Abstract
Background
Soil salinization is a worldwide environmental problem, especially in the arid and semiarid regions of northeastern China, which are heavily affected by soda saline–alkaline stress. At present, there is an urgent need to improve the soda saline–alkaline stress tolerance of rice.
Results
Stress-associated proteins are involved in regulating the abiotic stresses in plants. There are 18 members of the rice stress-associated protein (OsSAP) gene family. In this study, the expression levels of OsSAP6 in leaves and roots were upregulated with increasing NaHCO3 stress duration. OsSAP6 was located in nucleus and cytoplasm. The bud length and total root length of OsSAP6 overexpression rice were significantly longer than those of Lj11 (Oryza sativa longjing11) during germination stage, and the survival rates, plant height and malondialdehyde content at the seedling stage showed tolerance growth of saline–alkaline stress. The expression of OsCu/Zn-SOD, OsAPX2, and OsCAT1 in transgenic lines was increased significantly under SAE (soda saline–alkali soil eluent) stress. OsSAP6 interacts with OsPK5 according to yeast two-hybrid screening and luciferase complementation experiments. The expression of OsPK5 increased under NaHCO3 and H2O2 stress, and the overexpression of OsPK5 in rice improved soda saline–alkaline tolerance.
Conclusion
Overexpression of OsSAP6 in rice significantly enhanced saline–alkaline tolerance compared with the wild type. It is speculated that OsSAP6 responds to soda salinity stress and interacts with OsPK5 to positively regulate soda saline–alkaline tolerance through ROS homeostasis. This study revealed the features of OsSAP6 involved in response to soda saline–alkaline stress and the interaction with OsPK5, which provided resources for breeding aimed at improving the soda saline–alkaline stress tolerance of rice.
Similar content being viewed by others
Background
According to a survey, saline–alkali land is distributed in 36 countries, with a total area of 956 million hectares (Yun and Chen 2020). Among those countries, the existing saline–alkali land in China is as high as 9.913 × 106 hm2, mainly distributed in 23 provinces, including northeast, north, northwest and coastal areas. Those areas contain more than 10% of the total arable land in China with the potential for agricultural development. These lands are precious reserve arable land resources (Liu and Wang 2021; Zhu et al. 2018). The current research shows that rice cultivation is an effective method to control saline–alkali land. In the low-lying inland areas of the western Songnen Plain, the improvement measures for growing rice in saline–alkali land have achieved high economic benefits (Zhang and Wang 2016). Due to the expansion of rice cultivation areas and the requirement of high yield in the saline–alkali land of Northeast China, the rice varieties grown there should be more able to tolerate the saline–alkaline environment.
Stress-associated proteins (SAPs) are proteins containing A20/AN1 zinc finger domains with N-terminal A20 and/or C-terminal AN1 domains, and the expression of these genes changes when plants respond to stress (Evans et al. 2004; Huang et al. 2004). In recent years, studies have reported that many gene families and interaction networks are involved in regulating the response of rice to stress. There are 18 members of the rice stress-associated protein (OsSAP) gene family, which were determined based on a nucleotide alignment search of 1 kb of the A20/AN1 zinc finger domain, and quantitative expression analysis showed that most of these genes were induced by abiotic stress (Vij and Tyagi 2006). Other plants have been found that SAPs involved in abiotic stress tolerance responses, such as AtSAP5, AtSAP10, and AtSAP12 in Arabidopsis thaliana (Kang et al. 2011; Dixit and Dhankher 2011; Ströher et al. 2009), AlSAP in Aeluropus littoralis (Ben Saad et al. 2012a, b), MtSAP1 in Medicago truncatula (Gimeno-Gilles et al. 2011), ShSAP1 in Saccharum officinarum (Li et al. 2011), ZmAN13 in Zea mays (Xuan et al. 2011) and MusaSAP1 in banana (Sreedharan et al. 2012), and those are resistant to adversity. Overexpression of OsSAP1 in transgenic rice results in altered expression of several endogenous genes, including those coding for transcription factors, membrane transporters, signaling components, and genes involved in metabolism, growth and development (Dansana et al. 2014). The expression of OsSAP1 was induced under a variety of abiotic stresses, including cold, salt, dehydration, submersion, heavy metals, ABA (abscisic acid) and injury, and involved in the regulation of multiple abiotic stresses, including the ROS (reactive oxygen species) pathway (Mukhopadhyay et al. 2004). OsSAP8, OsSAP9/ZFP177 and OsSAP11 have also been reported to have the ability to enhance abiotic tolerance (Kanneganti and Gupta 2008; Huang et al. 2008; Giri et al. 2011). Overexpression of OsSAP8 in both transgenic tobacco and rice conferred tolerance to salt, drought, and cold stress at the germination and seedling stage, as reflected by the percentage of germination and gain in fresh weight after stress recovery, the transgenic plants were tolerant to salt and drought during the anthesis stage without any yield penalty compared to unstressed transgenic plants (Kanneganti and Gupta 2008). ZmAN11, the homolog of OsSAP8 in maize, increased expression under NaCl, cold and heat-shock stresses and decreased under drought stress (Xuan et al. 2015). OsSAP7 exhibits E3 ubiquitin ligase activity in vitro. Overexpression of OsSAP7 in Arabidopsis was insensitive to ABA at germination stage and sensitive to water-deficit stress at an advanced stage compared to the wild type. OsSAP7 was also impaired in ABA and stress-responsive gene expression, and acts as a negative regulator by acting as an E3 ubiquitin ligase (Sharma et al. 2015). The wheat stress-associated protein 5 (TaSAP5) ubiquitinated substrate is HSP90C (chloroplast heat shock protein 90), and it is involved in drought regulation in wheat and Arabidopsis as an E3 ubiquitin ligase for the degradation of DRIP (dehydration-responsive element binding protein2a interacting protein) and MBP-1 (c-myc binding protein) (Zhang et al. 2017, 2019). The ubiquitin–proteasome system is an important protein regulatory mechanism that affects many cellular processes in plant growth, including stress responses (Lyzenga and Stone 2012; Dreher and Callis 2007), hormonal signaling (Kelley and Estelle 2012), embryogenesis (Hellmann et al. 2003), and intracellular transport (Lee et al. 2009). Two mutants with overexpression of OsSAP16 showed reduced stomatal conductance in rice leaves, but there were no differences in stomatal development or morphology in either of the mutants. This phenotype limited CO2 uptake and the rate of photosynthesis, resulting in less biomass accumulation in the two mutants. Whole transcriptome analysis showed that overexpression of OsSAP16 led to global changes in gene expression. These results show that OsSAP16 was involved in modulating the response of rice to drought stress by regulating the expression of a set of stress-associated genes (Wang et al. 2016).
