):977–982. https://doi.org/10.1038/nbt.4199
Reardon S (2020) Step aside CRISPR, RNA editing is taking off. Nature 578:24–27. https://doi.org/10.1038/d41586-020-00272-5
Galonska C, Charlton J, Mattei AL, Donaghey J, Clement K, Gu H, Mohammad AW, Stamenova EK, Cacchiarelli D, Klages S, Timmermann B, Cantz T, Scholer HR, Gnirke A, Ziller MJ, Meissner A (2018) Genome-wide tracking of dCas9-methyltransferase footprints. Nat Commun 9(1):597. https://doi.org/10.1038/s41467-017-02708-5
Enriquez P (2016) CRISPR-mediated epigenome editing. Yale J Biol Med 89(4):471–486
Lalonde S, Stone OA, Lessard S, Lavertu A, Desjardins J, Beaudoin M, Rivas M, Stainier DYR, Lettre G (2017) Frameshift indels introduced by genome editing can lead to in-frame exon skipping. PLoS ONE 12(6):e0178700. https://doi.org/10.1371/journal.pone.0178700
Mou H, Smith JL, Peng L, Yin H, Moore J, Zhang XO, Song CQ, Sheel A, Wu Q, Ozata DM, Li Y, Anderson DG, Emerson CP, Sontheimer EJ, Moore MJ, Weng Z, Xue W (2017) CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol 18(1):108. https://doi.org/10.1186/s13059-017-1237-8
Tuladhar R, Yeu Y, Tyler Piazza J, Tan Z, Rene Clemenceau J, Wu X, Barrett Q, Herbert J, Mathews DH, Kim J, Hyun Hwang T, Lum L (2019) CRISPR-Cas9-based mutagenesis frequently provokes on-target mRNA misregulation. Nat Commun 10(1):4056. https://doi.org/10.1038/s41467-019-12028-5
Hahn F, Nekrasov V (2019) CRISPR/Cas precision: do we need to worry about off-targeting in plants? Plant Cell Rep 38(4):437–441. https://doi.org/10.1007/s00299-018-2355-9
Kapahnke M, Banning A, Tikkanen R (2016) Random splicing of several exons caused by a single base change in the target exon of CRISPR/Cas9 mediated gene knockout. Cells 5(4):45. https://doi.org/10.3390/cells5040045
Ramirez-Sanchez O, Perez-Rodriguez P, Delaye L, Tiessen A (2016) Plant proteins are smaller because they are encoded by fewer exons than animal proteins. Genomics Proteomics Bioinform 14(6):357–370. https://doi.org/10.1016/j.gpb.2016.06.003
Sharpe JJ, Cooper TA (2017) Unexpected consequences: exon skipping caused by CRISPR-generated mutations. Genome Biol 18(1):109. https://doi.org/10.1186/s13059-017-1240-0
Skryabin BV, Kummerfeld D-M, Gubar L, Seeger B, Kaiser H, Stegemann A, Roth J, Meuth SG, Pavenstädt H, Sherwood J, Pap T, Wedlich-Söldner R, Sunderkötter C, Schwartz YB, Brosius J, Rozhdestvensky TS (2020) Pervasive head-to-tail insertions of DNA templates mask desired CRISPR-Cas9–mediated genome editing events. Sci Adv 6(7):2941. https://doi.org/10.1126/sciadv.aax2941
Reiner G (2016) Genetic resistance—an alternative for controlling PRRS? Porcine Health Manag 2:27. https://doi.org/10.1186/s40813-016-0045-y
Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J (2018) CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. https://doi.org/10.1038/s41591-018-0049-z
Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K, Kommineni S, Chen J, Sondey M, Ye C, Randhawa R, Kulkarni T, Yang Z, McAllister G, Russ C, Reece-Hoyes J, Forrester W, Hoffman GR, Dolmetsch R, Kaykas A (2018) p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med 24:939–946. https://doi.org/10.1038/s41591-018-0050-6
Yoshiyama KO, Kimura S, Maki H, Britt AB, Umeda M (2014) The role of SOG1, a plant-specific transcriptional regulator, in the DNA damage response. Plant Signal Behav 9(4):e28889. https://doi.org/10.4161/psb.28889
Li Z, Liu ZB, Xing A, Moon BP, Koellhoffer JP, Huang L, Ward RT, Clifton E, Falco SC, Cigan AM (2015) Cas9-guide RNA directed genome editing in soybean. Plant Physiol 169(2):960–970. https://doi.org/10.1104/pp.15.00783
