Background
The phase 3 randomized PERSIST study demonstrated the efficacy and tolerability of galcanezumab, a humanized anti-calcitonin gene-related peptide (CGRP) monoclonal antibody for prevention of episodic migraines. We present findings from the open-label extension (OLE) of PERSIST, which evaluated the long-term efficacy and safety of galcanezumab in patients from China, India, and Russia.
Methods
Patients completing the 3-month double-blind period of PERSIST were eligible for the 3-month OLE. Patients previously randomized to galcanezumab (GMB/GMB group) continued to receive galcanezumab 120 mg at all three visits during the OLE whereas patients randomized to placebo received a 240 mg loading dose of galcanezumab and then two 120 mg doses (PBO/GMB group). The primary outcome was the mean change (from double-blind baseline) in the number of monthly migraine headache days (MHDs) to month 6. Other endpoints included percent reduction in monthly MHDs from double-blind baseline to month 6, functional outcomes, safety and tolerability.
Results
Overall, 99% of patients completing the double-blind period entered the OLE, and 96% completed through month 6. Patients in the GMB/GMB group achieved continued improvements in efficacy, with the reduction from baseline in the mean number of monthly MHDs, and slightly increasing from 4.01 days at the end of the double-blind period to 4.62 at the end of the OLE. Of patients who were ≥ 50% responders to galcanezumab at month 3, 66% maintained this response through to month 6. Patients in the PBO/GMB group experienced a rapid reduction in the number of monthly MHDs after initiation of galcanezumab, with a mean reduction from baseline of 4.56 days by month 6. The long-term benefits of galcanezumab were also supported by improvements in other efficacy and functional endpoints. All safety findings were consistent with the known long-term safety profile of galcanezumab; no patients experienced a treatment-related serious adverse event.
Conclusions
Galcanezumab was efficacious and well-tolerated in patients with episodic migraine from China, India and Russia, for up to 6 months.
Trial registration
ClinicalTrisABSTRACT_pals.gov NCT03963232, registered May 24, 2019.
Similar content being viewed by others
Background
Migraine was estimated to affect more than one billion individuals worldwide in 2019 [1], with an age-standardized point prevalence of 14.1% globally and 11.7% in China [1]. Migraine is an important cause of disability worldwide and the number of years lived with disability due to migraine globally was 42.1 million in 2019. For Central, East, Southeast and South Asia, the number of years lived with a disability due to migraine was estimated to be 0.5 million, 7.3 million, 4.2 million and 9.8 million, respectively [2].
Among individuals affected by migraines, around one-third (34–39%) may be candidates for preventive therapy [3, 4]; however, most current preventive therapies were initially developed for other therapeutic uses. Therefore, these therapies may have low adherence and persistence due to poor efficacy and tolerability [5, 6].
Calcitonin gene-related peptide (CGRP) has a key pathophysiological role in migraine and is expressed widely in the central and peripheral nociceptive system [7], representing a novel therapeutic target for prevention of episodic migraine. A number of monoclonal antibodies against CGRP or its receptor have been shown to provide a preventive effect for migraine and may have an improved benefit-risk profile versus historical preventive treatments [8, 9].
Galcanezumab has been assessed in multiple phase 3 placebo-controlled trials for episodic, chronic, and treatment resistant migraine [10,11,12,13,14,15]. The phase 3, randomized, double-blind, placebo-controlled PERSIST study in China, India, and Russia randomized 520 patients and evaluated galcanezumab 120 mg in patients with episodic migraine [16]. Findings from the 3-month double-blind period demonstrated that galcanezumab 120 mg resulted in significantly higher overall mean reductions in migraine headache days (MHDs) per month compared with placebo; by 3.81 days versus 1.99 days (p < 0.0001) [16]. Galcanezumab was also associated with acceptable tolerability, with low rates of serious adverse events (SAEs) and few discontinuations due to treatment-emergent adverse events (TEAEs). After completion of the double-blind period of the PERSIST trial, patients could enter a 3-month open-label extension (OLE) period. Here, we report results of the OLE study, which further evaluated the efficacy and safety of galcanezumab in patients with episodic migraine from China, India and Russia up to 6 months.