Transcription factors played important regulatory roles in plant responses to different stresses (Singh et al. 2002). OsSAPs are involved in abiotic stress response pathways as transcriptional regulators and have different regulatory roles. Vij and Tyagi (2006) showed that OsSAP4, OsSAP6, OsSAP8, OsSAP9, OsSAP15, and OsSAP16 seemed to have a high level of expression in the unstressed state, and the mRNA level of all the OsSAPs showed an increase in response to salt and dehydration stress treatment for 6 h relative to the unstressed control. We detected the expression of OsSAP4, 6, 8, and 9 in the same clade by quantitative real-time PCR (qRT–PCR) under 60 mM NaHCO3 stress and found that the expression of OsSAP6 increased the most. To determine the regulatory function of OsSAP6 in response to saline–alkaline stress, the OsSAP6 gene was cloned. Subcellular location of OsSAP6 was verified in onion epidermal cells. The resistance to SAE (soda saline–alkali soil eluent) stress at germination and seedling stages was analyzed with overexpression of OsSAP6 in Lj11 (Oryza sativa longjing11). The interacting protein OsPK5 was screened by yeast two-hybrid (Y2H) assay and then verified. The increased expression and enhanced tolerance to alkaline stress in rice overexpressing OsPK5 were investigated. In this study, we investigated the function of OsSAP6 regulating soda salinity resistance in rice, revealed the synergistic relationship between OsSAP6 and interacting protein in regulating salinity resistance, and provided genetic resources and new insights for soda saline–alkaline resistance breeding.
Results
Expressional Patterns of OsSAP4, 6, 8, and 9 Under NaHCO3 Stress
Based on the A20 and AN1 domains of OsSAPs, 18 homologous genes were found in NCBI GenBank database (https://www.ncbi.nlm.nih.gov/), details are shown in Additional file 2: Table S1. Phylogenetic analysis of OsSAPs showed that OsSAP4, 6, 8, and 9 are placed in the same clade (Additional file 1: Fig. S1, S2). This group has been reported to be closely related to abiotic stress responses in plants. Further expressional analysis results showed that all these four genes were responsive to NaHCO3 stress (Fig. 1). OsSAP6 was the most upregulated one, which was increased by 8.8 times compared to the control at 12 h. The increased expression of the four genes in the roots varied considerably. OsSAP4, 8, and 9 were less increased than OsSAP6. The expression level of OsSAP6 at 24 h was 15.2 times higher than that of the control. The strong response of the OsSAP6 to NaHCO3 stress in roots suggests that the main biological function of this gene may be the regulation of NaHCO3 stress.
The expression of OsSAP4, 6, 8, and 9 under 60 mM NaHCO3. Lj11 seedlings at the three-leaf stage were treated with 60 mM NaHCO3 for 0, 6, 12, 24, and 48 h to detect the expression of OsSAP4, 6, 8, and 9 in leaves (A) and roots (B). The expression level of the four genes at 0 h was set to 1, and the Os18sRNA gene was used as an internal reference control. The fold change was analyzed by the 2−ΔΔCT method. Values are the mean ± standard deviation of three biological replicates. Statistical differences are labeled with different letters using Duncan test (p < 0.05, one-way ANOVA)
Subcellular Localization and Organizational Expression of OsSAP6 Protein
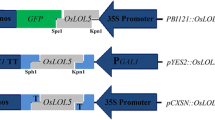
Subcellular localization of the protein encoded by OsSAP6 was predicted using PSORT (http://psort1.hgc.jp/form.html). The result indicated OsSAP6 may be located in the cytoplasm with a score of 0.65. The prediction scores of chloroplast stroma, chloroplast thylakoid membrane, and chloroplast thylakoid space were all 0.20. However, TargetP (https://services.healthtech.dtu.dk/service.php?TargetP-2.0) did not predict the signal peptide of the secretory pathway. The subcellular location of OsSAP6 was then experimentally verified by transient transformation of CaMV35S-OsSAP6-GFP in onion epidermis. Green fluorescent signals were detected in both cytoplasm and nucleus (Fig. 2 and Additional file 1: Fig. S3), indicating that OsSAP6-GFP was located in both cytoplasm and nucleus. In addition, a portion of the localization exhibited separate nucleus expression, speculating that intracytoplasmic localization may be caused by targeting proteins within the cytoplasm and they interacted and functioned. Although the expression differed from the software prediction, it reflected the properties of the transcription factor protein.
Subcellular localization of OsSAP6 in onion epidermal cells and expression of OsSAP6 promoter-GUS. A GFP and OsSAP6-GFP driven by the 35S promoter under green fluorescence, bright field, and merged views. Bar, 50 μm. B Image of stereomicroscope after immersion in GUS stain and decolorization in 95% ethanol, Bar, 1 mm
To understand the expression pattern of OsSAP6 in rice, we generated transgenic lines that express a β-glucuronidase (GUS) reporter driven by the OsSAP6 promoter. GUS staining of young seedlings of OsSAP6 promoter-GUS transgenic lines showed blue roots, leaves, and leaf sheaths, indicating that OsSAP6 was expressed in the whole plant.
Overexpression of OsSAP6 in Rice Enhances Tolerance to Soda Saline–Alkaline Stress During Germination and Vegetative Stages
OsSAP6 was integrated into rice genome by Agrobacterium tumefaciens mediated genetic transformation (Additional file 1: Fig. S4A). The relative expression of OsSAP6 in seedlings of T3 generation lines was investigated using qRT–PCR (Additional file 1: Fig. S4B). The results indicated that OsSAP6 was overexpressed in these transgenic lines. The overexpressing lines T3-#7, 10, and 11 were selected for harvesting T3 generation seeds in preparation for subsequent experiments.
