Abstract
The four vertebrate R-spondin proteins are secreted agonists of the canonical Wnt/β-catenin signaling pathway. These proteins are approximately 35 kDa, and are characterized by two amino-terminal furin-like repeats, which are necessary and sufficient for Wnt signal potentiation, and a thrombospondin domain situated more towards the carboxyl terminus that can bind matrix glycosaminoglycans and/or proteoglycans. Although R-spondins are unable to initiate Wnt signaling, they can potently enhance responses to low-dose Wnt proteins. In humans, rare disruptions of the gene encoding R-spondin1 cause a syndrome of XX sex reversal (phenotypic male), palmoplantar keratosis (a thickening of the palms and soles caused by excess keratin formation) and predisposition to squamous cell carcinoma of the skin. Mutations in the gene encoding R-spondin4 cause anonychia (absence or hypoplasia of nails on fingers and toes). Recently, leucine-rich repeat-containing G-protein-coupled receptor (Lgr)4, Lgr5 and Lgr6, three closely related orphans of the leucine-rich repeat family of G-protein-coupled receptors, have been identified as receptors for R-spondins. Lgr5 and Lgr6 are markers for adult stem cells. Because R-spondins are potent stimulators of adult stem cell proliferation in vivo and in vitro, these findings might guide the therapeutic use of R-spondins in regenerative medicine.
Similar content being viewed by others
Gene organization and evolutionary history
The R-spondins are members of a superfamily of thrombospondin type 1 repeat (TSR-1)-containing proteins. The prototype member (discovered in 1971) was isolated from platelets that had been stimulated with thrombin, and was therefore designated 'thrombin-sensitive protein' [1]. The TSR-1 repeat (also known as properdin repeat) was then characterized in the thrombospondin proteins (TSPs), in which it is repeated three times [2]. TSP1 and TSP2 are secreted multimeric matricellular proteins that, in addition to the TSP repeat, share homology in an amino-terminal globular region, von Willebrand factor domain, type II repeats (epidermal growth factor (EGF)-like), type III repeats (calcium binding) and the carboxy-terminal region. These modular proteins act by bringing together cytokines, growth factors, membrane receptors and extracellular proteases. Several proteins involved in the complement pathway (properdin, C6, C7, C8A, C8B, C9) and extracellular matrix proteins, such as mindin, F-spondin and SCO-spondin, contain one or more TSR-1 repeats.
The prefix R in the R-spondin subfamily of TSR-1-containing proteins derives from the expression of the gene encoding murine R-spondin1. This gene is transiently expressed in the neural tube at 10 and 12 days post-conception, in the boundary region between the roof plate and neuroepithelium, hence its name R(oof plate specific)-spondin [3]. In addition to the presence of the TSR-1 domain, all four R-spondin members are characterized by the presence of a carboxy-terminal region with positively charged amino acids and, importantly, two furin-like cysteine-rich repeats near the amino terminus of the mature protein. Furin repeats (first seen in the endoprotease furin) are also present in receptors for growth factors such as EGF, insulin, hepatocyte growth factor (HGF) and neurotrophic factors. The R-spondin family was discovered over a 4-year period. R-spondin3 was discovered in 2002 [4], whereas descriptions of R-spondin1 [3] and R-spondin2 followed in 2004 [5]. Finally, R-spondin4 was characterized in 2006 [6].
R-spondin homologs (defined by two Fu domains followed by a TSP1 domain) are present in all vertebrates, in primitive chordates such as the lancelet Branchiostoma floridae, in the hemichordate acorn worm Saccoglossus kowalevskii and in the echinodermate sea urchin Strongylocentrotus purpuratus (Figure 1). No homologs with an R-spondin domain composition are found in invertebrate model organisms such as Drosophila or Caenorhabditis, or any other primitive animal. Given this phylogenetic distribution, an R-spondin-like gene was likely to have been present in the deuterostome ancestor and, given its absence outside the deuterostome clade, also originated there. An evolutionary tree of these sequences rooted on the primitive deuterostomes clearly shows the two successive genome duplications that generated present day R-spondin diversity in vertebrate species such as fish and mammals (Figure 1).
Evolutionary history of R-spondins. Homologs of R-spondin (each contains two Fu domains followed by a thrombospondin protein 1 (TSP1) domain) are shown. Two non-chordate R-spondin sequences with its characteristic domain architecture were detected in the hemichordate acorn worm and the echinodermate sea urchin. These two were used to root the gene phylogeny. The tree clearly shows the two successive whole genome duplications that generated present day R-spondin diversity in vertebrate species such as fish and mammals. Sequences were aligned using MAFFT [80]. The tree was constructed using neighbor-joining as implemented in the clustalx package and visualized using iToL [81, 82]. SkRspo is D1LXC5_SACKO from the hemichordate acorn worm Saccoglossus kowalevskii, BfRspo is C3Y1K8_BRAFL from the primitive chordate amphioxus Branchiostoma floridae, and SpRspo is XP_796266.2 from the echinodermate sea urchin Strongylocentrotus purpuratus. Vertebrate protein identifiers used for this tree are as follows: HsRspo4 ENSP00000217260, MmRspo4 ENSMUSP00000041578, DrRspo4 ENSDARP00000123862, DrRspo2 ENSDARP00000100941, HsRspo2 ENSP00000276659, MmRspo2 ENSMUSP00000067325, HsRspo1 ENSP00000348944, MmRspo1 ENSMUSP00000030687, DrRspo1 ENSDARP00000058458, HsRspo3 ENSP00000349131, MmRspo3 ENSMUSP00000090287 and DrRspo3 ENSDARP00000058577.