Michno JM, Virdi K, Stec AO, Liu J, Wang X, Xiong Y, Stupar RM (2020) Integration, abundance, and transmission of mutations and transgenes in a series of CRISPR/Cas9 soybean lines. BMC Biotechnol 20(1):10. https://doi.org/10.1186/s12896-020-00604-3
Carlson DF, Lancto CA, Zang B, Kim ES, Walton M, Oldeschulte D, Seabury C, Sonstegard TS, Fahrenkrug SC (2016) Production of hornless dairy cattle from genome-edited cell lines. Nat Biotechnol 34(5):479–481. https://doi.org/10.1038/nbt.3560
Gelvin SB (2017) Integration of Agrobacterium T-DNA into the plant genome. Annu Rev Genet 51:195–217. https://doi.org/10.1146/annurev-genet-120215-035320
Forsbach A, Schubert D, Lechtenberg B, Gils M, Schmidt R (2003) A comprehensive characterization of single-copy T-DNA insertions in the Arabidopsis thaliana genome. Plant Mol Biol 52(1):161–176. https://doi.org/10.1023/a:1023929630687
Makarevitch I, Svitashev SK, Somers DA (2003) Complete sequence analysis of transgene loci from plants transformed via microprojectile bombardment. Plant Mol Biol 52(2):421–432. https://doi.org/10.1023/a:1023968920830
Jupe F, Rivkin AC, Michael TP, Zander M, Motley ST, Sandoval JP, Slotkin RK, Chen H, Castanon R, Nery JR, Ecker JR (2019) The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet 15(1):e1007819. https://doi.org/10.1371/journal.pgen.1007819
Wilson AK, Latham JR, Steinbrecher RA (2006) Transformation-induced mutations in transgenic plants: analysis and biosafety implications. Biotechnol Genet Eng Rev 23:209–237. https://doi.org/10.1080/02648725.2006.10648085
Latham JR, Wilson AK (2006) Steinbrecher RA (2006) The mutational consequences of plant transformation. J Biomed Biotechnol 2:25376. https://doi.org/10.1155/JBB/2006/25376
Windels P, De Buck S, Van Bockstaele E, De Loose M, Depicker A (2003) T-DNA integration in Arabidopsis chromosomes. Presence and origin of filler DNA sequences. Plant Physiol 133(4):2061–2068. https://doi.org/10.1104/pp.103.027532
Kim SR, Lee J, Jun SH, Park S, Kang HG, Kwon S, An G (2003) Transgene structures in T-DNA-inserted rice plants. Plant Mol Biol 52(4):761–773. https://doi.org/10.1023/a:1025093101021
Claros MG, Bautista R, Guerrero-Fernandez D, Benzerki H, Seoane P, Fernandez-Pozo N (2012) Why assembling plant genome sequences is so challenging. Biology (Basel) 1(2):439–459. https://doi.org/10.3390/biology1020439
Pele A, Rousseau-Gueutin M, Chevre AM (2018) Speciation success of polyploid plants closely relates to the regulation of meiotic recombination. Front Plant Sci 9:907. https://doi.org/10.3389/fpls.2018.00907
Lu K, Wei L, Li X, Wang Y, Wu J, Liu M, Zhang C, Chen Z, Xiao Z, Jian H, Cheng F, Zhang K, Du H, Cheng X, Qu C, Qian W, Liu L, Wang R, Zou Q, Ying J, Xu X, Mei J, Liang Y, Chai YR, Tang Z, Wan H, Ni Y, He Y, Lin N, Fan Y, Sun W, Li NN, Zhou G, Zheng H, Wang X, Paterson AH, Li J (2019) Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat Commun 10(1):1154. https://doi.org/10.1038/s41467-019-09134-9
International Wheat Genome Sequencing Consortium (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. https://doi.org/10.1126/science.aar7191
Mohan C (2016) Genome editing in sugarcane: challenges ahead. Front Plant Sci 7:1542. https://doi.org/10.3389/fpls.2016.01542
Kawall K (2019) New possibilities on the horizon: genome editing makes the whole genome accessible for changes. Front Plant Sci 10:525. https://doi.org/10.3389/fpls.2019.00525
Mao Y, Zhang H, Xu N, Zhang B, Gou F, Zhu JK (2013) Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol Plant 6(6):2008–2011. https://doi.org/10.1093/mp/sst121
Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, Xie Y, Shen R, Chen S, Wang Z, Chen Y, Guo J, Chen L, Zhao X, Dong Z, Liu YG (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8(8):1274–1284. https://doi.org/10.1016/j.molp.2015.04.007
Wang W, Akhunova A, Chao S, Trick H, Akhunov E (2018) Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J 1(1):65–74. https://doi.org/10.1089/crispr.2017.0010
Liang G, Zhang H, Lou D, Yu D (2016) Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Sci Rep 6:21451. https://doi.org/10.1038/srep21451
Cai Y, Chen L, Liu X, Guo C, Sun S, Wu C, Jiang B, Han T, Hou W (2018) CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnol J 16(1):176–185. https://doi.org/10.1111/pbi.12758
Morineau C, Bellec Y, Tellier F, Gissot L, Kelemen Z, Nogue F, Faure JD (2017) Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol J 15(6):729–739. https://doi.org/10.1111/pbi.12671
Kagale S, Koh C, Nixon J, Bollina V, Clarke WE, Tuteja R, Spillane C, Robinson SJ, Links MG, Clarke C, Higgins EE, Huebert T, Sharpe AG, Parkin IA (2014) The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploid genome structure. Nat Commun 5:3706. https://doi.org/10.1038/ncomms4706
Hutcheon C, Ditt RF, Beilstein M, Comai L, Schroeder J, Goldstein E, Shewmaker CK, Nguyen T, De Rocher J, Kiser J (2010) Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol 10:233. https://doi.org/10.1186/1471-2229-10-233
Schmidt C, Schindele P, Puchta H (2019) From gene editing to genome engineering: restructuring plant chromosomes via CRISPR/Cas. aBIOTECH 1:21–31. https://doi.org/10.1007/s42994-019-00002-0
Cobb JN, Biswas PS, Platten JD (2019) Back to the future: revisiting MAS as a tool for modern plant breeding. Theor Appl Genetics 132:647–667. https://doi.org/10.1007/s00122-018-3266-4
Ricroch A, Clairand P, Harwood W (2017) Use of CRISPR systems in plant genome editing: toward new opportunities in agriculture. Emerging Topics Life Sci 1(2):169–182. https://doi.org/10.1042/etls20170085
Price B, Cotter J (2014) The GM Contamination Register: a review of recorded contamination incidents associated with genetically modified organisms (GMOs), 1997–2013. FoodContamination 1:5. https://doi.org/10.1186/s40550-014-0005-8
Rodriguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB (2017) Engineering quantitative trait variation for crop improvement by genome. Cell 171(2):470–480. https://doi.org/10.1016/j.cell.2017.08.030
Hu Z, Lu SJ, Wang MJ, He H, Sun L, Wang H, Liu XH, Jiang L, Sun JL, Xin X, Kong W, Chu C, Xue HW, Yang J, Luo X, Liu JX (2018) A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol Plant 11(5):736–749. https://doi.org/10.1016/j.molp.2018.03.005
Yang Y, Zhu K, Li H, Han S, Meng Q, Khan SU, Fan C, Xie K, Zhou Y (2018) Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol J 16(7):1322–1335. https://doi.org/10.1111/pbi.12872
Huang C, Sun H, Xu D, Chen Q, Liang Y, Wang X, Xu G, Tian J, Wang C, Li D, Wu L, Yang X, Jin W, Doebley JF, Tian F (2018) ZmCCT9 enhances maize adaptation to higher latitudes. Proc Natl Acad Sci U S A 115(2):E334–E341. https://doi.org/10.1073/pnas.1718058115
Sanchez-Leon S, Gil-Humanes J, Ozuna CV, Gimenez MJ, Sousa C, Voytas DF, Barro F (2018) Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J 16(4):902–910. https://doi.org/10.1111/pbi.12837
Li X, Wang Y, Chen S, Tian H, Fu D, Zhu B, Luo Y, Zhu H (2018) Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front Plant Sci 9:559. https://doi.org/10.3389/fpls.2018.00559
