Abstract
Background
Benign prostatic hyperplasia (BPH) is on the increase placing a substantial burden on health care systems. Recent studies have shown that men with high body mass index (BMI) and central obesity, as denoted by waist circumference (WC) have bigger prostate volumes (PV) with subsequent increase in lower urinary tract symptoms (LUTS) than men with normal BMI. The purpose of this research was to investigate the correlation between Obesity and PV in patients with BPH.
Methods
The study included 178 men aged between 50 and 75 years with BPH seen at Charlotte Maxeke Johannesburg academic hospital (CMJAH) Urology Outpatient Department between September 2018 and February 2019. Weight and height measurements were obtained to calculate BMI. Furthermore, WC was measured using a measuring tape, while a transrectal ultrasound (TRUS) was used to measure PV. Patient demographics, clinical characteristics such as hypertension, diabetes, smoking and prostate specific antigen (PSA) were also noted.
Results
Patients in the study had a mean age of 64.87 ± 6.526 years and the mean BMI was 27.31 ± 3.933 kg/m2. The mean PV of each BMI group were 52.92 ± 38.49, 61.00 ± 33.10 and 64.86 ± 37.46 cm3 for normal, overweight and obese groups, respectively, and the average PV score was 59.36 ± 36.507 cm3. The mean PSA score was 4.30 ± 3.126 with a range of 1.3–6.4, while the mean WC was 98.67 cm. There was no correlation between BMI and PV (p value = 0.195) as well as between PV and WC, hypertension, diabetes or smoking. The results revealed that the relationship between PV with PSA level as well as age was significant (p value = 0.001, p value = 0.009, respectively).
Conclusion
The results showed no correlation between BMI and PV. Diabetes and hypertension as well had no positive correlation with PV. A follow-up study may be indicated to look at the correlation between obesity, LUTS and urinary flow rates to establish whether aggressive management of obesity would have significant impact on the management of BPH.
Similar content being viewed by others
1 Background
BPH is a common condition in older men with an extensive burden on the health care system. Up to 75% of men over the age of 60 are affected and health care expenditure is consumed on outpatient medication [1]. The prevalence of obesity also increases with age and prostatic growth may be accentuated with obesity [2].
There are many mechanisms that have been postulated by which obesity exacerbates BPH. Obesity increases intra-abdominal pressure, which raises intravesical pressure, in turn worsening or causing BPH symptoms such as hesitancy, poor urine stream and nocturia [3]. The inflammatory ensuing activity and oxidative stress associated with obesity are other proposed mechanisms by which obesity worsens BPH.
Central obesity accentuates microvascular disease and inflammation, leading to ischemia and oxidative stress favourable to BPH [4]. Chronic inflammation causes the release of pro-growth cytokines and various other growth factors [5]. Another hypothesis is alteration of endocrine status, with raised estrogen to androgen ratio. Increased adipose tissue increases aromatase activity and the conversion of androgen to estrogen. Increased estrogen by adipose tissue causes suppression of gonadotropins, leading to reduction in testosterone levels, a mechanism favoured in the development of BPH [6].
Although there are many criteria in classifying patient’s BMI and WC required to make the diagnosis of obesity, literature has shown similar outcomes, that obesity increases the risk of BPH while worsening LUTS [7,8,9,10,11,12]. Even though it is easy to calculate, BMI has its drawbacks, including its inability to differentiate between muscle and fat, especially in the elderly. Central obesity is shown to be more detrimental than general obesity and tends to correlate more with BPH than BMI due to its ability to accelerate hormonal and systemic changes that result in inflammation [5].
The Department of Urology at our institution treats a considerable number of patients with BPH both medically and surgically and the incidence is growing as the population ages. This continues to place a substantial burden on the health care system, in this otherwise manageable condition if obesity is reduced. Recent literature has shown that the impact of obesity on prostate size may also make detection of prostate cancer difficult, leading to under-diagnosis of prostate cancer amongst men with obesity who have large prostate size [13]. In addition, declining PSA levels with rising BMI could affect early detection of prostate cancer since PSA is used as a screening tool and trigger point for prostate biopsy. This study aimed to understand the correlation between obesity and prostatic enlargement and detect a potentially modifiable risk factor for LUTS that could contribute to the epidemiologic investigation of obesity and BPH. The correlation between obesity and PSA levels as well as PV with diabetes, hypertension and smoking was also investigated.