Methods
Study design and treatment
The phase 3 PERSIST study (NCT03963232) was conducted at 26 centers in China, 20 in India, and 4 in Russia (40 total). There were five study periods (Suppl. Figure 1): initial screening and washout; a prospective baseline period; a 3-month, randomized, double-blind, placebo-controlled treatment period; a 3-month OLE; and a 4-month post-treatment phase. As previously reported in detail [16], during the double-blind treatment period, patients were randomized (1:1) to galcanezumab 120 mg (as a monthly subcutaneous injection with a 240 mg loading dose) or matching placebo. All patients who completed the double-blind treatment period could enter the OLE and receive open-label study drug. Patients from the prior placebo group received a 240 mg loading dose of galcanezumab at Visit 7 and subsequently 120 mg at Visits 8 and 9. Patients from the prior galcanezumab group continued to receive galcanezumab 120 mg at all three visits during the open-label period. Sites and patients remained blinded to patients’ previous treatment assignment. To preserve blinding at Visit 7, all patients received two injections: the prior placebo group received two injections of galcanezumab 120 mg and the prior galcanezumab group received one injection of galcanezumab 120 mg and one injection of placebo.
Patients
Eligibility criteria for the PERSIST trial have been described previously [16]. In brief, the study included adults (18–65 years) with episodic migraine [17].
Assessments and endpoints
The primary efficacy measure was mean change in the number of monthly MHDs from double-blind baseline (the prospective baseline period) to month 6. Secondary endpoints included response rates (based on percent reduction in monthly MHDs from double-blind baseline to month 6), functional outcomes, safety and tolerability.
The mean change in monthly MHDs was derived from the ePRO system, in which patients recorded information about headaches (including medication used) and migraine-associated symptoms each day. Response rates were then estimated as the percentage of patients with reductions of ≥ 50%, ≥ 75% and 100% in monthly MHDs from double-blind baseline. A maintained ≥ 50% response was defined as a ≥ 50% reduction in monthly MHDs from baseline to month 3 that was maintained throughout the OLE. Functional outcomes were assessed as described previously [16] using the Migraine-Specific Quality of Life Questionnaire (MSQ) [18], the Patient Global Impression of Severity (PGI-S) scale [19], and the Migraine Disability Assessment (MIDAS) score [20]. The MSQ was conducted at randomization and monthly until month 6, whereas the PGI-S and MIDAS score questionnaires were administered at baseline, month 3 (end of double-blind period) and month 6 (end of OLE).
Safety was assessed by monitoring TEAEs, SAEs, deaths, adverse events leading to discontinuation, laboratory tests, electrocardiograms, vital signs, and body weight. Levels of antidrug antibodies (ADA) and neutralizing ADAs were measured to assess immunogenicity. Treatment-emergent ADAs (TE-ADAs) were defined as a negative baseline ADA result followed by a positive post-baseline ADA result with a titer ≥ 1:20 (treatment-induced) or positive baseline and post-baseline ADA results with a ≥ fourfold increase in titer (treatment-boosted ADA).
Statistical analysis
Efficacy and safety analyses were performed in all randomized patients who received at least one dose of the study drug in groups defined by treatment assignment during the double-blind period, i.e., patients previously randomized to galcanezumab (GMB/GMB group) or to placebo (PBO/GMB group). The efficacy analysis included data from both the double-blind and OLE periods. Patients who have a baseline (from the double-blind phase) and at least one post-baseline observation were included in the analysis. Continuous efficacy endpoints were analyzed using a restricted maximum likelihood-based mixed model for repeated measures (MMRM). Binary efficacy endpoints with repeated measurements were analyzed with a categorical, pseudo-likelihood-based repeated measures analysis implemented using a generalized linear mixed model procedure (GLIMMIX). Except for the efficacy analyses on MHDs or categorical analysis of response rate (such as 50% response rate) derived from MHDs, in which the continuous value of baseline MHDs was used as a covariate, all other efficacy analyses included baseline number of MHDs category (< 8 vs ≥ 8) as a covariate in the MMRM and GLIMMIX model.
Comparisons were claimed to be statistically significant if two-sided p-values were less than 0.05. TEAEs were summarized descriptively. Immunogenicity was assessed in all patients who received galcanezumab. All statistical analyses were performed using SAS version 9.4 or higher.
Results
Patient disposition and demographics
Almost all of the 487 patients who completed double-blind treatment (N = 484, 99.4%) entered the OLE (Fig. 1). This included 243 and 241 patients previously randomized to galcanezumab (GMB/GMB) or placebo (PBO/GMB), respectively. The majority of patients (466/484, 96.3%) completed the OLE: 95.5% in the GMB/GMB and 97.1% in the PBO/GMB group. The mean duration of exposure to treatment was 92.2 days during the OLE and mean treatment compliance was > 97%. In total, 18 patients discontinued treatment during the OLE; three due to an adverse event, two due to a protocol deviation, 12 withdrew from the study, and one due to pregnancy.