Zhou X, Liao H, Chern M, Yin J, Chen Y, Wang J, Zhu X, Chen Z, Yuan C, Zhao W, Wang J, Li W, He M, Ma B, Wang J, Qin P, Chen W, Wang Y, Liu J, Qian Y, Wang W, Wu X, Li P, Zhu L, Li S, Ronald PC, Chen X (2018) Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc Natl Acad Sci U S A 115(12):3174–3179. https://doi.org/10.1073/pnas.1705927115
Nekrasov V, Wang C, Win J, Lanz C, Weigel D, Kamoun S (2017) Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci Rep 7(1):482. https://doi.org/10.1038/s41598-017-00578-x
Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D (2017) Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J 91(4):714–724. https://doi.org/10.1111/tpj.13599
Duan YB, Li J, Qin RY, Xu RF, Li H, Yang YC, Ma H, Li L, Wei PC, Yang JB (2016) Identification of a regulatory element responsible for salt induction of rice OsRAV2 through ex situ and in situ promoter analysis. Plant Mol Biol 90(1–2):49–62. https://doi.org/10.1007/s11103-015-0393-z
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15(2):207–216. https://doi.org/10.1111/pbi.12603
Hummel AW, Chauhan RD, Cermak T, Mutka AM, Vijayaraghavan A, Boyher A, Starker CG, Bart R, Voytas DF, Taylor NJ (2018) Allele exchange at the EPSPS locus confers glyphosate tolerance in cassava. Plant Biotechnol J 16(7):1275–1282. https://doi.org/10.1111/pbi.12868
Sun Y, Zhang X, Wu C, He Y, Ma Y, Hou H, Guo X, Du W, Zhao Y, Xia L (2016) Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol Plant 9(4):628–631. https://doi.org/10.1016/j.molp.2016.01.001
Zhou X, Jacobs TB, Xue LJ, Harding SA, Tsai CJ (2015) Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial Populus reveals 4-coumarate:CoA ligase specificity and redundancy. New Phytol 208(2):298–301. https://doi.org/10.1111/nph.13470
Andersson M, Turesson H, Nicolia A, Falt AS, Samuelsson M, Hofvander P (2017) Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep 36(1):117–128. https://doi.org/10.1007/s00299-016-2062-3
Bruce A (2017) Genome edited animals: learning from GM crops? Transgenic Res 26(3):385–398. https://doi.org/10.1007/s11248-017-0017-2
European Medicines Agency (2019) ATryn. European Public Assessment Report—summary for the public. https://www.ema.europa.eu/en/documents/overview/atryn-epar-summary-public_en.pdf. Accessed 29 Apr 2020
European Medicines Agency (2019) Withdrawal of the marketing authorisation in the European Union. https://www.ema.europa.eu/en/documents/public-statement/public-statement-atryn-withdrawal-marketing-authorisation-european-union_en.pdf. Accessed 29 Apr 2020
Eckerstorfer MF, Engelhard M, Heissenberger A, Simon S, Teichmann H (2019) Plants developed by new genetic modification techniques-comparison of existing regulatory frameworks in the EU and non-EU countries. Front Bioeng Biotechnol 7:26. https://doi.org/10.3389/fbioe.2019.00026
Ledford H (2016) Gene-editing surges as US rethinks regulations. Nature 532(7598):158–159. https://doi.org/10.1038/532158a
US Food and Drug Administration (2020) Understanding new plant varieties. https://www.fda.gov/food/food-new-plant-varieties/understanding-new-plant-varieties. Accessed 12 May 2020
Hilbeck A, Meyer H, Wynne B, Millstone E (2020) GMO regulations and their interpretation: how EFSA’s guidance on risk assessments of GMOs is bound to fail. Environ Sci Eur. https://doi.org/10.1186/s12302-020-00325-6
Eriksson D, Custers R, Edvardsson Bjornberg K, Hansson SO, Purnhagen K, Qaim M, Romeis J, Schiemann J, Schleissing S, Tosun J, Visser RGF (2020) Options to reform the European Union legislation on GMOs: scope and definitions. Trends Biotechnol 38(3):231–234. https://doi.org/10.1016/j.tibtech.2019.12.002
European Commission (2001) Directive 2001/18/EC of The European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC, Recital 17. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32001L0018&from=EN. Accessed 28 Apr 2020