2 Methods
The study was a prospective cohort analytical study. Ethics approval to conduct the study was obtained from the Human Research Ethics Committee (HREC) of the University of the Witwatersrand (ethics no: M180641). In total, 178 patients between the ages of 50 and 75 seen consecutively at CMJA urology outpatient department between September 2018 and February 2019 were recruited following informed consent. The patients were divided into three subgroups according to their BMI (normal BMI 20–24.9 kg/m2, overweight BMI 25–29.9 kg/m2 and Obese BMI > 30 kg/m2). Patients diagnosed with prostate cancer, on 5α-reductase inhibitors for the treatment of BPH, with previous prostate surgery or with of BMI < 20 kg/m2 were excluded from the study.
Measurement of body weight and height were done and BMI was derived by dividing weight in kilograms by height in meters. Waist circumferences were measured using a tape measure, while PV was measured using transrectal ultrasound. In addition, patient age, clinical characteristics such as hypertension, diabetes, smoking and PSA were also recorded.
2.1 Statistical analysis
The results were summarised by percentages and frequencies (categorical variables) and means, percentiles, or standard deviations (numerical variables). Associations between variables and outcome were investigated using contingency tables with relative risks and appropriate hypothesis testing. A one-way analysis of variance (ANOVA) was used to assess the relationship between BMI and PV. The relationship between variables was considered positive when the Pearson correlation (r) was greater than zero. A p value of < 0.05 was considered significant.
3 Results
3.1 Patients’ characteristics
The sample was made up of 178 men aged between 50 and 75 years with BPH seen consecutively at CMJAH Urology Outpatient Department. Patients in the study had a mean age of 64.87 ± 6.526 years and the mean BMI was 27.31 ± 3.933 kg/m2 with the average PV score of 59.36 ± 36.507 ml. The mean PSA score was 4.30 ± 3.126 ng/ml, while the mean WC was 98.67 cm. Baseline characteristics of the participants is shown in Table 1.
Of the 178 patients, 49% (n = 88/178) had hypertension, 29% (n = 51/178) had diabetes and 21% (n = 37/178) were smokers as shown in Fig. 1.
It was noted that 35% (n = 62/178) of the patients had a normal BMI, another 35% (n = 62/178) were overweight and 30% (n = 54/178) were obese indicating an approximate 1:1:1 ratio (Fig. 2).
3.2 Relationships among variables
3.2.1 BMI and prostate volume
The mean PV of each BMI group were 52.92 ± 38.49, 61.00 ± 33.10 and 64.86 ± 37.46 cm3 for normal, overweight and obese groups, respectively, and the average PV score was 59.36 ± 36.507 cm3. Although the PV values were increasing with an increase in the BMI category, these was not statistically significant (p = 0.195) (as shown in Table 2).
3.2.2 Correlation between PV with WC, PSA and age
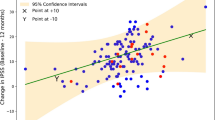
Correlation between PV and each of PSA, Age, and WC was conducted using Pearson correlation statistics for all 178 participants. Pearson’s correlation coefficient showed no correlation between PV and WC (r = 0.131, p value = 0.081 > 0.05). However, there was a positive correlation between PV and PSA level (r = 0.247, p value = 0.001) as well as PV and age (r = 0.195, p value = 0.009).
3.2.3 Correlation between PV with hypertension, diabetes, and smoking independently
The relationship between PV and hypertension, diabetes or smoking is shown in Table 3. The average PV values and p values are shown. There were no significant differences in PV in patients with or without hypertension, diabetes or those who were smokers versus non-smokers (p > 0.05).