The mammalian R-spondins have a similar five-exon gene organization and protein domain structure. The human family members share a pair-wise amino acid similarity of 40% to 60% (Figure 2). The amino-terminal hydrophobic signal peptide ensures that secretion is encoded by the first exon, whereas the two cysteine-rich furin-repeat domains are encoded by exons 2 and 3, and a single TSP1 domain is encoded by exon 4. Exon 5 encodes a region in the protein that is solely characterized by its high density of basic amino acids.
Protein domain architecture and chromosome location of human R-spondins. Schematic representations are shown for all four human R-spondin proteins. The total lengths of R-spondin1, 2, 3 and 4 are 263, 243, 292 and 234 amino acids, respectively. Three types of domains are detected: two cysteine-rich furin-like repeats, a single thrombospondin domain, and a basic amino-acid-rich domain. The relative protein sequence conservation, as a percentage of identical amino acids, within these domains is indicated. The two furin repeats jointly contain 15 conserved cysteines, conforming to the consensus sequence for this domain in each repeat. Twelve out of 60 amino acid residues are highly conserved in thrombospondin protein 1 (TSP1) domains, six of which are cysteines. Secretion is mediated by an amino-terminal endoplasmic reticulum signal peptide. Putative N-linked glycosylation sites are indicated (N).
Characteristic structural features
The four R-spondin proteins share a common domain architecture. An amino-terminal endoplasmic reticulum signal peptide ensures entry into the secretory pathway. The processed mature protein has two cysteine-rich furin-like repeats at the amino terminus. The central TSR-1 domain is followed by a region with a high number of basic amino acids at the carboxyl terminus (Figure 2). The two furin-like repeats near the amino terminus are related to a domain seen in the subtilisin-like proprotein convertase family member furin. Although the function of this domain in furin is unknown, its prevalence in a number of important receptors for growth factors, such as EGF, insulin, HGF and neurotrophic factors, suggests it makes a significant functional contribution.
Mass spectrometry approaches have provided some insight into the molecular structure of the furin domains in R-spondins [7]. That study by Li et al. recorded the pattern of disulfide bonds between the 15 available cysteine residues present in these domains. In a purified peptide containing both furin-like repeats of R-spo2, they determined the free and interconnected cysteine residues. In total, five free cysteine residues were found: three in furin repeat 1 and two in furin repeat 2. All interconnected cysteine residues appeared to be separated by only two or three intervening amino acids. No crystallographic study of furin-like repeats in R-spondins is yet available. However, such analyses have been performed for the EGF receptor and insulin growth factor receptor 1 [8, 9]. These revealed the existence of three pairs of linked cysteine residues in furin-like repeat 1 that successively bridge 5, 8 and 18 intervening residues. No unbound cysteine residues remained. It is unclear whether these divergent outcomes reflect consequences of the techniques used or structural differences underlying the specific roles of these domains in the proteins studied.
The second domain that is common to all four R-spondins is a TSR-1 domain. The human genome harbors 41 proteins that contain TSR-1 domains. The number of the TSR-1 domains in these proteins varies from 1 to 18. All of the TSRs occur either in secreted proteins or in the extracellular portion of transmembrane proteins. The TSR-1 domain in R-spondin may have a role related to glycosaminoglycan (GAG)/proteoglycan binding. Several observations supporting such a role have been made in other TSR-1-domain-containing proteins. Multiple amino acid sequence alignments of TSRs show that a typical TSR domain consists of 60 amino acids, of which 12 are highly conserved [10, 11]. X-ray crystallography of the TSR-1s of human TSP1 led to the discovery of the CWR layer, an architecture composed of three antiparallel strands. Strand A assumes a rippled conformation, whereas strands B and C assume regular β-sheets. The side chains of the tryptophan residues in the A strand make up two W-layers. Two arginine residues in the B strand comprise the R-layers. The alternate stacking of the cationic guanidinium groups of the arginine residues with the aromatic side chains of the tryptophan residues provide vital stabilization in the structure of this small domain. Additional solidity derives from the C-layers, disulfide bonds capping the amino-terminal and carboxy-terminal ends of the strands. The exposed tryptophan residues and arginine residues define the front face of the domain and are likely to contact the negatively charged repeating disaccharide units of GAGs and proteoglycans. Moreover, the disaccharide units in GAGs span approximately 9 Å, enabling two units to fit into the recognition groove of the TSR-1 [12]. The three-dimensional structure of R-spondins is not yet available, but molecular modeling techniques have also predicted a GAG-binding site for the TSR of R-spondin 4 [13]. A recently reported binding of R-spondin3 to the transmembrane proteoglycan syndecan-4 is consistent with these findings [14]. It will be of interest to determine the GAG-binding specificity of the four R-spondin TSR-1s and to translate this knowledge into functional models.