Eriksson D, Kershen D, Nepomuceno A, Pogson BJ, Prieto H, Purnhagen K, Smyth S, Wesseler J, Whelan A (2019) A comparison of the EU regulatory approach to directed mutagenesis with that of other jurisdictions, consequences for international trade and potential steps forward. New Phytol 222(4):1673–1684. https://doi.org/10.1111/nph.15627
Bratlie S, Halvorsen K, Myskja BK, Mellegard H, Bjorvatn C, Frost P, Heiene G, Hofmann B, Holst-Jensen A, Holst-Larsen T, Malnes RS, Paus B, Sandvig B, Sjoli SI, Skarstein B, Thorseth MB, Vagstad N, Vage DI, Borge OJ (2019) A novel governance framework for GMO: a tiered, more flexible regulation for GMOs would help to stimulate innovation and public debate. EMBO Rep. https://doi.org/10.15252/embr.201947812
Scientific Advisory Mechanism (2018) A scientific perspective on the regulatory status of products derived from gene editing and the implications for the GMO Directive. https://op.europa.eu/en/publication-detail/-/publication/a9100d3c-4930-11e9-a8ed-01aa75ed71a1/language-en/format-PDF/source-94584603. Accessed 13 May 2020
Fraser PD, Aharoni A, Hall RD, Huang S, Giovannoni JJ, Sonnewald U, Fernie AR (2020) Metabolomics should be deployed in the identification and characterization of gene-edited crops. Plant J. https://doi.org/10.1111/tpj.14679
Kannan B, Jung JH, Moxley GW, Lee SM, Altpeter F (2018) TALEN-mediated targeted mutagenesis of more than 100 COMT copies/alleles in highly polyploid sugarcane improves saccharification efficiency without compromising biomass yield. Plant Biotechnol J 16(4):856–866. https://doi.org/10.1111/pbi.12833
Gasperskaja E, Kucinskas V (2017) The most common technologies and tools for functional genome analysis. Acta Med Litu 24(1):1–11. https://doi.org/10.6001/actamedica.v24i1.3457
Manzoni C, Kia DA, Vandrovcova J, Hardy J, Wood NW, Lewis PA, Ferrari R (2018) Genome, transcriptome and proteome: the rise of omics data and their integration in biomedical sciences. Brief Bioinform 19(2):286–302. https://doi.org/10.1093/bib/bbw114
van der Greef J, van Wietmarschen H, van Ommen B, Verheij E (2013) Looking back into the future: 30 years of metabolomics at TNO. Mass Spectrom Rev 32(5):399–415. https://doi.org/10.1002/mas.21370
Heinemann JA, Kurenbach B, Quist D (2011) Molecular profiling–a tool for addressing emerging gaps in the comparative risk assessment of GMOs. Environ Int 37(7):1285–1293. https://doi.org/10.1016/j.envint.2011.05.006
van Dijk JP, de Mello CS, Voorhuijzen MM, Hutten RC, Arisi AC, Jansen JJ, Buydens LM, van der Voet H, Kok EJ (2014) Safety assessment of plant varieties using transcriptomics profiling and a one-class classifier. Regul Toxicol Pharmacol 70:297–303. https://doi.org/10.1016/j.yrtph.2014.07.013
European Food Safety Agency (2018) Omics in risk assessment: state of the art and next steps. EFSA Supporting Publ. https://doi.org/10.2903/sp.efsa.2018.en-1512
Rodriguez PA, Rothballer M, Chowdhury SP, Nussbaumer T, Gutjahr C, Falter-Braun P (2019) Systems biology of plant-microbiome interactions. Mol Plant 12(6):804–821. https://doi.org/10.1016/j.molp.2019.05.006
Agler MT, Ruhe J, Kroll S, Morhenn C, Kim ST, Weigel D, Kemen EM (2016) Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol 14(1):e1002352. https://doi.org/10.1371/journal.pbio.1002352
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17(8):478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA, Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332(6033):1097–1100. https://doi.org/10.1126/science.1203980
