Abstract
Background
Head-to-head comparator trials between first-line oral migraine preventatives and the new monoclonal antibodies (mAbs) blocking the calcitonin gene-related peptide (CGRP) pathway have not been published to date.
Objectives
This study aimed to indirectly compare the clinical efficacy and safety of mAbs against CGRP or its receptor (CGRPR) and topiramate in episodic migraine prophylaxis using meta-analysis.
Methods
We included controlled trials testing efficacy and safety of erenumab, galcanezumab, fremanezumab, eptinezumab, and topiramate in adults diagnosed with episodic migraine. We searched PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov from January 2000 to November 2020. We used the Risk of Bias 2 (RoB2) tool to assess the risk of bias and report pooled mean effects (mean difference and risk ratio) as estimated in a random effect model. For efficacy analysis, we determined the reduction of monthly migraine days (MMDs), reduction of days with acute medication (AMDs), and 50% responder rates (50% RR). For safety, we determined adverse events (AEs) occurring in ≥ 2% of study participants and the number of patients who discontinue treatment due to AEs (DAEs). The number needed to treat (NNT) and to harm (NNH) were estimated as well as the likelihood to help or harm (LLH).
Results
We included 13 trials involving 7557 patients: three trials with erenumab, two trials with galcanezumab, two trials with fremanezumab, one trial with eptinezumab, and five trials with topiramate, for the prophylaxis of episodic migraine in adults. The placebo-subtracted reduction (pooled mean difference) of MMDs were − 1.55 (95% CI − 1.86 to − 1.24; active drug n = 3326 vs placebo n = 2219, 8 studies) for the CGRP(R) mAb and − 1.11 (95% CI − 1.62 to − 0.59; active drug n = 1032 vs placebo n = 543, 4 studies) for topiramate (p for subgroup difference = 0.15). ‘Cognitive’ and ‘sensory & pain’-related adverse events occurred more often in patients treated with topiramate compared with those treated with a CGRP(R) mAb (p for subgroup difference 0.03 and < 0.001, respectively). Based on the 50% RR and DAE, the NNT, NNH, and LHH for the CGRP(R) mAbs were 6, 130, and 24.3:1, respectively. For topiramate, these values were 7, 9, and 1.8:1, respectively.
Conclusion
The efficacy of CGRP(R) mAbs to reduce migraine days does not differ from topiramate. However, the safety profile is in favor of the CGRP(R) mAbs, with a higher likelihood to help than to harm compared with topiramate. The diversity of endpoint determination and the heterogeneity between studies for some endpoints cause some limitations for this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our results suggest a favorable efficacy and safety profile of the new CGRP pathway drug class compared with topiramate for episodic migraine prophylaxis. |
Based on the likelihood to help or harm, patients treated with a CGRP receptor (R) monoclonal antibody (mAb) are 19.2 times more likely to be helped compared with patients treated with topiramate. |
Patients treated with topiramate have a higher risk of experiencing adverse reactions and discontinuing treatment compared with patients treated with a CGRP(R) mAb (placebo-subtracted risk: 12% and 1%, respectively; p = 0.005). |
1 Introduction
Migraine is one of the most disabling neurological diseases and often requires preventive therapy to reduce attack frequency [1]. Recently, migraine-specific prophylactic agents targeting the calcitonin gene-related peptide (CGRP) pathway have been introduced into the field [2]. Eptinezumab, fremanezumab, and galcanezumab bind to CGRP while erenumab blocks the canonical CGRP receptor (CGRPR) [3]. These monoclonal antibodies (mAbs) are approved by numerous authorities for the preventive treatment of migraine in patients with at least four monthly migraine days. However, reimbursement restrictions limit the use of mAbs targeting the CGRP pathway in many countries.
The antibodies have shown efficacy in multiple placebo-controlled, randomized, double-blind clinical trials. Their safety and tolerability profile has also been established. Clinical use led to the impression that CGRP antibody medications have a better tolerability profile than the standard of care (SoC) oral migraine prophylactic agents. However, adverse event (AE) rates in mAbs trials range between 60 and 70% [4,5,6].
Some patients have a tremendous response to mAbs therapy. For example, ~ 39% of patients treated with galcanezumab in the EVOLVE studies had at least 1 month without any migraine day during the 6-month double-blind (DB) trial phase. A reduction of ≥ 75% monthly migraine days (MMDs) was achieved by > 40% of participants in month 6 [7]. These parameters have typically not been analyzed in previous clinical trials with oral medications.
Topiramate is a first-choice oral SoC medication for migraine prophylaxis [8]. It is the most frequently prescribed preventative in the US and among the most frequently used anti-migraine drugs in Europe. Other first-line, non-specific preventatives include β-blockers, flunarizine, and amitriptyline [8]. All these SoC medications are inexpensive treatment options, which are effective in most patients.
The therapeutic benefit, which indicates the difference of an active drug versus placebo in the reduction of MMDs, does not seem to be different between mAbs and SoC medications. For example, differences for mAbs range between − 1.00 and − 1.99 MMDs, which is similar to data from clinical trials with oral SoC medications [1, 9, 10].