4 Discussion
BPH is a prevalent condition with an estimated 90% of men in their 80 s demonstrating histologic evidence of the disease [14]. Previous studies have established a link between obesity and BPH [3, 7, 9, 15, 16]. Worldwide, obesity is on the rise even in countries with low socio-economic index implying it is no longer a disease of the affluent [17, 18]. This necessitated further research into the effects of obesity on PV, PSA levels and LUTS. A recent systematic review re-affirmed the aggravating effects of obesity on BPH [15].
BMI and Central obesity, as determined by the WC, are modifiable risk factors to the development of prostate disease—including BPH, prostatitis and prostate cancer [3, 10, 12, 19, 20]. Other studies have gone further and demonstrated that central obesity was the most significant predictor of PV than overall obesity [15, 21]. Our data suggest that there is no correlation between WC and PV (r = 0.131, p = 0.081). This was also true for the effect of BMI on PV whereby; obese patients generally had larger PV but the results were not statistically significant (5% CI, p = 0.195).
Lee et al. and Kim et al. arrived at a similar conclusion, that there was no link between BMI and prostate volume, leading to the conclusion that central obesity as denoted by WC > 90 cm rather than overall obesity is a predictor of prostate growth and LUTS. These findings were a result of a multicentre, prospective, cross-sectional study at four urology centres in Korea from July 2007 to May 2008 recruiting 602 patients with BPH. Men with waist circumference > 90 cm had increased prostate volume; however, they had lower PSA values than men with WC < 90 cm [21, 22].
In contrast, Lee et al. showed that prostate volume was bigger in obese and central obesity patients than in those with normal BMI. The authors analysed 146 men over the age of 40 and separated them into three groups according to their BMI and into two groups according to their WC. Significantly increased prostate size was noticeable in the obesity group than in the normal group as well as in the central obesity group than in the group with normal waist size [7]. Similarly to this Matsuda et al. and Kim et al. showed a positive correlation between BMI and prostate size; however in both these studies there was a negative correlation between BMI and prostate specific antigen (PSA) levels, whereby men with high BMI had low PSA [8, 9].
The study showed that hypertension, diabetes and smoking were quite prevalent in our population. There was no statistically significant difference in PV between the hypertensive and the non-hypertensive (p = 0.454). Similarly to our study, Li-Peng showed no correlation between BPH and hypertension [11]. This is in contrast to the findings of a 2013 South Korean study in which men with hypertension had a higher International Prostate Symptom Score as well as larger PV putting them at an increased risk of LUTS compared to the non-hypertensive [23].
Our data showed diabetic patients had slightly higher mean PV. However, the results were not statistically significant at 5% CI (p = 0.832). This finding differs with Ahmed Elabbady’s study findings in which diabetes was significantly associated with lower serum PSA and testosterone, but larger PV [24]. We did not include the correlation with ethnicity and testosterone levels as an endpoint in our study, which could explain the findings in Elabbady’s study.
Smoking is a known trigger of prostate inflammation—a mechanism contributing to BPH [25]. However, previous studies have shown conflicting results on the effects of smoking on PV [23, 26]. This study demonstrated that smoking had no statistically significant effect on PV (5% CI, p = 0182) despite smokers having minimally larger prostates than non-smokers. This was corroborated by a study done by Platz et al. which showed that smoking had no correlation with PV, and that it may in fact reduce PV, thus protecting against BPH [27].
The current evidence trends towards the level of both total and free PSA being directly proportional to PV [28,29,30,31]. This study confirms this relationship as our data showed a direct correlation between the level of PSA and PV (r = 0.247, p = 0001). Our findings further suggest that increasing age was associated with increased PV (r = 0.195, p = 0.009) which was in keeping with evidence from previous studies [29]. However, not all previous studies agree with these findings. Stacy Loeb et. al., found that PV was highly variable in men with BPH and a good number of aging men had stable or decreasing prostate size [32].
Currently, the medical management of BPH related LUTS is hugely reliant on the prostatic smooth muscle relaxation effects of alpha-adrenergic receptor blockers [17, 33]. This has led to a hunt for alternate therapies that may aid LUTS improvement. With the previously established link between obesity and BPH, there have been proposals to promote and incorporate lifestyle modifications and appropriate medical management as an adjunct to diseases prevention, diagnosis and therapy [15, 17, 33].