We investigated germination abilities of OsSAP6 overexpression lines and Lj11 exposed to different ratios of SAE. The results indicated that seed germination abilities of OsSAP6 overexpression lines were better than that of the WT (Lj11) (Fig. 3A). The germination rates in water (control) and low soda saline–alkaline stress (H2O:SAE = 5:1) was not significant difference. However, when the stress increased to H2O:SAE = 3:1 and 2:1, all the germination rates decreased, but the transgenic lines were higher than that of Lj11 (Fig. 3D). The bud and total root length showed a significant difference under the high level of SAE stress. When the ratios of H2O:SAE were 3:1 and 2:1, the bud length of OsSAP6 overexpression lines was approximately 1 cm longer than Lj11, and the total root length was 2–3 times longer than that of Lj11 (Fig. 3B, C). This finding suggests that the overexpression of OsSAP6 confers tolerance to soda saline–alkaline stress during seed germination and regulates the response mechanisms to soda saline–alkaline stress.
Overexpression of OsSAP6 in rice enhanced tolerance to soda saline–alkaline stress during germination period. A Germination phenotypes of OsSAP6 overexpression lines (T3-#7, 10, and 11) and Lj11 under different concentrations of SAE stress (H2O:SAE = 5:1, 3:1, and 2:1, water as control). B bud length; C total root length; D germination rate. Data show the mean ± SD of three independent replicates. At least 40 seeds per genotype were measured in each replicate. Statistical differences are labeled with different letters using LSD test (p < 0.05, one-way ANOVA)
To further investigate the molecular functions of OsSAP6, the overexpression lines were treated with soda saline–alkaline stress during growth period. Four-week-old OsSAP6 overexpression line T3-#10 and Lj11 seedlings were treated with different ratios of SAE. Significant difference in growth was observed after 14 days. Lj11 had more leaves curling and wilting, and the fresh weight was significantly lower than transgenic lines (Fig. 4A, B). The survival rates of the OsSAP6 overexpression line were higher than that of Lj11 at H2O:SAE = 5:1, 3:1, and 2:1 (Fig. 4C). The MDA (malondialdehyde) content were both increased under stress, but transgenic lines were significantly less than Lj11 (Fig. 4D). These results indicated that the overexpression of OsSAP6 enhanced the tolerance to soda saline–alkaline stress in rice, and the OsSAP6 protein may be a transcription factor involved in positive regulation of alkaline salt stress.
Overexpression of OsSAP6 in rice enhances tolerance to soda saline–alkaline stress during growth period. Four-week-old Lj11 (three rows on the left) and OsSAP6 overexpression line T3-#10 (three rows on the right) seedlings were treated with different concentrations of SAE with H2O:SAE = 5:1, 3:1, and 2:1, water as a control, and the growth were investigated after 14 days. A Phenogram of growth; B fresh weight of individual plants; C survival rate, asterisks indicate significant mean differences between OsSAP6 overexpression lines and Lj11 (*p < 0.05 and **p < 0.01); D MDA contents. Data show the mean ± SD of three replicates. Statistical differences are labeled with different letters using LSD test (p < 0.05, one-way ANOVA)
Relative Expression of OsCu/Zn-SOD, OsAPX2, and OsCAT1 Under Soda Saline–Alkaline Stress in Transgenic Rice
Soda saline–alkali stress disrupts ROS balance, and the expression of genes encoding key enzymes in its scavenging pathway reflects the level of clearing excess ROS. The expression levels of OsCu/Zn-SOD in OsSAP6 overexpression lines T3-#7, 10, and 11 were 5 times higher than that of Lj11 in the unstressed state. OsCu/Zn-SOD was significantly increased under SAE stress, with the transgenic lines having expression more than 20 times higher than that of Lj11 at H2O: SAE = 2:1. The expression of OsAPX2 increased significantly with increasing SAE concentration and differed between lines. OsAPX2 was increased more significantly in T3-#7 than the other lines, and increased most at H2O:SAE = 2:1. The expression of OsCAT1 increased and then decreased with increasing SAE ratios, peaking at H2O:SAE = 3:1 and decreasing at H2O:SAE = 2:1, but it is still a high level relative to Lj11 (Fig. 5). Significant overexpression of the functional genes in ROS balance pathway suggests that resistance of OsSAP6 overexpression lines under soda saline–alkaline stress may be related to the ROS balance.
Expression of OsCu/Zn-SOD, OsAPX2, and OsCAT1 in OsSAP6 overexpression rice under soda saline–alkaline stress. 4-week-old OsSAP6 overexpression lines and Lj11 seedlings were treated with different ratios of SAE (H2O:SAE = 5:1, 3:1, and 2:1, water as a control) for 7 days. The expression levels of OsCu/Zn-SOD (A), OsAPX2 (B), and OsCAT1 (C) were detected by qRT–PCR. The expression level of Lj11 was set to 1, and the Os18sRNA gene was used as an internal reference control. Values are the mean ± standard deviation of three replicates. Statistical differences are labeled with different letters using Duncan test (p < 0.05, one-way ANOVA)
Interactional Analysis Between OsSAP6 and OsPK5
To identify OsSAP6 interactors we used a yeast two-hybrid system screen for a total of 16 colonies (Additional file 1: Fig. S5). According to sequencing results, pyruvate kinase 5 (PK5, EU267984) was identified from the NCBI BLAST database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). OsPK5 was cloned and constructed into pGADT7 vector, then cotransformed into Y2HGold with pGBKT7-OsSAP6 plasmids for validation. The results revealed that OsPK5 showed a strong interaction with OsSAP6 on SD/-Trp-Leu-His (synthetic drop-out medium lacking Trp, Leu, and His) containing X‐α‐gal (Fig. 6A). LCI (LUC complementation imaging) assays showed that OsSAP6 interacted with OsPK5 in N. benthamiana leaves (Fig. 6B), indicating that they also interacted in vivo. It is speculated that OsSAP6 is involved in saline–alkaline stress resistance pathway in synergy with the OsPK5.