The side effects of oral SoC medications lead to treatment termination in approximately half of the patients within the first 2 months of therapy [11]. The lack of efficacy also contributes to the > 80% of patients who stop preventive therapy within 1 year after initiating therapy. In contrast, dropout rates in mAbs episodic migraine prophylactic trials are below 5% within a DB treatment period of 6 months [12,13,14].
Only head-to-head comparator trials of mAbs and SoC medications will allow a true comparison and estimation of the efficacy and tolerability of these new prophylactic agents. These data do not exist to date.
To assess the benefit of this new class of CGRP-targeted antibodies for migraine prophylaxis we performed this meta-analysis. Based on the availability of trials, we decided to focus primarily on episodic migraine. The number of topiramate chronic migraine trials is limited and their sample size is small, which may be at the expense of a valid comparison. Therefore, we aimed to indirectly compare the clinical efficacy and safety of the CGRP(R) mAbs (erenumab, galcanezumab, fremanezumab, and eptinezumab) and topiramate in episodic migraine prophylaxis using meta-analysis.
2 Methods
2.1 Literature Search
This meta-analysis was conducted according to the recommendations of the “Preferred Reporting Items for Systematic reviews and Meta-Analysis” (PRISMA). The International Headache Society (IHS) has set out guidelines for the conduction of clinical trials for migraine prevention [15]. These recommendations form the basis for the selection of inclusion and exclusion criteria of this analysis.
We systematically searched PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov using the keywords ‘erenumab’, ‘AMG334’, ‘galcanezumab’, ‘LY2951742’, ‘fremanezumab’, ‘TEV-48125’, ‘eptinezumab’, ‘ALD403’, ‘topiramate’, ‘Topamax’, and ‘episodic migraine’. The detailed search strategy can be found in the electronic supplementary material [ESM], page 5–8. The study search was performed by two authors (LO and TK) independently. The literature search was conducted on June 8, 2020.
2.2 Study Selection
Study inclusion criteria were (1) randomized, DB, placebo-controlled, parallel-group trials of phase IIb, III, or IV and (2) assessing the efficacy and/or safety of erenumab, galcanezumab, fremanezumab, eptinezumab, or topiramate in episodic or high-frequency episodic migraine patients.
The following criteria led to the exclusion of studies: (1) studies performed in minors (< 18 years of age), (2) studies including patients with chronic migraine, (3) migraine diagnosis was not assessed according to IHS guidelines, and (4) no reporting of any outcome of our interest.
Studies were selected based on title and abstract but deemed suitable for inclusion only after full-text review. The screening process and study selection were independently performed by two authors (LO and TK). Disagreements were resolved through discussion with a third author (UR).
2.3 Data Extraction and Outcome Measures
Study information (design, duration, DB phase, assessment of primary outcome, study arms, and sample size), and demographic information (sex, age, and baseline migraine days) were extracted.
The primary efficacy outcome included the reduction of mean MMDs in the active study group compared with placebo. The secondary efficacy outcomes were reduction of acute anti-migraine medication days (AMDs), which includes specific and non-specific substances, and the 50% responder rate (50%RR, which indicates the number of patients with a reduction of at least 50% of MMDs versus baseline).
The primary safety outcome was the number of adverse events (AEs) occurring in at least 2% of the study population. We then clustered AEs into the following six categories to create an organ system-related illustration: ‘cognitive’, ‘sensory & pain’, ‘gastrointestinal’, ‘infection & infestation’, ‘administration site condition’, and ‘general & other’ related AEs. All AEs per category are listed in the supplement (Additional Table 1 in the ESM). The secondary safety outcome was the discontinuation rate of patients due to AEs (DAEs). Data were extracted for all outcomes from each study independently (LO and JM). After evaluation, a consensus was obtained. In case a publication reported data for several time points, we used the time point of the study’s primary endpoint.
2.4 Assessment for the Risk of Bias and Quality Assessment
To estimate the risk of bias of the included studies, we used the Risk of Bias 2.0 Tool, which assesses the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results for each study separately [16]. The assessment was performed by LO and BR independently. Disagreements were resolved through discussion with a third author (UR).
Heterogeneity was estimated using a Chi-square test and I2 statistic, indicating the percentage of the variance between studies. Significant heterogeneity was assumed if the p value for the Chi-square test was significant (p < 0.05). Cochrane gives rough interpretations for heterogeneity as follows: “0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity” [17].
We assumed the random-effect model as more appropriate for analysis because of differences between study settings (e.g. duration, location, and dose). We used the random-effects for all outcomes to account for heterogeneity [17]. In case of significant heterogeneity, sensitivity analyses were performed to identify the studies causing the heterogeneity. Publication bias was not assessed because the number of studies in each meta-analysis group was insufficient, the Egger’s test will in this case not have enough power to distinguish between change and real asymmetry [17]. Funnel plots, however, are reported.