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
de Vries FT, Griffiths RI, Knight CG, Nicolitch O, Williams A (2020) Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 368(6488):270–274. https://doi.org/10.1126/science.aaz5192
Sergaki C, Lagunas B, Lidbury I, Gifford ML, Schafer P (2018) Challenges and approaches in microbiome research: from fundamental to applied. Front Plant Sci 9:1205. https://doi.org/10.3389/fpls.2018.01205
Busby PE, Soman C, Wagner MR, Friesen ML, Kremer J, Bennett A, Morsy M, Eisen JA, Leach JE, Dangl JL (2017) Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol 15(3):e2001793. https://doi.org/10.1371/journal.pbio.2001793
Colombo SM, Campbella LG, Murphy EJ, Martin SL, Arts MT (2018) Potential for novel production of omega-3 long-chain fatty acids by genetically engineered oilseed plants to alter terrestrial ecosystem dynamics. Agric Syst 164:31–37. https://doi.org/10.1016/j.agsy.2018.03.004
Hixson SM, Shukla K, Campbell LG, Hallett RH, Smith SM, Packer L, Arts MT (2016) Long-chain omega-3 polyunsaturated fatty acids have developmental effects on the crop pest, the cabbage white butterfly Pieris rapae. PLoS ONE 11(3):e0152264. https://doi.org/10.1371/journal.pone.0152264
Helliwell R, Hartley S, Pearce W, O’Neill L (2017) Why are NGOs sceptical of genome editing? NGOs’ opposition to agricultural biotechnologies is rooted in scepticism about the framing of problems and solutions, rather than just emotion and dogma. EMBO Rep 18:2090–2093. https://doi.org/10.15252/embr.201744385
Wickson F, Binimelis R, Herrero A (2016) Should organic agriculture maintain its opposition to GM? New techniques writing the same old story. Sustainability. 8:1105. https://doi.org/10.3390/su8111105
Ledford H (2019) CRISPR conundrum: strict European court ruling leaves food-testing labs without a plan. Nature 572(7767):15. https://doi.org/10.1038/d41586-019-02162-x
Sedlazeck FJ, Lee H, Darby CA, Schatz MC (2018) Piercing the dark matter: bioinformatics of long-range sequencing and mapping. Nat Rev Genet 19(6):329–346. https://doi.org/10.1038/s41576-018-0003-4
Bauer-Panskus A, Miyazaki J, Kawall K, Then C (2020) Risk assessment of genetically engineered plants that can persist and propagate in the environment. Environ Sci Eur 32:32. https://doi.org/10.1186/s12302-020-00301-0
Millstone E (2009) Science, risk and governance: radical rhetorics and the realities of reform. Res Policy 38(4):624–636. https://doi.org/10.1016/j.respol.2009.01.012
Millstone E (2014) The contributions of science and politics to global food safety law. In: Freeman MSH, Bennett B (eds) Law and global health: current legal issues, vol 16. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199688999.003.0036
Council Decision (2002) 2002/811/EC: Council Decision of 3 October 2002 establishing guidance notes supplementing Annex VII to Directive 2001/18/EC of the European Parliament and of the Council on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32002D0811&from=EN. Accessed 29 Apr 2020
Jordan NR, Dorn KM, Smith TM, Wolf KE, Ewing PM, Fernandez AL, Runck BC, Williams A, Lu Y, Kuzma J (2017) A cooperative governance network for crop genome editing: the success of governance networks in other areas could help to find common ground for applying genome editing in agriculture. EMBO Rep 18(10):1683–1687. https://doi.org/10.15252/embr.201744394
Hartley S, Gillund F, van Hove L, Wickson F (2016) Essential features of responsible governance of agricultural biotechnology. PLoS Biol 14(5):e1002453. https://doi.org/10.1371/journal.pbio.1002453