Interactional analysis between OsSAP6 and OsPK5. A OsSAP6 was found to interact with OsPK5 in a yeast two-hybrid assay. pGADT7-OsPK5 was validated by cotransforming Y2HGold yeast cells with pGBKT7-OsSAP6 plasmid, and strong interaction were found in medium SD/-Trp-Leu-His containing X‐α‐gal. B Interaction of OsSAP6 and OsPK5 in N. benthamiana leaves was analyzed by LCI assay
Expression of OsPK5 in Response to NaHCO3 and H2O2 Stress in Rice
To investigate the response of OsPK5 to saline–alkali and oxidative stress, qRT–PCR was performed to detect the expression level of OsPK5 in leaves and roots of Lj11 under 60 mM NaHCO3 and 5 mM H2O2 stress. The expression of OsPK5 was induced upregulation by NaHCO3. The maximum expression of OsPK5 in leaves and roots was 5.9 and 3.8 times higher than the control at 24 h, then slightly decreased at 48 h (Additional file 1: Fig. S6A, B). OsPK5 responded strongly in roots under H2O2 stress, rising to 14.1 times than that of the control at 24 h. Its expression in leaves increased to 1.7 times at 6 h and then decreased (Additional file 1: Fig. S6C, D). This result indicated that OsPK5 was responsive to NaHCO3 and H2O2 stress, which was speculated to be related to saline–alkaline tolerance and ROS balance.
Overexpression of OsPK5 in Rice Enhances Tolerance to Soda Saline–Alkaline Stress at Seedling Stage
OsPK5 was cloned into pGWB11 vector and integrated into Lj11 genome by Agrobacterium tumefaciens mediated genetic transformation and detected by RT-PCR (reverse transcription PCR) (Fig. 7A, B). 7-day-old OsPK5 overexpression lines and Lj11 seedlings were treated with SAE stress, and showed growth differences after 7 days (Fig. 7C). OsPK5 overexpression lines T3-#1, 2, and 3 showed stronger growth and more fresh weight than Lj11 under treatments of H2O:SAE = 4:1 and 3:1. Although the fresh weight between lines were differences under SAE treatment of 2:1, the transgenic lines were significantly heavier than Lj11 (Fig. 7D). The plant height of the transgenic lines was significantly higher than Lj11 under stress (Fig. 7E). These results indicated that the overexpression of OsPK5 in rice enhances the tolerance to soda saline–alkaline stress at seedling stage. The expression level of OsCu/Zn-SOD, OsAPX2, and OsCAT1 were investigated using qRT–PCR (Additional file 1: Fig. S7). The results revealed that those ROS scavenging genes were significantly expressed in OsPK5 overexpression lines T3-#1, 2, and 3 under different ratios of SAE treatment, indicating that OsPK5 regulates soda saline–alkaline tolerance may be related to the ROS homeostasis.
Overexpression of OsPK5 in rice enhances tolerance to soda saline–alkaline stress at seedling stage. A Schematic diagram of the T-DNA insertion site in OsPK5 overexpression lines. B RT–PCR detection of OsPK5 transcripts in 6 independent transgenic lines of rice; M, Marker, DL2000; CK + , positive control, pGWB11-OsPK5; NT, Nontransgenic lines, Lj11. C Phenotypes of Lj11 and OsPK5 overexpression lines T3-#1, 2 and 3 under SAE stress (H2O:SAE = 5:1, 3:1 and 2:1, water as control). D fresh weight of 5 seedings; E plant height. Data show the mean ± SD of three replicates. Statistical differences are labeled with different letters using LSD test (p < 0.05, one-way ANOVA)
Discussion
Saline–alkaline stress affects plant growth and development, especially alkaline salt damage more severely. Currently, the mechanisms of plant response to biotic and abiotic stresses have become the key to the breeding of stress-resistant crop varieties (Chen et al. 2021). Studies about the resistance of OsSAPs have been reported in various aspects, however, there is no previous report on the resistance of OsSAPs under soda saline–alkaline stress. In this study, we investigated the expressional patterns of OsSAP4, 6, 8, and 9 in the same branch in response to alkaline salt NaHCO3 stress using qRT–PCR (Fig. 1). All these four genes were increased in both roots and leaves under NaHCO3 stress. The increased expression of the four genes was significantly different and OsSAP6 was the highest one, which was increased by 8.7875 and 15.18 times in leaves and roots compared to the control at 12 h. These four genes have the same upregulated expression as most of the 18 genes of OsSAPs under NaCl stress. The expression of OsSAP6 in 7-day-old rice seedlings under salt (200 mM) for 6 h was increased by about 2.7 times, and about 3.5 times of the control under dehydration for 6 h. (Vij and Tyagi 2006). The strong induction of OsSAP6 specificity to alkaline salt in this study suggested that it might be involved in NaHCO3 stress response regulation. Studies have shown that the subcellular location of OsSAP1 is in nucleus. A20, AN1, and A20/AN1 of OsSAP7 localized in nucleus of onion epidermal cells with YFP and colocalized with AtCOP1 in nucleus (Sharma et al. 2015). GFP and OsSAP8 fusion protein is localized in cytoplasm (Kanneganti and Gupta 2008), it is speculated that it may not act as a transcription factor like OsSAP1, because it lacks the nuclear localization sequence and DNA binding domain, and realizes its function through protein interactions (Mukhopadhyay et al. 2004). In this study, the OsSAP6-GFP fusion protein was localized in cytoplasm and nucleus (Fig. 2), speculating that it functions both as a transcription factor to bind DNA and through protein–protein interactions. OsSAP (R12H780) studied by Ubaidillah et al. (2013) is localized in mitochondria, which represents a new type of Bax suppressor-related gene and endows multiple stress tolerance in yeast.