Baseline demographics and disease characteristics for the patients who enrolled in the OLE are presented in Table 1; the majority were female (73.3%) and from China (76.9%), with a mean age of 36.9 (standard deviation [SD]: 9.6) years and a mean migraine illness duration of 12.6 (SD: 8.6) years. At baseline, the mean monthly MHDs was 8.25 days and about half (55.4%) were experiencing ≥ 8 monthly MHDs, indicating a balance of low- and high-frequency MHD patients. Patients typically had moderately impaired daily functioning (mean MSQ-Role Function Restrictive score of 56.1), very severe disability (mean total MIDAS score of 46.9), and had moderate migraine severity (mean PGI-S score of 4.4).
Efficacy
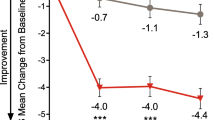
At the end of the OLE (month 6), patients in the PBO/GMB and GMB/GMB groups had a least squares (LS) mean reduction from baseline in monthly MHDs of 4.56 and. 4.62, respectively (Table 2; Fig. 2). In the GMB/GMB group, patients demonstrated sustained improvements throughout the OLE, with the LS mean reduction in the number of monthly MHDs from baseline increasing from 4.01 days at the end of the double-blind period to 4.62 at the end of the OLE (Fig. 2). In the PBO/GMB group, patients experienced a fast decrease in the number of monthly MHDs after initiating galcanezumab treatment, reaching that achieved by the GMB/GMB group by month 4, and subsequently maintaining the reduction (Fig. 2).
Response rates in patients in the GMB/GMB group increased from the end of the double-blind period to the end of the OLE. The proportion of patients achieving a ≥ 50% response increased from 59.7% to 70.9%, the proportion achieving a ≥ 75% response increased from 28.3% to 46.1%. and the proportion achieving a 100% response increased from 13.4% to 21.5% (Table 2, Fig. 3). After starting treatment with open-label galcanezumab, the percentage of patients in the PBO/GMB group achieving all three thresholds of response had increased by month 6.
Percentage of patients with ≥ 50%, ≥ 75%, and 100% reductions in monthly migraine headache days at month 3 (end of double-blind period) and month 6 (end of open-label period)
DB, double-blind; GMB, galcanezumab; OL, open-label; PBO, placebo; SE, standard error
*p < 0.05 versus placebo; ***p < 0.0001 versus placebo
Overall, 142 patients who were randomized to the galcanezumab group and were ≥ 50% responders at month 3 continued into the OLE. Of these, 66.2% (94/142) maintained a ≥ 50% response through to month 6. At the end of the OLE (month 6), patients in the GMB/GMB group had a further mean reduction in the number of monthly MHDs with acute headache medication; a reduction was also observed for the PBO/GMB group. Mean changes in the functional endpoints (MSQ-RFR, MIDAS, PGI-S) showed a similar pattern, indicating that further improvements were observed in the GMB/GMB group at month 6, and a rapid improvement occurred for patients in the PBO/GMB group (Table 2). MSQ-RFR domain scores increased by 23.72 following 6 months of treatment with galcanezumab, indicating a change from “moderately impaired” to “mildly impaired” performance of daily activities limited by migraine.
Safety
During the open-label period, TEAEs were reported by 94 (38.7%) and 83 (34.4%) patients in the GMB/GMB and PBO/GMB groups, respectively (Table 3). Most TEAEs (96.0%) were reported to be either mild or moderate in severity. For patients in the GMB/GMB group, the most common TEAEs during the open-label period were injection site reaction, injection site pruritus and upper respiratory tract infection, affecting 4.9%, 3.3% and 2.9% of patients, respectively. For patients in the PBO/GMB group, the most common TEAEs during the open-label period were injection site pain, upper respiratory tract infection and injection site erythema, affecting 2.5%, 2.5% and 2.1% of patients, respectively (Table 3). Fourteen SAEs were reported by 12 patients in total during the OLE. Three patients in the GMB/GMB group reported four SAEs (intestinal obstruction, mucosal infection, traumatic ulcer, and uterine polyp). Nine patients in the PBO/GMB group reported ten SAEs (borderline personality disorder, COVID-19 pneumonia, carpal tunnel syndrome, ectopic pregnancy, hemorrhoids, ligament sprain, limb injury, migraine, pain in extremity, and tension headache) and no SAEs were considered related to study treatment by the investigator. No patients died during the total study period. TEAEs leading to treatment discontinuation during the OLE occurred in two patients in the GMB/GMB group (abdominal discomfort and iritis) and one patient in the PBO/GMB group (an SAE of COVID-19 pneumonia).