2.5 Data Synthesis and Analysis
All meta-analyses of eligible results were conducted using Review Manager Version 5.4.0. Meta-analysis was carried out for all outcomes of interest. For the continuous variables MMDs and AMDs, we calculated the pooled mean difference (PMD) with corresponding 95% confidence intervals (95% CI). Data are illustrated as mean (SD) and 95% CI. Studies were weighted by the use of the inverse variance method. For the categorical variables 50%RR, DAEs, and our categories of AEs as described above, we calculate the pooled risk ratio (PRR) with corresponding 95% CI. Data are illustrated as count (%). Studies were weighted by the use of the Mantel–Haenszel method. Additionally, we calculated the pooled risk difference (PRD) for the 50%RR and DAE with corresponding 95% CI, to calculate the number needed to treat (NNT: 1/RD of 50%RR) and the number needed to harm (NNH: 1/RD of DAE). The range of the 95% CI for the NNT and NNH is from 1 NNT to benefit (NNTB) to 1 NNT to harm (NNTH), where RD = 0 is NNT = infinity [18]. From the calculations of the NNT and NNH (including 95% CI), we calculated the likelihood to be helped or harmed (LHH: [1/NNT]/[1/NNH]).
We only analyzed available data (i.e. missing data was ignored) [17]. We considered p values of 5% or lower to be statistically significant.
For our efficacy and safety outcomes, we used one model (main comparison) in which we pooled all CGRP(R) mAbs studies into one subgroup and all topiramate studies in another subgroup. A subgroup comparison was performed to assess the difference between the CGRP(R) mAbs and topiramate. The comparisons we made are indirect.
For our safety outcomes, we additionally used a second model in which we pooled studies together per substance and per dose of the experimental groups. Here we divided the ‘shared’ placebo group into two or more groups to be able to perform two or more comparisons [17]. Subgroup comparisons were made between the pooled data of the CGRP mAbs, the CGRPR mAbs, and topiramate and not between the different mAbs. The benefit of model 1 is the aggregation of studies, which leads to higher statistical discrimination and robust effect estimates. Model 2 provides in-depth safety information about single drugs and doses.
3 Results
3.1 Eligible Studies
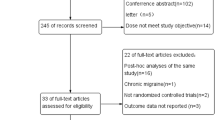
Our search until June 8, 2020, identified 305 records through database and trial registry screening (n = 114); after the removal of duplicates, 394 records remained. After screening titles and abstracts, we excluded 291 records, leaving 102 records for a full reading. Full reading led to exclusion of an additional 89 records, which left 13 records for qualitative and quantitative synthesis (Figure 1 in the ESM). All studies were published between 2004 and 2019. All studies compared active treatment with placebo. One study included an active control arm with propranolol [19].
3.2 Characteristics of the Included Studies
Our search led to 13 studies on the prophylaxis of episodic migraine with 7557 patients for analysis (4670 receiving an active drug and 2887 receiving placebo). These trials had a multicenter, randomized, DB, parallel-group, placebo-controlled design. We included one phase IIb trial [20], 11 phase III [4, 12,13,14, 19, 21,22,23,24,25,26], and one phase IV trial [27]. All study characteristics are reported in Table 1. All CGRP(R) mAb studies were of high quality with a low risk of bias, where the topiramate studies had some concerns. We included the risk of bias to all forest plots in the ESM).
3.3 Efficacy Analysis
3.3.1 CGRP(R) mAbs
All studies reported the difference of MMDs and AMDs between the CGRP(R) mAb (n = 3326) and placebo (n = 2219). The PMD for the reduction of MMDs was − 1.55 (95% CI − 1.86 to − 1.24; p < 0.001 | heterogeneity: p = 0.06; I2 = 49%) in favor of the CGRP(R) mAbs versus placebo (Fig. 1). The reduction of AMDs was also greater for the CGRP(R) mAbs than placebo with a difference (PMD) of − 1.26 (95% CI − 1.70 to − 0.81; p < 0.001 | heterogeneity: p < 0.001; I2 = 89%) days (Fig. 1). Sensitivity analyses identified the studies ‘Arise’ (for erenumab) and ‘Promise-1’ (for eptinezumab) as causing the heterogeneity. Exclusion of these studies resulted in a PMD reduction of AMDs of − 1.51 (95% CI − 1.78 to − 1.23; p < 0.001 | heterogeneity: p = 0.13; I2 = 41%).
Comparison between the calcitonin gene-related peptide (receptor) [CGRP(R)] monoclonal antibodies and topiramate of the efficacy outcomes monthly migraine days and acute medication days†. SD standard deviation, IV inverse variance, df degrees of freedom, CI confidence interval. †The top eight studies of each analysis involved the CGRP(R) studies, and the bottom four studies involved the topiramate studies
3.3.2 Topiramate
The differences of MMDs and AMDs were reported in only four trials with topiramate (n = 1032) versus placebo (n = 543). Patients treated with topiramate showed a larger reduction of MMDs compared with placebo (PMD − 1.11; 95% CI − 1.62 to − 0.59; p < 0.001 | heterogeneity: p = 0.08; I2 = 46%) (Fig. 1). Topiramate showed a greater reduction of AMDs (PMD − 0.78; 95% CI − 1.13 to − 0.44; p < 0.001 | heterogeneity: p = 0.28; I2 = 22%) (Fig. 1).
