Abstract
In order to eradicate an infecting organism, it is necessary to achieve or maintain concentrations of an antibiotic in vivo that exceed the minimum inhibitory concentration (MIC) for the organism. Duration of exposure, or time above MIC, was recognized as being important for β-lactam antibiotics more than 60 years ago. Continuous infusion regimens are associated with higher clinical response rates, improvement in surrogate markers of outcome, and lower cost of therapy compared with intermittent infusion regimens, because the MIC can be exceeded for an entire dosing interval. However, for carbapenem antibiotics, it appears that the MIC must only be exceeded for 40% of the dosing interval for bactericidal activity in vivo. Therefore, a promising strategy to optimize carbapenem use is to administer the same dose at the same frequency of administration but to extend the infusion time. Extended infusion regimens take full advantage of a drug's exposure potential within the context of in vivo potency without altering the dose or dosing schedule and with no increase in toxicity or cost. Administering higher doses by extended infusion allows one to manage organisms with high MICs. Optimizing the pharmacokinetics/pharmacodynamics of an antibiotic allows one to 'make good drugs better'.
Similar content being viewed by others
Introduction
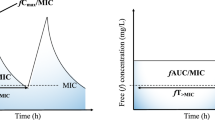
Utilizing pharmacokinetic/pharmacodynamic principles to optimize antibiotic administration can improve outcomes. The in vitro potency of antibiotics is measured as the minimum inhibitory concentration (MIC). In contrast, the in vivo potency of an antibiotic refers to its ability to achieve and maintain concentrations in the body that are necessary to eradicate infecting organisms. The goal of antibacterial therapy is generally to maximize in vivo concentrations or the duration of exposure, depending on the class of antibiotic (Figure 1). Duration of exposure was recognized as being important for β-lactam antibiotics more than 60 years ago, when it was noted that the most effective way to treat an infection with penicillin was to maintain inhibitory concentrations in the body throughout treatment [1–3]. Intramuscular injections of penicillin were recognized as producing high peak concentrations that could not be maintained, whereas continuous intravenous infusions produced constant and sustained concentrations of penicillin. However, continuous intravenous infusions of antibiotics fell out of favor as a treatment strategy. Contributing to the shift in antibiotic administration regimens were a more plentiful drug supply, the convenience of intermittent dosing regimens, and a general population of very susceptible organisms.
In vivo potency as a pharmacodynamic parameter for antibiotic therapy. Maintaining antibiotic concentrations above the minimum inhibitory concentration (MIC) is necessary to achieve in vivo potency and is dependent on the pharmacokinetics of the drug. AUC, area under the concentration-time curve; Cmax, maximum plasma concentration; T > MIC, time spent above the MIC.
Although pharmacokinetic/pharmacodynamic principles have not changed over the past 60 years, antibiotic administration strategies are in flux, as the prevalence of resistance increases and as effective antibiotics become less plentiful, particularly for Gram-negative organisms. For β-lactams, old philosophies are being revisited to increase the duration of the dosing interval that antibiotic concentrations exceed the MIC. The various methods to achieve this goal include administering doses more frequently and administering higher doses. However, dose escalation strategies usually are not cost-effective. Administering two times the dose neither doubles the time above the MIC nor optimizes exposure to the antibiotic, but it does increase the cost of therapy (Figure 2).
Dose escalation as a strategy to improve antibiotic efficacy. Dose escalation either by administering higher doses or administering a dose more frequently achieves only small increments in efficacy because there is little increase in the time during which the drug concentration is above the minimum inhibitory concentration (MIC).
Infusion strategies
Continuous infusion
During the 1940s, bacterial endocarditis was effectively treated with penicillin administered as a continuous infusion [4]. There is a resurgence of interest in using a continuous infusion of an antibiotic dose administered via pump over 24 hours after a loading dose. The goal of continuous infusion therapy is to maintain drug concentrations above the MIC for the entire 24-hour interval by selecting the appropriate dose. Several studies have demonstrated that continuous infusion of β-lactam antibiotics is an effective dosing strategy.
The efficacies of administering carbenicillin plus tobramycin by continuous infusion, carbenicillin plus cefamandole by continuous infusion, and carbenicillin plus cefamandole by intermittent infusion were compared in a randomized study of 490 febrile episodes (235 documented infections) in neutropenic cancer patients [5]. Carbenicillin was administered over 2 hours every 4 hours, whereas tobramycin was administered as a loading dose followed by continuous infusion, and cefamandole was administered either as a continuous infusion after a loading dose or as an intermittent infusion every 6 hours. Overall, the carbenicillin plus cefamandole regimens were more effective than carbenicillin plus tobramycin, with cure rates of 65% for the carbenicillin plus cefamandole continuous infusion, 57% for the carbenicillin plus cefamandole intermittent infusion, and 54% for carbenicillin plus tobramycin. In profoundly neutropenic patients (initial neutrophil count <100/mm3 and no increase during the infection), the continuous infusion cefamandole regimen was three times more effective than the intermittent regimen (65% versus 21%; P = 0.03). Although these agents are no longer drugs of choice for treating these infections, the study does support the pharmacokinetic/pharmacodynamic principle of maintaining drug concentration above the MIC throughout the dosing interval to improve efficacy.