Localization and function
Extensive functional analysis of the R-spondin proteins, using Wnt reporter assays in 293T cells, uncovered a link with the canonical Wnt/β-catenin pathway [5] (Figure 3). The latter plays a central role in cellular proliferation, differentiation and stem cell maintenance. Activity is initiated when secreted proteins of the Wnt family bind to Frizzled (Fzd) receptors and the low-density lipoprotein receptor related protein 5 or 6 (LRP5/6) co-receptors. At this level, the pathway is controlled by a series of extracellular antagonists (Figure 3). R-spondins uniquely synergize with Wnt proteins. Accordingly, R-spondin activation showed sensitivity to the presence of the extracellular Wnt inhibitor Dickkopf-1 (DKK1) and no synergy could be induced by overexpression of any of the known intracellular components of the pathway. Protein domain analysis showed that furin repeats are essential and sufficient to mediate the Wnt-potentiating effect of the R-spondins [5, 7, 15]. The first in vivo experiments documenting this Wnt potentiating phenomenon were performed in early frog embryos [5]. Depletion of R-spondin2 in one blastomere at the eight-cell stage resulted in disorganized somites and a reduction in myotomes at the injected site. Depletion at the gastrula stage resulted in a failure to transcriptionally activate the myoD and myf5 genes, later leading to impaired muscle development. Manipulation of Wnt activity at this developmental stage, in chick and mammals, strikingly phenocopies these effects [16, 17]. Canonical Wnt pathway potentiation by R-spondins has also been seen in experimentally induced tumors. A sustained high level of Wnt activity in the tumor was explained by the finding that mammary tumor virus integration sites were seen in both genes for Wnt family members and the gene for R-spondin2 [18].
Simplified overview of the canonical Wnt signaling pathway. The typical mammalian genome harbors 19 genes encoding Wnt secretory factors and 10 Frizzled (Fzd) genes encoding their receptors. Two low-density lipoprotein receptor-related proteins (Lrp) 5 or 6 act as Fzd co-receptors. Activating combinations of Fzd/Lrp/Wnt initiate signaling activity by silencing the activity of a dedicated β-catenin (βcat) destruction complex. Dvl gene products are instrumental in achieving this. (a) In the absence of Wnt signals, constitutively synthesized cytoplasmic βcat is the immediate target of this complex. Essential components of this complex are two tumor suppressor proteins: Apc (adenomatous polyposis coli) and axin, which act as scaffolds to capture newly synthesized βcat and allow its phosphorylation by the constitutively active kinases casein kinase-1 (Ck1) and glycogen synthase kinase 3 (GSK3), also residing in this complex. (b) The Wnt-binding-induced cytoplasmic accumulation of βcat leads to import into the nucleus and binding to T-cell transcription factor (Tcf)/Lef transcription factors, upon replacement of the transcriptional Groucho repressors. Bipartite Tcf/Lef-βcat complexes are the ultimate effectors of this signaling cascade. A series of secreted antagonists control signaling activity at the level of ligand perception. Secreted Frizzled-related proteins (Sfrp1, 2, 4 and 5), Frzb and Wnt inhibitory factor (Wif) can bind Wnt directly and prevent it from activating their receptors [83–86]. The other Wnt antagonists, Dickkopf 1 (Dkk1) [87] and Wise [88], inhibit by binding to the Lrp co-receptor. R-spondins, also operating at this level, are unique in enhancing Wnt activity. The seven transmembrane Lgr (4, 5 and 6) receptors mediating their action were recently uncovered [48, 89, 90].
R-spondins operate during embryogenesis
Wnt signaling is important in almost every fate decision during embryonic development throughout the animal kingdom [19]. The knowledge obtained of the Wnt-enhancing ability of R-spondins together with their dynamic expression patterns in embryonic tissues (Additional file 1, Table S1) predicts pleiotropic roles for R-spondins during embryogenesis.
R-spondin 1: sex phenotype reversal
R-spondin controls the most fundamental difference between individuals: their sex phenotype (Figure 4). The phenotypic sex of the embryo depends on gonadal sex determination. XY male to female sex reversal is relatively frequent, whereas XX male sex reversal is rare and usually caused by translocation of the sex-determining region Y (SRY) gene. Mutations in RSPO1 (encoding R-spondin1) lead to an extremely rare human syndrome that combines SRY-independent XX male sex reversal with palmoplantar hyperkeratosis (PPK; an abnormal thickening of the palms and sole), and a predisposition to squamous cell carcinoma (SCC) of the skin. Parma et al. [20] described an Italian family with 11 46, XX individuals in two sibships. All affected individuals were phenotypically male. The seven genetic females did not show signs of the PPK/SCC phenotype or sexual ambiguity. Parma et al. postulated that homozygosity for a single mutational event causes both PPK and SCC in XY and XX individuals, and sex reversal in XX individuals. A genetic analysis of this family, complemented with an individual from a family with an independent mutation, proved the presence of mutations in RSPO1. Two types of mutations appeared to result in an absence of functional protein. PKK and SCC could be explained by fibroblast-derived R-spondin1 stimulation of keratinocytes, leading to a reduced level of β-catenin in the affected keratinocytes [20]. The sex reversal appeared to be caused by a failure to mount high R-spondin1 levels in the gonads of affected individuals. This increase in R-spondin production, normally at embryonic day (E)18.5, occurs only in XX gonads and is required to promote oocyte differentiation. A later analysis of Rspo1-/- mice [21] confirmed that an absence of R-spondin1 at the gonadal differentiation stage leads to partial sex-reversed phenotypes. Similar phenomena are also seen in Wnt4-/- mice, probably because of the action of R-spondin1 upstream of Wnt4 [22]. In summary, the Wnt4/R-spondin1 axis is operational in bipotential gonads of XX individuals, driving ovarian development. In XY individuals, the HMG-box-containing transcription factor SRY induces transcription of the SOX9 gene, another member of this HMG box family. This transcription factor then activates the program for testis development. The activation of Wnt4/R-spondin1 in XX gonads not only drives ovarian differentiation, but also suppresses the fibroblast growth factor (FGF)9-stabilization of SOX9 production. In the absence of strong Wnt signaling, the resulting SOX9 production is sufficient to drive at least partial testis development (Figure 4) [23].