3.3.3 Efficacy Differences Between CGRP(R) mAbs and Topiramate
Our analysis (model 1) did not reveal differences between the CGRP(R) mAbs versus topiramate (p = 0.15) for the reduction of MMDs (Fig. 1). For AMDs, we also did not find any difference between the CGRP(R) mAbs versus topiramate (p = 0.10) for the reduction of AMDs (Fig. 1). In the sensitivity analyses, excluding the studies ‘Arise’ (erenumab) and ‘Promise-1’ (eptinezumab), which caused heterogeneity between studies, a difference between the CGRP(R) mAbs versus topiramate (p = 0.001) for the reduction of AMDs was identified. This was in favor of the CGRP(R) mAbs.
Subgroup analysis for dose (model 2) and the reduction of MMDs revealed that erenumab 140 mg (p = 0.04) and galcanezumab 120 mg (p = 0.02) and 240 mg (p = 0.04) are superior to topiramate 50 mg. Galcanezumab 120 mg is also superior to topiramate 100 mg (p = 0.04). For AMDs reduction, erenumab 140 mg and galcanezumab 120 mg and 240 mg are superior to topiramate 50 mg as well as the 100 mg dose (for all, p < 0.05; Supplementary Tables 2–4 and Supplementary Figs 2–8, see ESM).
3.4 Safety Analysis
3.4.1 CGRP(R) mAbs
Patients receiving a CGRP(R) mAb had a higher risk for injection-site-related AEs (e.g. pain and bruising) compared with placebo (PRR 1.58; 95% CI 1.20–2.07; p = 0.001). Further differences between the active substance and placebo could not be identified (Table 2). Significant heterogeneity was present in the categories ‘sensory & pain’ (caused by the galcanezumab study ‘EVOLVE-1’), ‘Gastrointestinal’ (caused by the erenumab study ‘Strive’), ‘Administration-site condition’ (caused by the fremanezumab study ‘HALO-EM’), and ‘General & other’ (caused by the erenumab study ‘Strive’). The results after the exclusion of these studies that caused heterogeneity from the models are shown in Table 2.
3.4.2 Topiramate
Patients treated with topiramate had a higher risk than patients on placebo for the occurrence of AEs in four categories. The PRR were 2.21 (95% CI 1.84–2.64; p < 0.001) for cognitive AEs; 8.01 (95% CI 3.95–16.26; p < 0.001) for sensory & pain AEs; 1.66 (95% CI 1.16–2.35; p = 0.005) for gastrointestinal AEs; and 2.40 (95% CI 1.24–4.67; p = 0.010) for general and other AEs (Table 2). Heterogeneity was observed in the categories ‘sensory & pain’ (caused by the topiramate study ‘INTREPID’) and ‘general & other’ (caused by the topiramate study ‘INTREPID’). The results after the exclusion of these studies that caused heterogeneity from the models are shown in Table 2.
3.4.3 Safety Differences Between CGRP(R) mAbs and Topiramate
Patients treated with topiramate had a higher risk for cognitive-related AEs (p = 0.03) and sensory & pain-related AEs (p < 0.001) compared with patients treated with a CGRP(R) mAb. After the sensitivity analyses, the categories ‘gastrointestinal-related AEs’ (p = 0.02) and ‘general & other-related AEs’ (p = 0.02) became significant in the comparison between the CGRP(R) mAbs versus topiramate, in favor of the CGRP(R) mAbs (Table 2).
We also explored the risk difference for each drug by dose. Figure 2 shows all risk differences (active drug vs placebo) for each drug by dose per category. (Additional comparisons between the CGRP(R) mAbs and topiramate for each drug by dose can be found in the Supplementary Tables 5–11 and Supplementary Figs 9–29 in the ESM.
3.5 Assessment of the Likelihood of Help or Harm
3.5.1 CGRP(R) mAbs
For all CGRP(R) mAbs studies, the information on the 50% RR (active drug, n = 3326, and placebo, n = 2219) and the discontinuation rates (active drug, n = 3354, and placebo, n = 2245) were complete. The NNT was 6NNTB (95% CI 4.6NNTB to 6.4NNTB; p < 0.001 | heterogeneity: p = 0.21; I2 = 28%). The NNH was 130NNTH (95% CI 1000NNTB ∞ 64.9NNTH; p = 0.05 | heterogeneity: p = 0.38; I2 = 6%) (Fig. 3). The LLH was 24.3:1, indicating that for every patient harmed, 25 patients are helped by the treatment with a CGRP(R) mAb.