The interacting protein OsPK5 was identified by Y2H assay in this study and verified by LCI analysis (Fig. 6). OsSAP6 was hypothesized to be involved in rice physiological pathways together with OsPK5. Mutants with enhanced expression of OsSAP16 have reduced stomatal conductance, which limits CO2 uptake and photosynthetic rate, resulting in less biomass accumulation in the mutants, and this gene is involved in modulating the response of rice to drought stress by regulating the expression of a set of stress-related genes (Wang et al. 2016). In this study, OsSAP6 overexpression rice showed a regulatory ability to soda saline–alkaline stress tolerate during the germination period, the overexpression lines had approximately 1 cm more bud length and 2–3 times more total root length than Lj11 at H2O:SAE = 2:1 (Fig. 3). Seedlings of OsSAP6 overexpression lines showed stronger tolerance than Lj11 under soda saline–alkaline stress, indicating that OsSAP6 may positively regulate proteins or genes related to resistance to alkaline salt stress. OsSAP6 regulates downstream genes related to ROS (Fig. 8). The reduction in MDA detected in transgenic lines also facilitates ROS homeostasis (Fig. 4). Lei et al. (2020) conducted QTL (quantitative trait locus) mapping and map-based cloning for salt tolerance at the rice bud burst stage, and the results showed that OsSAP16 was a candidate gene of qRSL7 (quantitative trait locus related to relative shoot length on chromosome 7) for improving rice salt tolerance. This study detected an increased expression of OsPK5 in response to soda saline–alkaline (NaHCO3) and H2O2 stress, echoing the effect of PKs on the metabolism of phosphoenolpyruvate and pyruvate in plastids (Weber 2004). Fourteen pyruvate kinase encoding genes have been identified in Arabidopsis with different functions (Arabidopsis Genome Initiative 2000). In this study, the soda saline–alkaline resistance analysis of OsPK5 overexpression rice showed that OsPK5 driven by 35S promoter improve the tolerant growth of transgenic lines, and the plant height under stress was significantly higher than Lj11 (Fig. 7), indicating that OsPK5 is involved in the regulation of soda saline–alkaline stress resistance in rice.
A brief pathway of OsSAP6 regulation under soda saline–alkaline stress. Overexpression of OsSAP6 upregulates genes involved in ROS scavenging. The expression of these genes leads to a reduction in ROS accumulation, which results in improved tolerance to soda saline–alkaline stress. OsSAP6 also interacts with OsPK5 to positively regulate soda saline–alkaline tolerance through ROS homeostasis
Conclusion
IN this study, we identified a stress-associated protein 6 (OsSAP6) gene from rice, which was upregulated expression under NaHCO3 stress. Overexpression of OsSAP6 in rice improved tolerance to soda saline–alkaline stress, and the expression of ROS-related genes was significantly upregulated in overexpression lines under SAE treatment. Moreover, we identified OsPK5 that interacts with OsSAP6, which was responsive to NaHCO3 and H2O2 stress, and played a positive role in soda salinity tolerance of rice. This study revealed that OsSAP6 responds to soda saline–alkaline stress and interacts with OsPK5 to positively regulate soda saline–alkaline tolerance through ROS homeostasis in rice.
Materials and Methods
Plant material and Rice Transformation
Oryza sativa longjing11 (Lj11) seeds were donated by the research group of Qingyun Bu, Chinese Academy of Sciences. According to the rice transgenic method of Toki et al. (2006) and Upadhyaya et al. (2000), dehulled Lj11 seeds were surface-sterilized and planted on a medium supplemented with 2,4-D to induce calli. Full-length sequences of OsSAP6 and OsPK5 were cloned into pGWB11 vector using Gateway LR Clonase II (Invitrogen) and introduced into Agrobacterium tumefaciens EHA105 by electroporation to infect rice calli for transformation. The integration of the OsSAP6 and OsPK5 was detected by PCR with special primers (Additional file 2: Table S2). qRT–PCR was used to detect the relative expression of OsSAP6 T3 generation transgenic rice seedlings. The seeds of the T3 generation from the overexpression lines were harvested as the material for subsequent experiments.
Soda Saline–Alkali Soil Eluent (SAE)
This study obtained soda saline–alkaline stress using soda saline–alkali soil eluent according to the preparation method of Wang et al. (2018). At the Anda field test station, the soil of the 0–10 cm soil layer of the severe alkaline patch was dried in the shade, passed through a fine sieve of 5 mm × 5 mm, and mixed thoroughly. Then, 4 L of water was poured into 2 L of saline–alkali soil, stirred well, and left for 12 h with stirring every 4 h. Filter with filter paper to remove impurities, and the experimental SAE was obtained. The leachate’s essential characteristics used in this study are shown in Tab S3.
Phylogenetic Analysis
The OsSAPs proteins were aligned using BioEdit software, then used for the phylogenetic analysis, and grouped by the NJ (neighbor-joining) method.
Analysis of Gene Expression
Three-leaf stage Lj11 seedlings were treated with 60 mM NaHCO3 and 5 mM H2O2 (0 h treatment was the control group). Leaves and roots were taken at different periods (0 h, 6 h, 12 h, 24 h, and 48 h) to extract total RNA and reverse transcribed it into cDNA, the tenfold diluted cDNA was used as a detection template. qRT–PCR using the SYBR Green PCR master mix (TransStart) in a fluorescent quantitative PCR instrument (Agilent Mx3000p) and analyzed by the 2−ΔΔCT method (Livak and Schmittgen 2001). The reaction system and procedure were described by Guan et al. (2016). All expressions were normalized against the Os18sRNA gene. The primers used are listed in Additional file 2: Table S2.
Subcellular Localization
The CDS (coding sequence) of the OsSAP6 was inserted into the pBI121-35S-GFP vector to construct the plant expression plasmid pBI121-35S-OsSAP6-GFP, then transformed into onion epidermal cells by a gene gun (GDS-80) and cultured for 18–24 h. The green fluorescence of OsSAP6-GFP fusion proteins was detected by scanning observation under a focusing microscope (Olympus), and the subcellular location of OsSAP6 was indicated by GFP.
Histochemical Analysis of GUS Activity
The promoter sequence of the OsSAP6 was identified through NCBI (LOC_Os03g57890), and primers (Additional file 2: Table S2) were designed to obtain a full length of 800 bp. The pGWB3-OsSAP6 promoter-GUS was generated by LR reactions with the OsSAP6 promoter driving GUS reporter gene expression. Then, transformed into Lj11 under the mediation of Agrobacterium tumefaciens EHA105, and the GUS activity was detected by staining with X-Gluc. The samples were destained with 95% ethanol and then examined.