Overview of sex determination in mice. During mouse embryogenesis, bipotential gonads arise from the genital ridges by 10.5 days post-conception (dpc). In somatic cells of XY genital ridges, Sry expression (dark blue line at lower part of figure) starts at 10.5 dpc, reaches a peak at 11.5 dpc and then wanes by 12.5 dpc. A few hours later, Sox9 expression (light blue line at the lower part of the figure) is upregulated to induce differentiation of Sertoli cells. Sox9 expression peaks at 11.5 to 12.5 dpc, continues to be expressed postnatally and is supported by several positive-feedback loops (including fibroblast growth factor 9 (FGF9), prostaglandin D2 (PGD2) and SOX9 itself), and SOX9 subsequently activates many male-specific genes, including the gene encoding anti-Müllerian hormone (Amh). At 12.5 dpc, morphological differences between testis and ovary are evident. In the absence of SRY, genes such as Wnt4, Rspo1 and Foxl2 are expressed in a female-specific manner and induce ovarian development, as characterized by the expression of follistatin and many other ovary-specific genes. FOXL2, forkhead box L2; SOX9, SRY box containing gene 9; SRY, sex-determining region on the chromosome Y. This figure is adapted with permission from [23].
R-spondin2: necessary for development of limbs, lungs and hair follicles
Limb buds in the early embryo show production of R-spondin2 and 3, while lung buds exclusively produce R-spondin2. The matching requirement of R-spondin2 for lung and limb development was unveiled in a mouse insertion mutant, termed 'footless' (Rspo2Tg/Tg), and in animals homozygous for a targeted inactivation of the Rspo2 gene [24–26]. The reported overlapping phenotypes in limb development are explained by an absence of functional R-spondin2 protein in the apical ectodermal ridge (AER). The resulting impaired Wnt signal leads to defective expression of the important AER maintenance factors FGF4 and FGF8. The lung defects seen in Rspo2Tg/Tg mice are associated with reduced branching of bronchioles. However, this developmental defect can be rescued by culturing ex vivo explants in R-spondin2-conditioned medium. Several additional observations imply involvement of canonical Wnt signaling. First, the effects seen are exacerbated if Rspo2Tg/Tg mice are intercrossed with Lrp6 mutant mice [27]. Second, mating of 'footless' mice with Wnt reporter mice detected a significant drop in Wnt activity at the distal tips of the branching epithelium. A corresponding reduction of expression of the Wnt target gene Irx3, required for branching, further explained the phenotype [28]. A study investigating the genes responsible for coat features in domestic dogs showed that R-spondin2 is also involved in the Wnt-driven development of the hair follicle [29]. An insertion event in the 3' UTR of this gene appeared to affect mRNA stability in dogs with 'furnishings' (extra fur around the mouth and eyes), and they show a threefold increase in transcript expression.
R-spondin3: placenta development
Development of the mouse placenta starts at E8.5 with a fusion between the chorion and allantois, two extra-embryonic tissues. Subsequently, chorioallantoic branching occurs, resulting in a functional labyrinth enabling exchange of gases, nutrients and waste products between embryonic and maternal blood vessels. An insufficient penetration of fetal blood vessels in the labyrinthine zone is seen in Wnt2 and Frzd5 knockout mice [30, 31]. In studies analyzing the signals for vasculogenesis and angiogenesis, the targeted disruption of the Rspo3 gene leads to severe vascular defects, especially in the placenta [32, 33]. R-spondin3 production is detectable at the chorioallantoic interface. The chorioallantoic fusion appears normal in the absence of R-spondin3. However, fetal blood vessels present in the labyrinth do not properly align with the maternal blood sinus, causing death of the animals around day E10. The same phenomena were reported for Wnt/Fzd mutated animals, implying that R-spondin acts upstream of Wnt.