Comparison between the calcitonin gene-related peptide (receptor) [CGRP(R)] monoclonal antibodies and topiramate of the number needed to treat and number needed to harm†. 50%RR 50% responder rate, DAE discontinuation due to adverse events, IV inverse variance, CI confidence interval, NNTB number needed to treat to benefit, NNTH number needed to treat to harm, df degrees of freedom. †The top eight studies of each analysis involved the CGRP(R) studies, and the bottom four and five studies involved the topiramate studies
3.5.2 Topiramate
4 out of 5 topiramate trials reported the 50%RR (active drug, n = 1128, and placebo, n = 445), but all studies reported the AE-related drop-out rates (active drug, n = 1316, and placebo, n = 642). The NNT was 7NNTB (95% CI 4.4NNTB to 13.6NNTB; p < 0.001 | heterogeneity: p = 0.08; I2 = 56%). The NNH was 9NNTH (95% CI 23.6NNTH to 5.1NNTH; p = 0.002 | heterogeneity: p < 0.001; I2 = 83%) (Fig. 3). The LLH was 1.3:1, indicating that for every patient harmed, two patients are helped by the treatment with topiramate. After the sensitivity analyses, the NNH was 12NNTH (95% CI 31.1NNTH to 7.2NNTH; p = 0.002|heterogeneity: p = 0.08; I2 = 56%). The LLH ratio became 1.8:1. The topiramate study ‘MIGR-003’ caused the heterogeneity between studies.
3.5.3 Differences Between CGRP(R) mAbs and Topiramate Regarding the NNT, NNH, and LHH
In our analysis (model 1) we did not observe differences between the CGRP(R) mAbs versus topiramate (p = 0.39) for the NNT (Fig. 3). For the NNH, we observed a difference between the CGRP(R) mAbs versus topiramate (p = 0.005) in favor of the CGRP(R) mAbs. (Fig. 3). Regarding the LHH, patients treated with a CGRP(R) mAb are 19.2 times more likely to be helped (13.8 times after sensitivity analyses) compared with patients treated with topiramate.
3.6 Publication Bias
The funnel plots do not show any obvious asymmetry (Supplementary Figs 34–45, see ESM). These analyses indicate that there is limited publication bias. Because we were not able to test asymmetry due to the limited number of included studies, we might not exclude the possibility of some publication bias. We included the funnel plots to all forest plots in the ESM.
4 Discussion
This meta-analysis of 13 studies for the prophylaxis of episodic migraine with a total of 7557 patients revealed comparable efficacy of the CGRP mAbs galcanezumab, fremanezumab, and eptinezumab, the CGRPR mAb erenumab, and topiramate. In contrast, the tolerability and safety analysis showed favorable effects for the CGRP(R)-targeted antibody therapies in comparison with topiramate. The CGRP(R) antibody group has a much higher probability to help than to harm in comparison with topiramate based on the 50% responder rates and treatment discontinuation rates. Our analysis indicates an advantage, especially in tolerability, of these new medication groups in comparison with topiramate. A comparison between single substances within the group of CGRP(R) mAbs was not within the scope of our analysis, because we aimed to assess the possible benefits of this whole new CGRP pathway-targeted antibody medication class compared with an established SoC oral treatment.
Overall, topiramate and CGRP(R) mAbs have similar efficacy. The analysis by treatment dose revealed that only topiramate doses of 100 mg and higher effectively reduced the number of MMDs compared with placebo. These doses are often not reached in clinical practice. Many patients discontinue oral preventive treatments due to side effects before reaching the target dose [28, 29]. Treatment discontinuation with topiramate occurs often within the first 3 months [29]. In patients receiving antiepileptic drugs for migraine prophylaxis, < 30% show adherence after 6 months of treatment [30]. An observational study in patients with episodic migraine in Germany evaluated the preventive treatment with topiramate in a clinical setting. Of 366 patients, 22.6% discontinued treatment within the first 6 months, mainly due to side effects. The majority of patients who continued treatment reached only a dose of 50 mg or 75 mg [31]. Based on our data, there is no evidence for the superiority of the topiramate 50 mg dose versus placebo for the prevention of episodic migraine. In contrast, the CGRP(R) mAbs doses used in clinical practice demonstrated superior efficacy in clinical trials. Moreover, real-world data on CGRP(R) mAbs showed an efficacy and tolerability profile comparable to clinical trials or better [32,33,34,35,36]. Less than 12% of patients discontinued treatment due to side effects in these reports, which mainly focused on patients with chronic migraine and mostly on erenumab, as it was the first available mAb across several countries. There is no reason to expect different results from patients with episodic migraine and those treated with other CGRP antibodies.
To give a quick overview, we grouped AEs into six different categories. For topiramate, our analysis reveals a dose–response relationship for the risk of AEs. Patients treated with a higher dose are at higher risk to experience any side effects and to discontinue treatment due to AEs. We also identified that gastrointestinal-related AEs are more likely to occur in patients treated with erenumab 140 mg compared with placebo. This is in line with data from real-world studies [37, 38].
Topiramate is available in immediate-release and extended-release formulations. Our study only included data with the immediate-release topiramate formulation in the absence of clinical trials with the extended-release tablets in episodic migraine. A real-world assessment in a migraine cohort (n = 285) compared both formulations. Potentially greater tolerability was found for extended-release topiramate tablets than the immediate-release formulation [39].