Soda Saline–Alkaline Stress Tolerance Assay
Full-grown seeds were surface-sterilized and treated with different ratios of SAE including H2O:SAE = 5:1 (5 ml H2O + 1 ml SAE), 3:1 (4.5 ml H2O + 1.5 ml SAE), 2:1 (4 ml H2O + 2 ml SAE), and control (6 ml H2O). Seed germination was defined as the germ reaching half the length of the seed. The germination rate was calculated from the 3rd day, recorded every 24 h for 12 d, and detected the bud length and the total root length. To test the tolerance of transgenic lines to soda saline–alkaline stress, T3 generation transgenic lines T3-#10 and Lj11 were grown in 1/8 MS nutrient solution for 4 weeks, then stressed in different ratios of SAE with a total volume of 600 ml included 5:1, 3:1, 2:1 and the control. The phenotype, survival rates, and fresh weight were investigated after 14 days.
Seeds of overexpressing OsPK5 lines and Lj11 were germinated for 7 days, soaked in different ratios of H2O to SAE (4:1, 3:1, 2:1, water as control) for 7 days, and investigated for phenotype, fresh weight per plant and plant height.
To detect the expression of salinity-related genes, OsCu/Zn-SOD, OsAPX2, and OsCAT1 gene expression in transgenic lines under SAE stress were detected by qRT–PCR. The primers are shown in Additional file 2: Table S2.
Yeast Two-Hybrid Assay
The full-length ORF (open reading frame) sequence of OsSAP6 was fused into pGBKT7 vector as the bait and transformed into the yeast strain Y2HGold. Then cocultured with yeast containing cDNA from the japonica cultivar Nipponbare library (pGADT7-Rice) on SD/-Trp-Leu-His, SD/‐Ade-His-Leu-Trp, and SD/‐Trp‐Leu containing X‐α‐gal, and the blue strains were preserved (the experiment was repeated n = 3). The gene of the interacting protein was cloned with the primer T7/3-AD (Additional file 2: Table S2), and the interaction factor was obtained by sequencing. To validate the interaction, we searched for interacting factors in the NCBI Gene Bank according to the sequencing results, designed primers (Additional file 2: Table S2) based on the mRNA sequence and cloned them into the pGADT7 vector. Then cotransformed into Y2HGold with pGBKT7-OsSAP6 and cultured on synthetic drop-out medium SD/-His-Leu-Trp containing X‐α‐gal. The colonies with interactions were blue; otherwise, they were false-positive clones.
LCI Assays
The OsSAP6 coding sequence was inserted into pCAMBIA1300-nLUC vector, and the full-length OsPK5 sequence was inserted into pCAMBIA1300-cLUC vector using a seamless cloning and assembly kit, then electroporated into Agrobacterium tumefaciens strain EHA105. Agrobacterium containing pCAMBIA1300-nLUC-OsSAP6 was coinjected with Agrobacterium containing pCAMBIA1300-cLUC-OsPK5 into the same part of the N. benthamiana leaf and incubated for 48 h. LUC activity after luciferin injection was analyzed using chemiluminescence imaging (Tanon 5200).
Data Statistical Analysis Methods
Statistical analysis was carried out using Microsoft Excel 2013, SPSS 12.5, and DPS system for Windows, and one-way analysis of variance (ANOVA) was used for analysis. Statistical significance was defined as p < 0.05.
Availability of Data and Materials
All data supporting the findings of this study are available within the paper and within its supplementary materials published online.
Abbreviations
- SAP:
-
Stress-associated protein
- Lj11:
-
Oryza sativa longjing11
- SAE:
-
Soda saline–alkali soil eluent
- ABA:
-
Abscisic acid
- ROS:
-
Reactive oxygen species
- HSP90C:
-
Chloroplast heat shock protein 90
- DRIP:
-
Dehydration-responsive element binding protein2a interacting protein
- MBP-1:
-
C-myc binding protein
- qRT–PCR:
-
Quantitative real time PCR
- Y2H:
-
Yeast two hybrid
- GUS:
-
β-Glucuronidase
- MDA:
-
Malondialdehyde
- SD:
-
Synthetic drop-out medium
- LCI:
-
LUC complementation imaging
- RT-PCR:
-
Reverse transcription PCR
- QTL:
-
Quantitative trait locus
- qRSL7:
-
Quantitative trait locus related to relative shoot length on chromosome 7
- NJ:
-
Neighbor-joining
- CDS:
-
Coding sequence
- ORF:
-
Open reading frame
References
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408(6814):796–815. https://doi.org/10.1038/35048692
Ben Saad R, Ben Ramdhan W, Zouari N, Azaza J, Mieulet D, Guiderdoni E, Ellouz R, Hassairi A (2012a) Marker-free transgenic durum wheat cv. Karim expressing the AlSAP gene exhibits a high level of tolerance to salinity and dehydration stresses. Mol Breed 30(1):521–523. https://doi.org/10.1007/s11032-011-9641-3
Ben Saad R, Fabre D, Mieulet D, Meynard D, Dingkuhn M, Al-Doss A, Guiderdoni E, Hassairi A (2012b) Expression of the Aeluropus littoralis AlSAP gene in rice confers broad tolerance to abiotic stresses through maintenance of photosynthesis. Plant Cell Environ 35(3):626–643. https://doi.org/10.1111/j.1365-3040.2011.02441.x
Chen X, Ding Y, Yang Y, Song C, Wang B, Yang S, Guo Y, Gong Z (2021) Protein kinases in plant responses to drought, salt, and cold stress. J Integr Plant Biol 63(1):53–78. https://doi.org/10.1111/jipb.13061
Dansana PK, Kothari KS, Vij S, Tyagi AK (2014) OsiSAP1 overexpression improves water-deficit stress tolerance in transgenic rice by affecting expression of endogenous stress-related genes. Plant Cell Rep 33(9):1425–1440. https://doi.org/10.1007/s00299-014-1626-3
Dixit AR, Dhankher OP (2011) A novel stress-associated protein “AtSAP10” from Arabidopsis thaliana confers tolerance to nickel, manganese, zinc, and high temperature stress. PLoS ONE 6(6):e20921. https://doi.org/10.1371/journal.pone.0020921
Dreher K, Callis J (2007) Ubiquitin, hormones and biotic stress in plants. Ann Bot 99(5):787–822. https://doi.org/10.1093/aob/mcl255