R-spondin 4: nail development
Anonychia is a mild disorder, defined as the absence of fingernails and toenails. It is mostly seen in autosomal-dominant inherited syndromes. Isolated anonychia shows an autosomal-recessive inheritance. Recently, homozygous and compound heterozygous mutations in the gene encoding R-spondin4 were found in affected individuals [34–37]. The various genetic alterations all predicted severely impaired synthesis of functional R-spondin4 protein. A study monitoring the effects of these mutations, using R-spondin2 as a template, showed that at least two of these, C78Y and C113R, led to a defect in secretion [7]. The Q65R substitution did not affect secretion, but drastically reduced R-spondin2 activity. Involvement of the Wnt pathway in nail development was recently also deduced in patients that combine anonychia with brachydactyly (shortness of fingers and toes) [38]. The SOX9 transcription factor is essential for the normal development of the terminal phalanges, and associated 'Anlagen' like nails [39]. It must initially be induced, but silenced at later stages. Downregulation of SOX9, and subsequent inhibition of chondrogenesis, is mediated by canonical Wnt signaling. Mutual antagonistic activity between SOX9 production and canonical Wnt activity has been deduced from the analysis of gonad differentiation [23]. The phenomena seen in these anonychia/bracydactyly patients seem to be explained by an imbalance between these forces due to duplications of regulatory sequences 5' of the gene encoding SOX9 [38]. Several reports imply involvement of Wnt7a in this process of terminal phalange differentiation [40, 41]. A likely role for R-spondin, as also seen in gonad differentiation, has not been addressed so far.
Mechanisms
Receptors
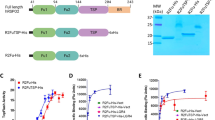
The identification of the membrane component mediating R-spondin signaling has proceeded with trial and error. Contradictory reports proposed that R-spondin bound to Fzds, LRPs, Kremen receptor and/or Wnts [42–44]. However, three recent reports [45–47] identified Lgr4, Lgr5 and Lgr6 as the receptors of the R-spondin protein family (Figure 3). Each of them can bind all four R-spondins in vitro [48]. RNA interference-mediated deletion of the endogenous Lgr4 in 293T cells resulted in effective removal of the R-spondin-mediated enhancement of Wnt signaling in these cells [48]. A specific rescue occurred by exogenously introducing Lgr4, Lgr5 and Lgr6 [48]. Recently, syndecan-4 was proposed as the receptor for R-spondin3 in the planar cell polarity pathway [14]. An earlier report had claimed a role for R-spondin3 in canonical Wnt signaling [33]. With the current knowledge that the Lgr proteins act as receptors for the furin domains in R-spondins, the R-spondin3/syndecan-4 interaction most likely involves the TSR-1 domain. The Lgr proteins appear to be physically associated with the Fzd/LRP complex. The R-spondin component in Wnt signaling may therefore be mediated by the LRP5/6 Frizzled co-receptors. Of note, R-spondin1 enhances LRP6 phosphorylation [43].
The R-spondin/Lgr axis
A variety of genetic studies were conducted to determine the locations of expression and the physiological roles of Lgr4, Lgr5 and Lgr6 during embryogenesis. Those experiments actually monitored locations of R-spondin-amplified Wnt signaling. Analysis of the Lgr4 receptor, using a variety of genetic models, detected strong expression in cartilage, kidney, adrenal gland, reproductive tracts, the eyes and nervous system cells. The associated phenotypes are diverse and extend over tissues derived from all germinal layers [49–60]. Lgr5, likewise, shows a dynamic and complex expression pattern during embryogenesis [61, 62]. Rare Lgr5+cells are seen in the adult eye, mammary gland, intestinal tract, skin and the reproductive organs [63–65]. Developmental Lgr6 expression is most prominent in the hair placodes, rare cells in the brain, the mammary gland, and the airways of the lungs [62, 66].
Importantly, R-spondin/Lgr signaling also operates in several self-renewing adult tissues. The best studied example is the mucosa of the digestive tract, consisting of a stomach, small intestine (Figure 4) and the colon. The first indication that Rspondin1 can act as a growth factor for intestinal epithelial cells, by agonizing canonical Wnt signaling, was found in a transgenic mouse model in which Rspo1 was under the control of the immunoglobulin locus [67]. The essential requirement of Wnt signaling for the physiological maintenance of the stem cells in these tissues was previously shown in a Tcf4 (T-cell transcription factor 4) ablation experiment and a DKK1 transgenic model [68, 69]. The involvement of R-spondin was indirectly uncovered by a Lgr5-driven GFP (green fluorescent protein) knock-in mouse model and a Lgr5/LacZ-driven lineage tracing model [70]. These studies identified the Wnt-target gene LGR5 as a unique marker for the stem cells feeding these tissues [63]. Lgr4 is co-expressed in stem cells, and in addition it is detectable in all other progenitor cells. Isolated Lgr5+ intestinal stem cells can be maintained in vitro and induced to continuously propagate organoids [64, 71]. Notably, addition of R-spondin and Wnt constitutes an absolute requirement for these cultures. In mouse intestinal organoids, deletion of these Lgr receptors phenocopies withdrawal of R-spondin. Moreover, absence of Lgr receptors can be compensated by providing cells with the strongest possible Wnt signals. Canonical Wnt/R-spondin signaling is, moreover, implied in establishing the hair follicle cycle and remains crucial for stem cell activity throughout life [63, 65, 72–76].