To our knowledge, this is the first meta-analysis comparing CGRP(R) mAbs with topiramate. Previous attempts to summarize the evidence on CGRP(R) mAbs and topiramate for migraine prophylaxis have been performed separately. These meta-analyses on mAbs often pooled clinical trial phase II CGRPR mAb and CGRP mAb studies together as well as studies in episodic and chronic migraine [40,41,42,43,44]. This study included only patients with episodic migraine and performed separate analyses for the CGRPR antagonist and the CGRP mAbs from phase III–IV clinical trials. Because phase III studies cover larger study populations, accuracy and confidence increase relative to phase II studies, which leads to more precise estimates of the treatment effects. The study of Drellia et al. compared the CGRP(R) mAbs with topiramate and others, albeit not in a meta-analysis [45]. The authors concluded that CGRP(R) mAbs have a more favorable benefit–risk ratio than topiramate in episodic and chronic migraine [45]. This is in line with our findings in episodic migraine. Our study adds pooled estimates and subgroup comparisons, thereby statically comparing the differences between the CGRP(R) mAbs and topiramate in an indirect fashion.
In addition to previous work, our study adds an indirect comparison between the CGRP mAbs and topiramate, and the estimated NNT, NNH, and LHH that indicate the favorability of treatment outcomes. Former meta-analysis on the efficacy and tolerability of topiramate included other oral prophylaxes (network meta-analysis) and generally used headache frequency as an outcome measure instead of migraine days. Nevertheless, a large Cochrane meta-analysis shows similar results to this analysis [46]. The authors did not find a significant reduction of headaches for topiramate 50 mg compared with placebo, but topiramate 100 mg and 200 mg were superior compared with the 50 mg dose in the reduction of headache frequency. The occurrence of AEs did not differ between topiramate 50 mg and placebo, but was in favor of placebo compared with topiramate 100 mg and 200 mg [46]. This analysis confirms that topiramate has a higher risk for adverse events versus placebo in four out of the five predefined AE categories. For sensory and pain-related AEs, this translates into a higher risk for patients on topiramate compared with the CGRP(R) antibody. In general, adverse events are more frequent in topiramate studies in the active substance and placebo groups than in all mAbs trials. Because both arms have an increased AE incidence in topiramate trials, we were not able to identify differences in risk ratios for side effects between topiramate and the CGRP mAbs. The high number of AEs reported in the placebo groups of topiramate trials are caused by the nocebo phenomena, which is substantially higher in topiramate trials than it is in anti-CGRP(R) trials [47], and probably led to an underestimation of the detected differences.
Based on the analysis of dropouts due to adverse events and the 50% responder rates, topiramate is more likely to harm than to help compared with all monoclonal antibody therapies. Although the use of dropouts due to AEs is a very rigid measure, it resembles clinical reality. With the lack of direct comparison (head-to-head study), a meta-analysis is currently the best possible way to compare the efficacy and safety of CGRP mAbs and topiramate. The current head-to-head trial HER-MES (NCT03828539) is incomplete in this regard, as this study compares the CGRPR antibody erenumab and topiramate.
Results from three mAb clinical trials in difficult-to-treat cohorts suggest that mAbs have some benefits compared with topiramate [14, 48, 49]. These studies included patients who previously failed treatment with up to four standard preventives and topiramate was the most frequently used non-successful previous oral preventative medication. Erenumab, fremanezumab, and galcanezumab showed good efficacy in this population compared with placebo with a significant reduction in monthly migraine days. We did not include these data in our meta-analysis because topiramate studies with a comparable patient population do not exist. The long-term safety of CGRP mAbs has been assessed in open-label studies [50,51,52]. Erenumab was studied in episodic and chronic migraine over 1 and 2 years (Liberty study) and in ~ 250 patients from the initial dose-finding study over 5 years [53, 54]. Galcanezumab and fremanezumab data are also available for 1 year. These studies found that the mAbs are safe and well tolerated. However, some concern still exists. Cardiovascular and cerebrovascular safety in compromised conditions is a matter of debate since CGRP is a potent vasodilator. A study in an experimental stroke model in mice found that the small molecule CGRP receptor antagonists (gepants) had a negative impact on infarct size [55]. One case report in a patient with a stroke after the first dose of erenumab also exists [56]. Ongoing post-marketing surveillance will shed further light on these important safety topics.
A limitation of this analysis is related to the primary endpoint analysis of the clinical trials. While some trials have a 3-month DB treatment phase duration, others analyzed data over a 6-month DB period or months 4–6 of the DB treatment phase. In addition, topiramate trials typically have a titration period as part of the DB phase. This heterogeneity between the studies may affect the estimated effect. In general, topiramate studies are older and of lower quality, which may affect data reliability. Also, the differences between inclusion and exclusion criteria between studies may affect the external validity. Due to the short duration of the clinical trials, the results of our meta-analysis may not accurately reflect the clinical practice. However, long-term open-label studies report similar results after 1 year [50,51,52, 57].