Evans PC, Ovaa H, Hamon M, Kilshaw PE, Hamm S, Bauer S, Ploegh HL, Smith TS (2004) Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem J 378:727–734. https://doi.org/10.1042/BJ20031377
Gimeno-Gilles C, Gervais ML, Planchet E, Satour P, Limami AM, Lelievre E (2011) A stress-associated protein containing A20/AN1 zinc-finger domains expressed in Medicago truncatula seeds. Plant Physiol Biochem 49(3):303–310. https://doi.org/10.1016/j.plaphy.2011.01.004
Giri J, Vij S, Dansana PK, Tyagi AK (2011) Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytol 191(3):721–732. https://doi.org/10.1111/j.1469-8137.2011.03740.x
Guan QJ, Ma HY, Wang ZJ, Wang ZY, Bu QY, Liu SK (2016) A rice LSD1-like-type ZFP gene OsLOL5 enhances saline–alkaline tolerance in transgenic Arabidopsis thaliana, yeast and rice. BMC Genomics 17:142. https://doi.org/10.1186/s12864-016-2460-5
Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M (2003) Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22(13):3314–3325. https://doi.org/10.1093/emboj/cdg335
Huang J, Teng L, Li L, Liu T, Li L, Chen D, Xu LG, Zhai Z, Shu HB (2004) ZNF216 is an A20-like and IkappaB kinase gamma-interacting inhibitor of NFkappaB activation. J Biol Chem 279(16):16847–16853. https://doi.org/10.1074/jbc.M309491200
Huang J, Wang MM, Jiang Y, Bao YM, Huang X, Sun H, Xu DQ, Lan HX, Zhang HS (2008) Expression analysis of rice A20/ AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene 420(2):135–144. https://doi.org/10.1016/j.gene.2008.05.019
Kang M, Fokar M, Abdelmageed H, Allen RD (2011) Arabidopsis SAP5 functions as a positive regulator of stress responses and exhibits E3 ubiquitin ligase activity. Plant Mol Biol 75(4–5):451–466. https://doi.org/10.1007/s11103-011-9748-2
Kanneganti V, Gupta AK (2008) Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol Biol 66(5):445–462. https://doi.org/10.1007/s11103-007-9284-2
Kelley DR, Estelle M (2012) Ubiquitin-mediated control of plant hormone signaling. Plant Physiol 160(1):47–55. https://doi.org/10.1104/pp.112.200527
Lee HK, Cho SK, Son O, Xu Z, Hwang I, Kim WT (2009) Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21(2):622–641. https://doi.org/10.1105/tpc.108.061994
Lei L, Zheng H, Bi Y, Yang L, Liu H, Wang J, Sun J, Zhao H, Li X, Li J, Lai Y, Zou D (2020) Identification of a major QTL and candidate gene analysis of salt tolerance at the bud burst stage in rice (Oryza sativa L.) using QTL-Seq and RNA-Seq. Rice (n Y) 13(1):55. https://doi.org/10.1186/s12284-020-00416-1
Li X, Cai W, Zhang S, Xu L, Chen P, Wang J (2011) Cloning and expression pattern of a zinc finger protein gene ShSAP1 in Saccharum officinarum. Sheng Wu Gong Cheng Xue Bao 27(6):868–875. https://doi.org/10.13345/j.cjb.2011.06.002
Liu L, Wang B (2021) Protection of halophytes and their uses for cultivation of saline–alkali Soil in China. Biology (basel) 10(5):353. https://doi.org/10.3390/biology10050353
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lyzenga WJ, Stone SL (2012) Abiotic stress tolerance mediated by protein ubiquitination. J Exp Bot 63(2):599–616. https://doi.org/10.1093/jxb/err310
Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci USA 101(16):6309–6314. https://doi.org/10.1073/pnas.0401572101
Sharma G, Giri J, Tyagi AK (2015) Rice OsiSAP7 negatively regulates ABA stress signalling and imparts sensitivity to water-deficit stress in Arabidopsis. Plant Sci 237:80–92. https://doi.org/10.1016/j.plantsci.2015.05.011
Singh K, Foley RC, Oñate-Sánchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5(5):430–436. https://doi.org/10.1016/s1369-5266(02)00289-3
Sreedharan S, Shekhawat UK, Ganapathi TR (2012) MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol Biol 80(4–5):503–517. https://doi.org/10.1007/s11103-012-9964-4
Ströher E, Wang XJ, Roloff N, Klein P, Husemann A, Dietz KJ (2009) Redox-dependent regulation of the stress-induced zinc-finger protein SAP12 in Arabidopsis thaliana. Mol Plant 2(2):357–367. https://doi.org/10.1093/mp/ssn084
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47(6):969–976. https://doi.org/10.1111/j.1365-313X.2006.02836.x
Ubaidillah M, Kim KA, Kim YH, Lee IJ, Yun BW, Kim DH, Loake GJ, Kim KM (2013) Identification of a drought-induced rice gene, OsSAP, that suppresses Bax-induced cell death in yeast. Mol Biol Rep 40(11):6113–6121. https://doi.org/10.1007/s11033-013-2723-z
Upadhyaya NM, Surin R, Ramm K, Gaudron J, Schünmann PHD, Taylor W (2000) Agrobacterium mediated transformation of Australian rice cultivars Jarrah and Amaroo using modified promoters and selectable markers. Plant Physiol 27(3):201–210. https://doi.org/10.1071/pp99078
Vij S, Tyagi AK (2006) Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger(s) in rice and their phylogenetic relationship with Arabidopsis. Mol Genet Genomics 276(6):565–575. https://doi.org/10.1007/s00438-006-0165-1
Wang F, Coe RA, Karki S, Wanchana S, Thakur V, Henry A, Lin HC, Huang J, Peng S, Quick WP (2016) Overexpression of OsSAP16 regulates photosynthesis and the expression of a broad range of stress response genes in rice (Oryza sativa L.). PLoS ONE 11(6):e0157244. https://doi.org/10.1371/journal.pone.0157244
Wang H, Takano T, Liu S (2018) Screening and evaluation of saline–alkaline tolerant germplasm of rice (Oryza sativa L.) in soda saline–alkali soil. Agronomy 8(10):205–205. https://doi.org/10.3390/agronomy8100205
Weber AP (2004) Solute transporters as connecting elements between cytosol and plastid stroma. Curr Opin Plant Biol 7(3):247–253. https://doi.org/10.1016/j.pbi.2004.03.008