Frontiers
Now that the Lgr proteins have been established as the receptors for R-spondins, directly funneling into the canonical Wnt pathway through Frizzled and Lrp, several gaps in our knowledge of R-spondins can be addressed. For example, crystallographic studies of R-spondin and R-spondin/Lgr complexes are required to understand how the interaction-induced information is transferred to the Wnt/Fzd/Lrp signaling unit. The increase in Lrp6 phosphorylation, associated with the presence of R-spondin in the Wnt receptor complex, needs to be understood in greater detail. In particular, the dedicated kinase and the specific substrate for this reaction among the five conserved PPPSPXS motifs in Lrp need to be identified [77]. Another challenge is to determine the exact composition of the operating Wnt receptor complexes. A key question here is whether the Lgr/R-spondin module constitutes a standard feature of canonical Wnt signals in vertebrates or an accessory option. It will also be important to determine to what extent preference in the cooperation between the various components in vivo plays a role. Another challenge will be to find out the specificity and site of synthesis of the R-spondins that control particular biological processes. Because the R-spondins are also stimulators of stem cell development, it is anticipated that future research will use R-spondin-based strategies for the manipulation of adult stem cells in regenerative medicine settings. The first findings, supporting the therapeutic potential of in vivo administered R-spondins, were found in a mouse model for inflammatory bowel diseases similar to Crohn's disease [78]. Future attempts to replenish disease-damaged epithelial tissue along the gastrointestinal tract, including Barrett's disease, will likely exploit R-spondin-mediated ex vivo expansion of the epithelia of interest [64, 71, 79].
Abbreviations
- AER:
-
apical ectodermal ridge
- DKK1:
-
Dickkopf-1
- E:
-
embryonic day
- EGF:
-
epidermal growth factor
- Fzd:
-
Frizzled
- GAG:
-
glycosaminoglycan
- HGF:
-
hepatocyte growth factor
- Lgr:
-
leucine-rich repeat-containing G-protein-coupled receptor
- Lrp:
-
lipoprotein-receptor-related protein
- PPK:
-
palmoplantar hyperkeratosis
- SCC:
-
squamous cell carcinoma
- TSP:
-
thrombospondin protein
- TSR-1:
-
thrombospondin type 1 repeat.
References
Baenziger NL, Brodie GN, Majerus PW: A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci USA. 1971, 68: 240-243. 10.1073/pnas.68.1.240.
Lawler J, Hynes RO: The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986, 103: 1635-1648. 10.1083/jcb.103.5.1635.
Kamata T, Katsube K, Michikawa M, Yamada M, Takada S, Mizusawa H: R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta. 2004, 1676: 51-62.
Chen JZ, Wang S, Tang R, Yang QS, Zhao E, Chao Y, Ying K, Xie Y, Mao YM: Cloning and identification of a cDNA that encodes a novel human protein with thrombospondin type I repeat domain, hPWTSR. Mol Biol Rep. 2002, 29: 287-292. 10.1023/A:1020479301379.
Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W: R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004, 7: 525-534. 10.1016/j.devcel.2004.07.019.
Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD: R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006, 5: 23-26. 10.4161/cc.5.1.2305.
Li SJ, Yen TY, Endo Y, Klauzinska M, Baljinnyam B, Macher B, Callahan R, Rubin JS: Loss-of-function point mutations and two-furin domain derivatives provide insights about R-spondin2 structure and function. Cell Signal. 2009, 21: 916-925. 10.1016/j.cellsig.2009.02.001.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S: Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002, 110: 775-787. 10.1016/S0092-8674(02)00963-7.
Garrett TP, McKern NM, Lou M, Frenkel MJ, Bentley JD, Lovrecz GO, Elleman TC, Cosgrove LJ, Ward CW: Crystal structure of the first three domains of the type-1 insulin-like growth factor receptor. Nature. 1998, 394: 395-399. 10.1038/28668.
Guo NH, Krutzsch HC, Negre E, Vogel T, Blake DA, Roberts DD: Heparin- and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion. Proc Natl Acad Sci USA. 1992, 89: 3040-3044. 10.1073/pnas.89.7.3040.
Guo NH, Krutzsch HC, Negre E, Zabrenetzky VS, Roberts DD: Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J Biol Chem. 1992, 267: 19349-19355.
Tan K, Duquette M, Liu JH, Dong Y, Zhang R, Joachimiak A, Lawler J, Wang JH: Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol. 2002, 159: 373-382. 10.1083/jcb.200206062.
Ayadi L: Molecular modelling of the TSR domain of R-spondin 4. Bioinformation. 2008, 3: 119-123. 10.6026/97320630003119.
Ohkawara B, Glinka A, Niehrs C: Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 20: 303-314.
Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A: R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008, 19: 2588-2596. 10.1091/mbc.E08-02-0187.
Cossu G, Borello U: Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999, 18: 6867-6872. 10.1093/emboj/18.24.6867.
Borycki AG, Emerson CP: Study of skeletal myogenesis in cultures of unsegmented paraxial mesoderm. Methods Mol Biol. 2000, 137: 351-357.
Lowther W, Wiley K, Smith GH, Callahan R: A new common integration site, Int7, for the mouse mammary tumor virus in mouse mammary tumors identifies a gene whose product has furin-like and thrombospondin-like sequences. J Virol. 2005, 79: 10093-10096. 10.1128/JVI.79.15.10093-10096.2005.
Nusse R: Wnt signaling in disease and in development. Cell Res. 2005, 15: 28-32. 10.1038/sj.cr.7290260.
Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G: R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006, 38: 1304-1309. 10.1038/ng1907.
Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M: R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008, 17: 1278-1291. 10.1093/hmg/ddn036.
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP: Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999, 397: 405-409. 10.1038/17068.
Kashimada K, Koopman P: Sry: the master switch in mammalian sex determination. Development. 2010, 137: 3921-3930. 10.1242/dev.048983.
Nam JS, Turcotte TJ, Yoon JK: Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns. 2007, 7: 306-312. 10.1016/j.modgep.2006.08.006.
Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA: R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008, 135: 1049-1058. 10.1242/dev.013359.
Nam JS, Park E, Turcotte TJ, Palencia S, Zhan X, Lee J, Yun K, Funk WD, Yoon JK: Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol. 2007, 311: 124-135. 10.1016/j.ydbio.2007.08.023.
DasGupta R, Fuchs E: Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999, 126: 4557-4568.
van Tuyl M, Liu J, Groenman F, Ridsdale R, Han RN, Venkatesh V, Tibboel D, Post M: Iroquois genes influence proximo-distal morphogenesis during rat lung development. Am J Physiol Lung Cell Mol Physiol. 2006, 290: L777-L789. 10.1152/ajplung.00293.2005.
Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, Vonholdt BM, Rhue A, Boyko A, Byers A, Wong A, Mosher DS, Elkahloun AG, Spady TC, André C, Lark KG, Cargill M, Bustamante CD, Wayne RK, Ostrander EA: Coat variation in the domestic dog is governed by variants in three genes. Science. 2009, 326: 150-153. 10.1126/science.1177808.
Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ: Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996, 122: 3343-3353.
Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM: Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001, 128: 25-33.
Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H: R-spondin3 is required for mouse placental development. Dev Biol. 2007, 301: 218-226. 10.1016/j.ydbio.2006.08.018.
Kazanskaya O, Ohkawara B, Heroult M, Wu W, Maltry N, Augustin HG, Niehrs C: The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development. 2008, 135: 3655-3664. 10.1242/dev.027284.
Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, Poblete-Gutierrez P, van Steensel M, Seelow D, Nurnberg G, Schild HH, Nürnberg P, Reis A, Frank J, Zerres K: Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet. 2006, 79: 1105-1109. 10.1086/509789.
Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP: The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet. 2006, 38: 1245-1247. 10.1038/ng1883.
Bruchle NO, Frank J, Frank V, Senderek J, Akar A, Koc E, Rigopoulos D, van Steensel M, Zerres K, Bergmann C: RSPO4 is the major gene in autosomal-recessive anonychia and mutations cluster in the furin-like cysteine-rich domains of the Wnt signaling ligand R-spondin 4. J Invest Dermatol. 2008, 128: 791-796. 10.1038/sj.jid.5701088.
Ishii Y, Wajid M, Bazzi H, Fantauzzo KA, Barber AG, Blaydon DC, Nam JS, Yoon JK, Kelsell DP, Christiano AM: Mutations in R-spondin 4 (RSPO4) underlie inherited anonychia. J Invest Dermatol. 2008, 128: 867-870. 10.1038/sj.jid.5701078.
Kurth I, Klopocki E, Stricker S, van Oosterwijk J, Vanek S, Altmann J, Santos HG, van Harssel JJ, de Ravel T, Wilkie AO, Gal A, Mundlos S: Duplications of noncoding elements 5' of SOX9 are associated with brachydactyly-anonychia. Nat Genet. 2009, 41: 862-863. 10.1038/ng0809-862.
Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B: The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16: 2813-2828. 10.1101/gad.1017802.
Parr BA, McMahon AP: Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995, 374: 350-353. 10.1038/374350a0.
Adamska M, Billi AC, Cheek S, Meisler MH: Genetic interaction between Wnt7a and Lrp6 during patterning of dorsal and posterior structures of the mouse limb. Dev Dyn. 2005, 233: 368-372. 10.1002/dvdy.20437.
Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK: Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006, 281: 13247-13257. 10.1074/jbc.M508324200.
Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X: R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem. 2007, 282: 15903-15911. 10.1074/jbc.M701927200.
Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A: R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA. 2007, 104: 14700-14705. 10.1073/pnas.0702305104.
de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H: Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011, 476: 293-297. 10.1038/nature10337.
Carmon KS, Gong X, Lin Q, Thomas A, Liu Q: R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2011, 108: 11452-11457. 10.1073/pnas.1106083108.
Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C: LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011, 12: 1055-1061. 10.1038/embor.2011.175.
de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H: Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011, 476: 293-297. 10.1038/nature10337.
Mazerbourg S, Bouley DM, Sudo S, Klein CA, Zhang JV, Kawamura K, Goodrich LV, Rayburn H, Tessier-Lavigne M, Hsueh AJ: Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol. 2004, 18: 2241-2254. 10.1210/me.2004-0133.
Van Schoore G, Mendive F, Pochet R, Vassart G: Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol. 2005, 124: 35-50. 10.1007/s00418-005-0002-3.
Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G: Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol. 2006, 290: 421-434. 10.1016/j.ydbio.2005.11.043.
Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K: LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod. 2007, 76: 303-313. 10.1095/biolreprod.106.054619.
Kato S, Matsubara M, Matsuo T, Mohri Y, Kazama I, Hatano R, Umezawa A, Nishimori K: Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp Nephrol. 2006, 104: e63-75. 10.1159/000093999.