Despite these limitations, our results are robust and comparable to previous work on topiramate. The comparisons between the CGRP(R) mAbs and topiramate lead to insights into the efficacy and safety differences between these medications. The assessment of safety measures has not been performed previously and is new in this regard, while the division of AEs into subcategories leads to a better understanding of the safety profiles. Because our comparison is indirect, the result should be interpreted with some caution. A firm recommendation for clinical practice should not be made based on these findings. However, clinicians should be aware of the tolerability profiles of topiramate and the CGRP(R) mAbs and should take this information into account when treating patients.
5 Conclusion
Our meta-analysis shows that topiramate has comparable efficacy to CGRP(R) mAbs, at least in doses of 100 mg and above. In contrast, mAbs have fewer adverse events than topiramate and lower discontinuation rates due to adverse events. Although this meta-analysis indicates some benefits for CGRP-targeted therapies over topiramate, only future head-to-head studies will allow a direct comparison of effects.
References
Valery LF, Amanuel AA, Kalkidan HA, Foad A, Abdishakur MA, Semaw FA, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;2017:390.
Raffaelli B, Neeb L, Reuter U. Monoclonal antibodies for the prevention of migraine. Expert Opin Biol Ther. 2019;19(12):1307–17.
Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765–74.
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008.
Lipton RB, Goadsby PJ, Smith J, Schaeffler BA, Biondi DM, Hirman J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):e1365–77.
Stauffer VL, Turner I, Kemmer P, Kielbasa W, Day K, Port M, et al. Effect of age on pharmacokinetics, efficacy, and safety of galcanezumab treatment in adult patients with migraine: results from six phase 2 and phase 3 randomized clinical trials. J Headache Pain. 2020;21(1):79.
Rosen N, Pearlman E, Ruff D, Day K, Jim NA. 100% Response rate to galcanezumab in patients with episodic migraine: a post hoc analysis of the results from Phase 3, randomized, double-blind, placebo-controlled EVOLVE-1 and EVOLVE-2 studies. Headache. 2018;58(9):1347–57.
Evers S. Treatment of migraine with prophylactic drugs. Expert Opin Pharmacother. 2008;9(15):2565–73.
Deng H, Li GG, Nie H, Feng YY, Guo GY, Guo WL, et al. Efficacy and safety of calcitonin-gene-related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine - an updated systematic review and meta-analysis. BMC Neurol. 2020;20(1):57.
Jackson JL, Cogbill E, Santana-Davila R, Eldredge C, Collier W, Gradall A, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One. 2015;10(7):e0130733.
Hepp Z, Dodick DW, Varon SF, Chia J, Matthew N, Gillard P, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–85.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–32.
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–37.
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7.
Diener HC, Tassorelli C, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020. https://doi.org/10.1177/0333102420941839.
Cochrane. 'Risk of bias 2.0' tool. 2019. https://methods.cochrane.org/risk-bias-20-tool. Accessed 27 Jun 2019.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. www.handbook.cochrane.org.
Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309–12.
Diener HC, Tfelt-Hansen P, Dahlöf C, Láinez MJ, Sandrini G, Wang SJ, et al. Topiramate in migraine prophylaxis–results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251(8):943–50.
Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14(11):1081–90.
Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–54.
Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291(8):965–73.
Silberstein SD, Hulihan J, Karim MR, Wu SC, Jordan D, Karvois D, et al. Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: a randomized, placebo-controlled, double-blind, 12-week pilot study. Clin Ther. 2006;28(7):1002–11.
Silberstein SD, Neto W, Schmitt J, Jacobs D. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol. 2004;61(4):490–5.
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of Galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Lipton RB, Silberstein S, Dodick D, Cady R, Freitag F, Mathew N, et al. Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia. 2011;31(1):18–30.
Blumenfeld AM, Bloudek LM, Becker WJ, Buse DC, Varon SF, Maglinte GA, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–55.
Woolley JM, Bonafede MM, Maiese BA, Lenz RA. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache. 2017;57(9):1399–408.
Berger A, Bloudek LM, Varon SF, Oster G. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12(7):541–9.
Nelles G, Schmitt L, Humbert T, Becker V, Sandow P, Bornhoevd K, et al. Prevention of episodic migraines with topiramate: results from a non-interventional study in a general practice setting. J Headache Pain. 2010;11(1):33–44.
Russo A, Silvestro M, Scotto di Clemente F, Trojsi F, Bisecco A, Bonavita S, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain. 2020;21(1):69.
Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21(1):61.
Ornello R, Casalena A, Frattale I, Gabriele A, Affaitati G, Giamberardino MA, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32.
Scheffler A, Messel O, Wurthmann S, Nsaka M, Kleinschnitz C, Glas M, et al. Erenumab in highly therapy-refractory migraine patients: first German real-world evidence. J Headache Pain. 2020;21(1):84.
Raffaelli B, Kalantzis R, Mecklenburg J, Overeem LH, Neeb L, Gendolla A, et al. Erenumab in chronic migraine patients who previously failed five first-line oral prophylactics and OnabotulinumtoxinA: a dual-center retrospective observational study. Front Neurol. 2020;11:417.
Kanaan S, Hettie G, Loder E, Burch R. Real-world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia. 2020;40(13):1511–22.
Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real-world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60(9):2014–25.
O’Neal W, Hur EE, Liranso T, Patel B. Real-world assessment of treatment with extended-release topiramate (Trokendi XR(®)) and comparison with previous immediate-release topiramate treatment. J Comp Eff Res. 2018;7(11):1095–105.
Huang IH, Wu PC, Lin EY, Chen CY, Kang YN. Effects of anti-calcitonin gene-related peptide for migraines: a systematic review with meta-analysis of randomized clinical trials. Int J Mol Sci. 2019;20(14).
Silberstein SD, McAllister P, Ning X, Faulhaber N, Lang N, Yeung P, et al. Safety and tolerability of fremanezumab for the prevention of migraine: a pooled analysis of phases 2b and 3 clinical trials. Headache. 2019;59(6):880–90.
Hou M, Xing H, Cai Y, Li B, Wang X, Li P, et al. The effect and safety of monoclonal antibodies to calcitonin gene-related peptide and its receptor on migraine: a systematic review and meta-analysis. J Headache Pain. 2017;18(1):42.
Lattanzi S, Brigo F, Trinka E, Vernieri F, Corradetti T, Dobran M, et al. Erenumab for preventive treatment of migraine: a systematic review and meta-analysis of efficacy and safety. Drugs. 2019;79(4):417–31.
Zhu C, Guan J, Xiao H, Luo W, Tong R. Erenumab safety and efficacy in migraine: a systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore). 2019;98(52):e18483.
Drellia K, Kokoti L, Deligianni CI, Papadopoulos D, Mitsikostas DD. Anti-CGRP monoclonal antibodies for migraine prevention: a systematic review and likelihood to help or harm analysis. Cephalalgia. 2021. https://doi.org/10.1177/0333102421989601.
Linde M, Mulleners WM, Chronicle EP, McCrory DC. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):Cd010610.
Kokoti L, Drellia K, Papadopoulos D, Mitsikostas DD. Placebo and nocebo phenomena in anti- CGRP monoclonal antibody trials for migraine prevention: a meta-analysis. J Neurol. 2020;267(4):1158–70.
Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–40.
Mulleners WM, Kim BK, Láinez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–25.
Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick D, Rippon GA, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39(11):1455–64.
Camporeale A, Kudrow D, Sides R, Wang S, Van Dycke A, Selzler KJ, et al. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018;18(1):188.
Goadsby PJ, Silberstein SD, Yeung PP, Cohen JM, Ning X, Yang R, et al. Long-term safety, tolerability, and efficacy of fremanezumab in migraine: a randomized study. Neurology. 2020;95(18):e2487–99.
Lanteri-Minet M, Goadsby PJ, Reuter U, Wen S, Hours-Zesiger P, Ferrari MD, et al. Effect of erenumab on functional outcomes in patients with episodic migraine in whom 2-4 preventives were not useful: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. 2021.
Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick DW, Xue F, et al. Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021.
Mulder IA, Li M, de Vries T, Qin T, Yanagisawa T, Sugimoto K, et al. Anti-migraine calcitonin gene-related peptide receptor antagonists worsen cerebral ischemic outcome in Mice. Ann Neurol. 2020;88(4):771–84.
Aradi S, Kaiser E, Cucchiara B. Ischemic stroke associated with calcitonin gene-related peptide inhibitor therapy for migraine: a case report. J Stroke Cerebrovasc Dis. 2019;28(10):104286.
Rapoport A, Mauskop A, Diener HC, Schwalen S, Pfeil J. Long-term migraine prevention with topiramate: open-label extension of pivotal trials. Headache. 2006;46(7):1151–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interest
LHO has nothing to disclose. BR has nothing to disclose related to the submitted work. BR reports grants from Novartis; personal fees from Novartis, TEVA, and Allergan. JM reports personal fees from Novartis, outside the submitted work. TH has nothing to disclose. LN has nothing to disclose related to the submitted work. LN reports personal fees from Novartis, Allergan, TEVA, and BIAL; personal fees from Hormosan, and Eli Lilly. UR has nothing to disclose related to the submitted work. UR reports personal fees from AbbVie, Allergan, Medscape, and StreaMedUp; personal fees and institutional fees from Amgen, Eli Lilly, and TEVA; grants, personal fees, and institutional fees from Novartis; institutional fees from Alder.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
UR initiated the project. Study concept and design: LHO, LN, and UR. Acquisition of data: LHO and TK. Analysis and interpretation of data: LHO, BR, JM and TK. Drafting of the manuscript: LHO, BR, JM, and UR. Statistical analysis: LHO and JM. All authors read and approved the final manuscript, and agree to the accountable for the data and the accuracy of the data analysis.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Overeem, L.H., Raffaelli, B., Mecklenburg, J. et al. Indirect Comparison of Topiramate and Monoclonal Antibodies Against CGRP or Its Receptor for the Prophylaxis of Episodic Migraine: A Systematic Review with Meta-Analysis. CNS Drugs 35, 805–820 (2021). https://doi.org/10.1007/s40263-021-00834-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00834-9