Immunogenicity
Among 482 evaluable patients treated with galcanezumab during the double-blind and/or OLE period, 71 (14.7%) had ADAs present at baseline, with 37 patients (7.7%) having neutralizing ADAs. TE-ADAs were detected during galcanezumab treatment in 63 (13.1%) patients, including 59 patients (12.2%) who developed neutralizing ADAs. During the entire study, including the 4-month post-treatment period, 188 galcanezumab-treated patients developed TE-ADAs, including 186 patients (37.6%) who developed neutralizing ADAs. No meaningful relationship was observed between ADAs and efficacy or tolerability of galcanezumab.
Discussion
In this 3-month OLE of the PERSIST study, galcanezumab 120 mg continued to be efficacious in patients from China, India and Russia with episodic migraine for up to 6 months, with a generally good safety profile.
The findings in predominantly Asian patients are consistent with published results from the OLE of the phase 3 CONQUER episodic migraine populations [15]. Although CONQUER study enrolled both chronic and episodic migraine populations, we compared here only the CONQUER episodic migraine population with the PERSIST study, and these indirect comparisons must be interpreted with caution. It is interesting to note that the efficacy of galcanezumab at the end of the open-label phase of the present study appeared to be higher compared with results from the predominantly Caucasian population with episodic migraine enrolled in CONQUER (reduction in monthly MHDs from baseline to Month 6: 4.6 vs 3.8; percentage achieving a ≥ 50% response rate at Month 6: 70.9% vs 57.3%) [15]. Furthermore, there appeared to be a greater placebo effect in PERSIST, with 35.9% of placebo-treated patients achieving a ≥ 50% response at the end of the double-blind phase compared with 20.8% of placebo-treated patients in CONQUER episodic migraine populations. However, other differences between the PERSIST and CONQUER episodic migraine populations should be noted, including a lower mean age (GMB/GMB 37.2/ PBO/GMB 36.5 years vs. 45.9/46.3 years), less mean monthly MHDs (8.2/8.3 days vs. 9.5/9.2 days), and a shorter mean duration of migraine illness (12.7/12.4 years vs. 21.7/22.9 years) at baseline [15].
In the present study, the PBO/GMB group showed a rapid improvement in all efficacy endpoints following their transition to open-label galcanezumab. This result is in-line with the findings from the double-blind periods of the PERSIST and other phase 3 trials [13, 15, 16]. Further noticeable improvements in most efficacy endpoints were observed from the end of the double-blind period through to the end of the OLE for patients who received galcanezumab throughout the study. This suggests that patients may achieve continued improvements with galcanezumab treatment for up to 6 months and suggests that longer treatment with galcanezumab may result in greater treatment benefits. However, the interpretability of these data is limited by the lack of a placebo comparator during the OLE, with patients being aware that they were receiving active treatment.
Galcanezumab also had a maintained response during this OLE study, with two-thirds of patients (66.2%) who achieved a clinically meaningful ≥ 50% response during the double-blind phase of the trial maintaining this level of response during the OLE. This suggests patients achieving a good initial response to galcanezumab are likely to continue to show a good response for up to 6 months of treatment.
Galcanezumab previously showed acceptable tolerability during the double-blind phase of the PERSIST trial and this continued throughout longer-term treatment in the OLE. No patients experienced a treatment-related SAE and only three patients (0.6%) discontinued treatment due to a TEAE during the OLE. Furthermore, treatment compliance was high (> 97%) during the OLE. Based on the high treatment adherence and acceptable tolerability observed in the PERSIST study it is likely that patient adherence would be high in a clinical setting, this has important clinical implications, as patients with migraine typically exhibit poor adherence to standard preventive treatments [5]. These data were comparable to the long-term safety profile of galcanezumab reported in previous phase 3 studies and their OLEs [10,11,12,13,14,15]. The long-term benefits of targeting the CGRP pathway have also been shown in studies of other monoclonal antibodies that target CGRP, including erenumab and fremanezumab [21, 22].
Limitations of this study included that, as for all OLE studies, there was no comparator arm or blinding, thus limiting the interpretability of results from open-label period. Furthermore, the study was only six months in duration, and the longer-term safety and efficacy of galcanezumab in Asian patients remain unknown.
Conclusions
Availability of data and materials
Lilly provides access to all individual participant data collected during the trial, after anonymization, except for pharmacokinetic or genetic data. Data are available upon reasonable request. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Abbreviations
- ADA:
-
Antidrug antibody
- CGRP:
-
Calcitonin gene-related peptide
- ePRO:
-
Electronic patient-reported outcomes
- ICHD:
-
International Classification of Headache Disorders
- LS:
-
Least squares
- MHDs:
-
Migraine headache days
- MIDAS:
-
Migraine Disability Assessment
- MMRM:
-
Mixed model repeated measures
- MSQ:
-
Migraine-Specific Quality of Life Questionnaire
- OLE:
-
Open-label extension
- PGI-S:
-
Patient Global Impression of Severity
- SAE:
-
Serious adverse event
- SD:
-
Standard deviation
- TEAE:
-
Treatment-emergent adverse event
References
Safiri S et al (2022) Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 163(2):e293–e309
Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2018. 17(11): p. 954–976.