The findings of this study suggest that the routine/aggressive medical management of obesity would be of little to no benefit with regard to BPH/LUTS as there was no correlation between PV and any of hypertension, diabetes, BMI, and WC. This is supported by another study which showed that modest weight loss might not prevent the onset or progression of LUTS [34].
4.1 Study limitations
Like many other previous studies, some limitations of this study were a small sample size as well as the fact that it was a single centre study. Larger multicentre studies are required to establish the true effects of obesity on BPH as related to PV and LUTS.
5 Conclusion
The urology department treats a significant number of patients with BPH annually. Obesity has been cited as one of the risk factors for the development of BPH/ LUTS. In our study, there was no correlation between PV and BMI. It must be noted though that the volume of the prostate does not always correlate with the severity of LUTS. Obesity has the potential to increase intra-abdominal pressure and intravesical pressure, in turn worsening BPH symptoms independent of the PV [3]. A follow-up study is warranted to look at the correlation between obesity and LUTS and urinary flow rates and to establish whether aggressive management of obesity would have significant impact on the management of BPH.
Availability of data and material
Not applicable.
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- LUTS:
-
Lower urinary tract symptoms
- PSA:
-
Prostate specific antigen
- WC:
-
Waist circumference
- TRUS:
-
Transrectal ultrasound
References
Wei JT, Calhoun E, Jacobsen SJ et al (2005) Urologic Diseases in America project: benign prostatic hyperplasia. J Urol 1256(4):156–159
Flegal KM, Carrol MD, Ogden CL, Johnson CL (2002) Prevalence and trends in obesity among US adults, 1999–2000. J Am Med Assoc 288:1723–1727
Wang S, Mao Q, Lin Y, Wu J, Wang X, Zheng X (2012) Body mass index and risk of BPH: a meta-analysis. Prostate Cancer Prostatic Dis 15:265–272
Chughtai B, Lee R, Te A, Kaplan S (2011) Role of inflammation in benign prostatic hyperplasia. Rev Urol 13:147–150
Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G (2009) Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immune-mediated inflammation. J Immunol 182:4046–4064
Williams G (2012) Aromatase up-regulation, insulin and raised intracellular estrogens in men, induced adiposity, metabolic syndrome and prostate disease. Mol Cell Endocrinol 351:269–278
Lee S, Min HG, Choi SH, Kim YJ et al (2006) Central obesity as a risk factor for prostatic hyperplasia. Obesity 14:172–179
Matsuda T, Abe H, Suda K, Rinsho B (2004) Relation between benign prostatic hyperplasia and obesity and estrogen. Jpn J Clin Pathol 52(4):291–294
Kim JM, Song PH, Kim HT, Moon KH (2011) Effect of obesity on prostate-specific antigen, prostate volume, and international prostate symptom score in patients with benign prostatic hyperplasia. Korean J Urol 52(6):401–405
Fowke JH, Motley SS, Cookson MS, Conception R, Chang SS, Williams ML, Smith Jr JA (2007) The association between body size, prostate volume and prostate-specific antigen. Prostate Cancer Prostatic Dis 10:137–142
Xie LP, Bai Y, Zhang XZ, Zheng XY, Yao KS, Xu L, Zeegers MP (2007) Obesity and benign prostatic enlargement: a large observational study in China. Urology 69(4):680–684
Fowke JH, Koyama T, Fadare O, Clark PE (2016) Does inflammation mediate the obesity and BPH relationship? An epidemiologic analysis of body composition and inflammatory markers in blood, urine, and prostate tissue, and the relationship with prostate enlargement and lower urinary tract symptoms. PLoS ONE 11(6):e0156918. https://doi.org/10.1371/jounal.pone0156918.ecollection
Presti JC, Lee U, Brooks JD, Terris MK (2004) Lower body mass index is associated with a higher prostate cancer detection rate and less favourable pathological features in a biopsy population. J Urol 171:2199–2202
Lepor H (2005) Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol 7:3–11
Gacci M, Corona G, Vignozi L et al (2015) Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int 115:24–31