Xuan N, Liu X, Zhang H, Yang Y, Yao F (2015) Sequence analysis of maize zinc- finger protein gene ZmAN11 and its expression status under abiotic stress. Shandong Agric Sci 47(11):1-4+11. https://doi.org/10.14083/j.issn.1001-4942.2015.11.001
Xuan N, Jin Y, Zhang H, Xie Y, Liu Y, Wang G (2011) A putative maize zinc-finger protein gene, ZmAN13, participates in abiotic stress response. Plant Cell Tissue Organ Cult 107:101–112. https://doi.org/10.1007/s11240-011-9962-2
Yun X, Chen Y (2020) International development of saline–alkali land and its enlightenment to China. Territ Nat Resour Study 01:84–87. https://doi.org/10.16202/j.cnki.tnrs.2020.01.020
Zhang N, Xu J, Liu X, Liang W, Xin M, Du J, Hu Z, Peng H, Guo W, Ni Z, Sun Q, Yao Y (2019) Identification of HSP90C as a substrate of E3 ligase TaSAP5 through ubiquitylome profiling. Plant Sci 287:110170. https://doi.org/10.1016/j.plantsci.2019.110170
Zhang N, Yin Y, Liu X, Tong S, Xing J, Zhang Y, Pudake RN, Izquierdo EM, Peng H, Xin M, Hu Z, Ni Z, Sun Q, Yao Y (2017) The E3 Ligase TaSAP5 Alters Drought Stress Responses by Promoting the Degradation of DRIP Proteins. Plant Physiol 175(4):1878–1892. https://doi.org/10.1104/pp.17.01319
Zhang X, Wang N (2016) The development and management of saline–alkali land in Heilongjiang Province. Cognit Pract 03:106–110. https://doi.org/10.19309/j.cnki.zyx.2016.03.023
Zhu JF, Cui ZR, Wu CH, Deng C, Chen JH, Zhang HX (2018) Research advances and prospect of saline and alkali land greening in China. World Forestry Research 31(04):70–75. https://doi.org/10.13348/j.cnki.sjlyyj.2018.0034.y
Acknowledgements
We thank Dr. Qingyun Bu for providing Lj11 seeds and the japonica rice Nipponbare cDNA library.
Funding
This work is supported by the National Natural Science Foundation of China (32171989), Fundamental Research Funds for the Central Universities (2572021DS03), National Natural Science Foundation of China (31870300) and Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Author information
Authors and Affiliations
Contributions
FZ and KW performed most of the experiments. DL, ZL and ML participated in some parts of the study. QG, FZ and KW designed the experiments and wrote the paper. ML, ZW, XL and XL revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Homology analysis of OsSAP proteins. The amino acid sequence homology of the 18 OsSAP proteins was aligned by BioEdit software. Figure S2. Phylogenetic analysis of OsSAP proteins. The amino acid sequence homology of 18 OsSAP proteins was grouped by the neighbor-joining method phylogenetic analysis results, a bootstrap method with 1,000 replications was used for test of phylogeny. Scale bar indicates 0.2 amino acid substitution per site. Figure S3. Subcellular localization of OsSAP6-GFP at other excitation wavelengths. OsSAP6-GFP were observed at blue excitation group (383nm), red excitation group (587nm) and merge of green and red excitation group. Bar, 50 μm. Figure S4. Identification of OsSAP6 overexpression lines. A The integration of OsSAP6 into the genome of Lj11 in OsSAP6-overexpressing lines T0 was detected by PCR with specific primers. B The relative expression of OsSAP6 in seedlings of T3 generation transformed lines was measured using qRT–PCR. The values of Lj11 are set as 1. Values are mean ± SD; n = 3. Statistical analyses were performed using Student’s t test: *p < 0.05, **p < 0.01. Figure S5. Colonies interacting with OsSAP6 protein in the rice cDNA library. To identify the interactors of the OsSAP6 protein, a yeast two-hybrid system was used to preliminarily screen out a total of 16 colonies that turned blue on SD/‐Trp‐Leu-His containing X‐α‐gal. Four genes were identified according to Y2H assay, namely 2-phospho-D-glycerate hydrolase (PGH2, AK099342), voltage-dependent anion channel (VDAC1, AK071833), pyruvate kinase 5 (PK5, EU267984) and probable plastid-lipid-associated protein 2 (PAP2, AK104742). These proteins include proteins responsible for regulating ion channels and enzymes, and may cooperate with OsSAP6 proteins to participate in abiotic stress response mechanisms. Figure S6. Expression of OsPK5 in response to NaHCO3 and H2O2 stress in rice. qRT–PCR detected OsPK5 expression in leaves and roots of Lj11 treated with 60 mM NaHCO3 and 5 mM H2O2 stress. The values of 0 h (control) are set as 1. Values are mean ± SD; n = 3. Statistical analyses were performed using Student’s t test: *p < 0.05, **p < 0.01. Figure S7. Expression of OsCu/Zn-SOD, OsAPX2 and OsCAT1 in OsPK5 overexpression rice under soda saline-alkaline stress. 7-day-old OsPK5 overexpression lines and Lj11 seedlings were treated with different ratios of SAE (H2O:SAE = 4:1, 3:1, and 2:1, water as a control) for 7 days. The expression levels of OsCu/Zn-SOD, OsAPX2 and OsCAT1 were detected by qRT–PCR. The expression level of Lj11 was set to 1, and the Os18sRNA gene was used as an internal reference control. Values are the mean ± standard deviation of three replicates. Statistical differences are labeled with different letters using Duncan test (p < 0.05, one-way ANOVA)
Additional file 2: Table S1.
Distribution of OsSAP gene family encoding A20/AN1 zinc-Finger proteins in the rice Genes. Table S2. Primers used in this study. Table S3. The leachate’s essential characteristics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, F., Wang, K., Li, D. et al. OsSAP6 Positively Regulates Soda Saline–Alkaline Stress Tolerance in Rice. Rice 15, 69 (2022). https://doi.org/10.1186/s12284-022-00616-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-022-00616-x