Lipton RB et al (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68(5):343–349
Katsarava Z et al (2018) Poor medical care for people with migraine in Europe - evidence from the Eurolight study. J Headache Pain 19(1):10
Hepp Z, Bloudek LM, Varon SF (2014) Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 20(1):22–33
Rizzoli P, Loder EW (2011) Tolerance to the beneficial effects of prophylactic migraine drugs: a systematic review of causes and mechanisms. Headache 51(8):1323–1335
Villalón CM, Olesen J (2009) The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther 124(3):309–323
Overeem LH et al (2021) Indirect Comparison of Topiramate and Monoclonal Antibodies Against CGRP or Its Receptor for the Prophylaxis of Episodic Migraine: A Systematic Review with Meta-Analysis. CNS Drugs 35(8):805–820
Drellia K et al (2021) Anti-CGRP monoclonal antibodies for migraine prevention: A systematic review and likelihood to help or harm analysis. Cephalalgia 41(7):851–864
Stauffer VL et al (2018) Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol 75(9):1080–1088
Skljarevski V et al (2018) Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 38(8):1442–1454
Detke HC et al (2018) Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 91(24):e2211–e2221
Pozo-Rosich P et al (2022) Long-term treatment with galcanezumab in patients with chronic migraine: results from the open-label extension of the REGAIN study. Curr Med Res Opin 38(5):731–742
Mulleners WM et al (2020) Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 19(10):814–825
Reuter U et al (2021) Galcanezumab in Patients with Multiple Previous Migraine Preventive Medication Category Failures: Results from the Open-Label Period of the CONQUER Trial. Adv Ther 38(11):5465–5483
Hu B et al (2022) Galcanezumab in episodic migraine: the phase 3, randomized, double-blind, placebo-controlled PERSIST study. J Headache Pain 23(1):90
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia, 2018. 38(1): p. 1–211.
Speck, R., et al. The Migraine-Specific Quality of Life Questionnaire, Role Function Restrictive Domain: Defining Clinically Meaningful Categories of Functional Impairment Severity. in JOURNAL OF HEADACHE AND PAIN 2021, 22(Supp 1): P0226. BMC CAMPUS, 4 CRINAN ST, LONDON N1 9XW, ENGLAND.
W, G., ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch. 1976: p. 217–22.
Lipton RB et al (2001) Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache 41(9):854–861
Ashina M et al (2021) Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol 28(5):1716–1725
Goadsby PJ et al (2020) Long-term safety, tolerability, and efficacy of fremanezumab in migraine: A randomized study. Neurology 95(18):e2487–e2499
Acknowledgements
The authors wish to acknowledge Stephanie Carter PhD, CMPP and Jake Burrell PhD (Rude Health Consulting Ltd.) for medical writing support, which was paid for by Eli Lilly and Company. The authors thank Yu Mao from Eli Lilly and Company for project management and medical writing assistance. The authors thank Yan Cheng and Fei Ji from Eli Lilly and Company for review and critical suggestions.”
Funding
The PERSIST study was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
SY was involved in study design, data acquisition and interpretation. JZ, LZ, DC, KS, GL, XY, and MZ were involved in data acquisition and interpretation. LS, HL and CQ were involved in data analysis and interpretation. All the authors revised the manuscript critically and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, International Conference on Harmonisation guidelines for Good Clinical Practice, and applicable local laws and regulations. Patients provided written informed consent before undergoing any study procedures. The appropriate institutional review board for each participating site approved the study protocol, patient consent form and other relevant documents.
Consent for publication
Not applicable.
Competing interests
LS, HL and CQ are full-time employees of Eli Lily and Company. SY serves as associated editor of the Journal of Headache and Pain and as a member of the International Headache Society. MZ has no competing interests. JZ, LZ, DC, KS, GL, XY and SY declare receiving clinical research fees from Eli Lilly and Company for participating as investigators in the PERSIST study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, J., Zhong, L., Chowdhury, D. et al. Galcanezumab in patients with episodic migraine: results from the open-label period of the phase 3 PERSIST study. J Headache Pain 24, 103 (2023). https://doi.org/10.1186/s10194-023-01613-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01613-1