Kato S, Mohri Y, Matsuo T, Ogawa E, Umezawa A, Okuyama R, Nishimori K: Eye-open at birth phenotype with reduced keratinocyte motility in LGR4 null mice. FEBS Lett. 2007, 581: 4685-4690. 10.1016/j.febslet.2007.08.064.
Mohri Y, Oyama K, Akamatsu A, Kato S, Nishimori K: Lgr4-deficient mice showed premature differentiation of ureteric bud with reduced expression of Wnt effector Lef1 and Gata3. Dev Dyn. 2011, 240: 1626-1634. 10.1002/dvdy.22651.
Jin C, Yin F, Lin M, Li H, Wang Z, Weng J, Liu M, Da Dong X, Qu J, Tu L: GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Invest Ophthalmol Vis Sci. 2008, 49: 4245-4253. 10.1167/iovs.08-1860.
Oyama K, Mohri Y, Sone M, Nawa A, Nishimori K: Conditional knockout of Lgr4 leads to impaired ductal elongation and branching morphogenesis in mouse mammary glands. Sex Dev. 2011, 5: 205-212. 10.1159/000329476.
Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, Tu L, Ai D, Li D, Wang J, Martin JF, Amendt BA, Liu M: Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci USA. 2008, 105: 6081-6086. 10.1073/pnas.0708257105.
Song H, Luo J, Luo W, Weng J, Wang Z, Li B, Li D, Liu M: Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. J Biol Chem. 2008, 283: 36687-36697. 10.1074/jbc.M800721200.
Yamashita R, Takegawa Y, Sakumoto M, Nakahara M, Kawazu H, Hoshii T, Araki K, Yokouchi Y, Yamamura K: Defective development of the gall bladder and cystic duct in Lgr4- hypomorphic mice. Dev Dyn. 2009, 238: 993-1000. 10.1002/dvdy.21900.
Morita H, Mazerbourg S, Bouley DM, Luo CW, Kawamura K, Kuwabara Y, Baribault H, Tian H, Hsueh AJ: Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004, 24: 9736-9743. 10.1128/MCB.24.22.9736-9743.2004.
Leushacke M, Barker N: Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene.
Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgard R, Clevers H: Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb Symp Quant Biol. 2008, 73: 351-356. 10.1101/sqb.2008.72.003.
Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H: Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010, 6: 25-36. 10.1016/j.stem.2009.11.013.
Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R: Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008, 40: 1291-1299. 10.1038/ng.239.
Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G: Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004, 22: 411-417. 10.1038/nbt950.
Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K: Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005, 309: 1256-1259. 10.1126/science.1112521.
Pinto D, Clevers H: Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005, 306: 357-363. 10.1016/j.yexcr.2005.02.022.
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H: Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998, 19: 379-383. 10.1038/1270.
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H: The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002, 111: 241-250. 10.1016/S0092-8674(02)01014-0.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H: Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009, 459: 262-265. 10.1038/nature07935.
van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R: Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994, 8: 2691-2703. 10.1101/gad.8.22.2691.
Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E: Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005, 19: 1596-1611. 10.1101/gad.1324905.
Alonso L, Fuchs E: Stem cells of the skin epithelium. Proc Natl Acad Sci USA. 2003, 100 (Suppl 1): 11830-11835.
Alonso L, Fuchs E: Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003, 17: 1189-1200. 10.1101/gad.1086903.
Alonso LC, Rosenfield RL: Molecular genetic and endocrine mechanisms of hair growth. Horm Res. 2003, 60: 1-13.
Macdonald BT, Semenov MV, Huang H, He X: Dissecting molecular differences between Wnt Coreceptors LRP5 and LRP6. PLoS One. 2011, 6: e23537-10.1371/journal.pone.0023537.
Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, Abo A, Funk WD: R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007, 132: 1331-1343. 10.1053/j.gastro.2007.02.001.
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H: Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011, 141: 1762-1772. 10.1053/j.gastro.2011.07.050.
Katoh K, Kuma K, Toh H, Miyata T: MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33: 511-518. 10.1093/nar/gki198.
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD: Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31: 3497-3500. 10.1093/nar/gkg500.
Letunic I, Bork P: Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007, 23: 127-128. 10.1093/bioinformatics/btl529.
Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM: Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997, 88: 747-756. 10.1016/S0092-8674(00)81921-2.
Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J: A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999, 398: 431-436. 10.1038/18899.
Jones SE, Jomary C: Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002, 24: 811-820. 10.1002/bies.10136.
Kawano Y, Kypta R: Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003, 116: 2627-2634. 10.1242/jcs.00623.
Niehrs C: Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006, 25: 7469-7481. 10.1038/sj.onc.1210054.
Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R: Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003, 130: 4295-4305. 10.1242/dev.00674.
Carmon KS, Gong X, Lin Q, Thomas A, Liu Q: R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2011, 108: 11452-11457. 10.1073/pnas.1106083108.
Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C: LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011, 12: 1055-1061. 10.1038/embor.2011.175.
Acknowledgements
We would like to thank Johan van Es for critically reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
de Lau, W.B., Snel, B. & Clevers, H.C. The R-spondin protein family. Genome Biol 13, 242 (2012). https://doi.org/10.1186/gb-2012-13-3-242
Published:
DOI: https://doi.org/10.1186/gb-2012-13-3-242