Eriksson S, Jonas E, Rydhmer L, Röcklinsberg H (2018) Breeding and ethical perspectives on genetically modified and genome edited cattle. J Dairy Sci 101:1–17. https://doi.org/10.3168/jds.2017-12962
Shen L, Hua Y, Fu Y, Li J, Liu Q, Jiao X, Xin G, Wang J, Wang X, Yan C, Wang K (2017) Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice. Sci China Life Sci 60(5):506–515. https://doi.org/10.1007/s11427-017-9008-8
Roldan MVG, Perilleux C, Morin H, Huerga-Fernandez S, Latrasse D, Benhamed M, Bendahmane A (2017) Natural and induced loss of function mutations in SlMBP21 MADS-box gene led to jointless-2 phenotype in tomato. Sci Rep 7(1):4402. https://doi.org/10.1038/s41598-017-04556-1
Proudfoot C, Carlson DF, Huddart R, Long CR, Pryor JH, King TJ, Lillico SG, Mileham AJ, McLaren DG, Whitelaw CB, Fahrenkrug SC (2015) Genome edited sheep and cattle. Transgenic Res 24(1):147–153. https://doi.org/10.1007/s11248-014-9832-x
Wang X, Niu Y, Zhou J, Yu H, Kou Q, Lei A, Zhao X, Yan H, Cai B, Shen Q, Zhou S, Zhu H, Zhou G, Niu W, Hua J, Jiang Y, Huang X, Ma B, Chen Y (2016) Multiplex gene editing via CRISPR/Cas9 exhibits desirable muscle hypertrophy without detectable off-target effects in sheep. Sci Rep 6:32271. https://doi.org/10.1038/srep32271
Crispo M, Mulet AP, Tesson L, Barrera N, Cuadro F, dos Santos-Neto PC, Nguyen TH, Creneguy A, Brusselle L, Anegon I, Menchaca A (2015) Efficient generation of myostatin knock-out sheep using CRISPR/Cas9 technology and microinjection into zygotes. PLoS ONE 10(8):e0136690. https://doi.org/10.1371/journal.pone.0136690
He Z, Zhang T, Jiang L, Zhou M, Wu D, Mei J, Cheng Y (2018) Use of CRISPR/Cas9 technology efficiently targetted goat myostatin through zygotes microinjection resulting in double-muscled phenotype in goats. Biosci Rep. https://doi.org/10.1042/bsr20180742
Jin-Dan Kang SK, Zhu Hai-Ying, Jin Long, Guo Qing, Li Xiao-Chen, Zhang Yu-Chen, Xing Xiao-Xu, Xuan Mei-Fu, Zhang Guang-Lei, Luo Qi-Rong, Kim Yong Soo, Cui Cheng-Du, Li Wen-Xue, Cui Zheng-Yun, Kimd Jin-Soo, Yin Xi-Jun (2017) Generation of cloned adult muscular pigs with myostatin gene mutation by genetic engineering. RSC Adv 7:12541–12549. https://doi.org/10.1039/c6ra28579a
Li WR, Liu CX, Zhang XM, Chen L, Peng XR, He SG, Lin JP, Han B, Wang LQ, Huang JC, Liu MJ (2017) CRISPR/Cas9-mediated loss of FGF5 function increases wool staple length in sheep. FEBS J 284(17):2764–2773. https://doi.org/10.1111/febs.14144
Wang X, Cai B, Zhou J, Zhu H, Niu Y, Ma B, Yu H, Lei A, Yan H, Shen Q, Shi L, Zhao X, Hua J, Huang X, Qu L, Chen Y (2016) Disruption of FGF5 in cashmere goats using CRISPR/Cas9 results in more secondary hair follicles and longer fibers. PLoS ONE 11(10):e0164640. https://doi.org/10.1371/journal.pone.0164640
Burkard C, Lillico SG, Reid E, Jackson B, Mileham AJ, Ait-Ali T, Whitelaw CB, Archibald AL (2017) Precision engineering for PRRSV resistance in pigs: macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLoS Pathog 13(2):e1006206. https://doi.org/10.1371/journal.ppat.1006206
Burkard C, Opriessnig T, Mileham AJ, Stadejek T, Ait-Ali T, Lillico SG, Whitelaw CBA, Archibald AL (2018) Pigs lacking the scavenger receptor cysteine-rich domain 5 of CD163 are resistant to porcine reproductive and respiratory syndrome virus 1 infection. J Virol. https://doi.org/10.1128/jvi.00415-18
Whitworth KM, Rowland RR, Ewen CL, Trible BR, Kerrigan MA, Cino-Ozuna AG, Samuel MS, Lightner JE, McLaren DG, Mileham AJ, Wells KD, Prather RS (2016) Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat Biotechnol 34(1):20–22. https://doi.org/10.1038/nbt.3434