De Nunzio C, Aronso W, Freedland SJ, Giovannucci E, Parsons JK (2012) The correlation between metabolic syndrome and prostatic diseases. Eur Urol 61:560–570
Yoo TK, Cho HJ (2012) Benign prostatic hyperplasia: from bench to clinic. Korean J Urol 53:139–148
Saklayen MG (2018) The global epidemic of the metabolic syndrome. Glob Epidem Metab Syndr 9:1–8
Parikesit D, Mochtar CA et al (2016) The impact of obesity towards prostate diseases. Prostate Int 4(1):1–6
Jung JH, Ahn SV, Song JM et al (2016) Obesity as a risk factor for prostatic enlargement : a retrospective cohort study in Korea. Int Neurourol J 20:321–328
Kim GW, Doo SW, Yang WJ (2010) Effects of obesity on prostate volume and lower urinary tract symptoms in Korean men. Korean J Urol 51(5):344–347
Lee SH, Kim JC, Lee JY, Kim JH, Oh CY, Lee SW, Yoo SJ, Chung BH (2009) Effects of obesity on lower urinary tract symptoms in Korean BPH patients. Asian J Androl 11(6):663–668
Hwang EC, Kim S, Nam D, Yu HS, Hwang I (2015) Men with hypertension are more likely to have severe lower urinary tract symptoms and large prostate volume. LUTS 7:32–36
Elabbady A, Hashat MM, Kotb AF, Ghanem AE et al (2016) Studying the effect of type 2 diabetes mellitus on prostate-related parameters: a prospective single institutional study. Prostate Int 4(4):156–159
Moreira DM et al (2015) Smoking is associated with acute and chronic prostatic infl ammation: results from the REDUCE study. Cancer Prev Res 8:312–318
Smoking K (1997) The role of cigarette smoking in prostatic enlargement. Br J Urol 80:201–204
Platz EA, Rim EB, Kawachi I et al (1999) Alcohol consumption, cigarette smoking, and risk of benign prostatic hyperplasia. Am J Epidemiol 149(2):23–27
Coric J et al (2015) Prostate-specific antigen (PSA) and prostate volume: better predictor of prostate cancer for Bosnian and Herzegovina men. Open Biochem J 9:34–36
Deori R, Das B, Rahman MA (2017) A study of relationship of prostate volume. Prostate Specif Antigen Age Benign Prostatic Hyperpla 4(7):1582–1586
Mochtar CA et al (2003) European urology prostate-specific antigen as an estimator of prostate volume in the management of patients with symptomatic benign prostatic hyperplasia. Eur Urol 44:695–700
Putra IB et al (2016) Relationship of age, prostate-specific antigen, and prostate volume in Indonesian men with benign prostatic hyperplasia. Prostate Int 4(2):43–48
Loeb S et al (2016) Prostate volume changes over time: results from the baltimore longitudinal study of aging. HHS Public Access 182(4):1458–1462
Corona G et al (2014) Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunctions. Int J Endocrinol; 2014.
Sauver JL et al (2011) Associations between modest weight changes and onset and progression of lower urinary tract symptoms in two population-based cohorts. URL 78(2):437–441
Acknowledgements
Not applicable.
Funding
This study had no external funding from any resource.
Author information
Authors and Affiliations
Contributions
EM contributed to the conception of the study, to obtain ethical approval, drafting, reviewing for critical content and editing of the manuscript. PF and MH supervised, reviewed for critical content and edited the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Wits Human Research Ethics Committee, Reference number: M180641. All procedures performed in this study were in accordance with the ethical standards of the Wits Human Research Ethics Committee and with the Title page Click here to access/download;Title page;title page.docx 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This was a prospective study using patients who presented at Urology outpatient Department and written consent to participate was obtained. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Consent for publication
Written informed consent was obtained from the patient for publication of this manuscript. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mampa, E., Haffejee, M. & Fru, P. The correlation between obesity and prostate volume in patients with benign prostatic hyperplasia at Charlotte Maxeke Johannesburg Academic Hospital. Afr J Urol 27, 60 (2021). https://doi.org/10.1186/s12301-021-00160-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-021-00160-y