Xie Z, Pang D, Yuan H, Jiao H, Lu C, Wang K, Yang Q, Li M, Chen X, Yu T, Chen X, Dai Z, Peng Y, Tang X, Li Z, Wang T, Guo H, Li L, Tu C, Lai L, Ouyang H (2018) Genetically modified pigs are protected from classical swine fever virus. PLoS Pathog 14(12):e1007193. https://doi.org/10.1371/journal.ppat.1007193
Whitworth KM, Rowland RRR, Petrovan V, Sheahan M, Cino-Ozuna AG, Fang Y, Hesse R, Mileham A, Samuel MS, Wells KD, Prather RS (2019) Resistance to coronavirus infection in amino peptidase N-deficient pigs. Transgenic Res 28(1):21–32. https://doi.org/10.1007/s11248-018-0100-3
Hubner A, Petersen B, Keil GM, Niemann H, Mettenleiter TC, Fuchs W (2018) Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci Rep 8(1):1449. https://doi.org/10.1038/s41598-018-19626-1
Liu X, Wang Y, Tian Y, Yu Y, Gao M, Hu G, Su F, Pan S, Luo Y, Guo Z, Quan F, Zhang Y (2014) Generation of mastitis resistance in cows by targeting human lysozyme gene to beta-casein locus using zinc-finger nucleases. Proc Biol Sci 281(1780):20133368. https://doi.org/10.1098/rspb.2013.3368
Wu H, Wang Y, Zhang Y, Yang M, Lv J, Liu J, Zhang Y (2015) TALE nickase-mediated SP110 knockin endows cattle with increased resistance to tuberculosis. Proc Natl Acad Sci U S A 112(13):E1530–1539. https://doi.org/10.1073/pnas.1421587112
Gao Y, Wu H, Wang Y, Liu X, Chen L, Li Q, Cui C, Liu X, Zhang J, Zhang Y (2017) Single Cas9 nickase induced generation of NRAMP1 knockin cattle with reduced off-target effects. Genome Biol 18(1):13. https://doi.org/10.1186/s13059-016-1144-4
Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T (2016) Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep 6:23980. https://doi.org/10.1038/srep23980
Park TS, Lee HJ, Kim KH, Kim JS, Han JY (2014) Targeted gene knockout in chickens mediated by TALENs. Proc Natl Acad Sci U S A 111(35):12716–12721. https://doi.org/10.1073/pnas.1410555111
Acknowledgements
We would like to thank Wolfram Reichenbecher for critical reading and his helpful comments on the manuscript.
Funding
This paper is drawn from the report of the Risk Assessment of Genetically Engineered Organisms in the EU and Switzerland (RAGES) project funded by Stiftung Mercator Schweiz. KK was funded by the German Federal Agency for Nature Conservation (BfN) Research & Development (Grant No. 351783 1500 and 3519840300)
Author information
Authors and Affiliations
Contributions
JC and KK contributed equally to the manuscript. JC and KK drafted and wrote the manuscript. CT contributed to the draft and provided feedback to the manuscript. All authors read and approved the final manuscript.
Authors’ information
Dr. Janet Cotter received her Ph.D. in soil science from Imperial College, University of London and has worked as a senior scientist for an non-governmental organisation. Currently, she runs a scientific consultancy, Logos Environmental, for non-governmental organisations. Dr. Katharina Kawall received her doctoral degree in biology from the Free University Berlin and conducted her doctoral thesis at the Max-Planck Institute for infection biology in Berlin. She is currently working for the Fachstelle Gentechnik und Umwelt, a Research & Development project funded by the German Federal Agency for Nature Conservation. Dr. Christoph Then leads the non-profit organisation Testbiotech, Institute for Independent Impact Assessment in Biotechnology, Germany.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kawall, K., Cotter, J. & Then, C. Broadening the GMO risk assessment in the EU for genome editing technologies in agriculture. Environ Sci Eur 32, 106 (2020). https://doi.org/10.1186/s12302-020-00361-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-00361-2