Contemporary studies provide additional support for this pharmacokinetic/pharmacodynamic principle as a rationale for designing antibiotic regimens. An open-label study compared continuous versus intermittent administration of piperacillin plus tazobactam in 98 patients at a large community teaching hospital [2]. It found that clinical (94% versus 82%) and microbiologic (89% versus 73%) success rates were slightly higher with the continuous infusion regimen, although the difference was not statistically significant. The continuous infusion regimen, however, was associated with a significantly shorter time to temperature normalization (1.2 ± 0.8 days versus 2.4 ± 1.5 days; P = 0.012) and a trend toward faster normalization of the white blood cell count (2.8 ± 2.4 days versus 3.9 ± 2.2 days; P = 0.065). In addition, the drug acquisition cost per patient was less with the continuous infusion regimen ($291 ± 218 versus $371 ± 325; P = 0.054), and all costs associated with antibiotic use were significantly lower with the continuous infusion regimen ($399 ± 407 versus $523 ± 526; P = 0.028).
The results of a randomized, multicenter, open-label study comparing continuous infusion piperacillin/tazobactam (12 g/1.5 g administered continuously over 24 hours) with the standard intermittent infusion (3 g/0.375 g administered over 30 minutes intermittently every 6 hours) in patients with complicated intra-abdominal infections were recently reported [6]. Patient demographics were similar among groups, as was the percentage of those clinically cured or improved (86.4% of those treated with continuous infusion versus 88.4% for intermittent infusion; P = 0.817). Bacteriologic success was not statistically different between the two treatment groups (P = 0.597). Moreover, drug-related adverse events were similar, despite the differing administration techniques utilized.
Additional studies conducted at our institute [7, 8] have also illustrated the clinical and microbiologic attributes of continuous infusion regimens in the management of both community-acquired and nosocomial pneumonia.
A recently published trial [9] reported on the clinical utility of a β-lactam administered by continuous infusion. In this trial, a lower daily dose (2 g) of cefotaxime given by continuous infusion to patients with chronic obstructive pulmonary disease produced clinical and microbiological effects similar to those with a higher total daily dose of 1 g administered three times daily. Moreover, the continuous infusion regimen resulted in pharmacodynamic (time above MIC) optimization of cefotaxime therapy. These data further suggest that continuous infusion yields similar clinical outcomes in a cost-effective manner – an observation that has been noted in other studies utilizing this therapeutic approach [2, 8].
Studies also have demonstrated the efficacy of carbapenem antibiotics administered by continuous infusion in critically ill patients and in patients with resistant pathogens. In a small, prospective, crossover study conducted in critically ill patients [10], pharmacokinetic parameters for meropenem differed significantly between intermittent and continuous infusion. Although the area under the concentration-time curve was significantly lower after continuous infusion, the mean steady state concentration was above the MIC for common bacterial strains for the entire infusion interval. A retrospective study of patients with ventilator-associated pneumonia [11] found that the clinical cure rate was significantly higher after continuous infusion of meropenem than after intermittent infusion (90.5% versus 59.6%; P < 0.001). Because meropenem is stable for only 4 to 6 hours at room temperature, the drug supply was changed every 6 hours to achieve a continuous infusion. In a randomized, open-label study conducted in seven adults with cystic fibrosis [12] the investigators evaluated administration of meropenem (3 g/24 hours or 6 g/24 hours) over 12 hours by a continuous ambulatory drug-delivery infusion pump stored in a cold pouch between two freezer packs. Drug stability was maintained over 12 and 24 hours, and the pharmacokinetic parameters were dose dependent, with mean steady-state drug concentrations of 8.3 μg/ml and 18.5 μg/ml for the 3 g and 6 g doses at 12 hours. These serum concentrations were twofold to fourfold greater than the MICs of organisms considered susceptible (MIC ≤ 4 μg/ml) or intermediately resistant (MIC of 8 μg/ml) to meropenem.
Continuous infusion regimens have therefore been associated with enhanced clinical response rates, improvement in surrogate markers of outcome, and a lower cost of therapy compared with intermittent infusion regimens. Various strategies may be used successfully to overcome drug stability issues.
Extended infusion
Continuous infusion of β-lactam antibiotics has the potential to maintain drug concentrations above the MIC over a 24-hour interval. However, it is not necessary to exceed the MIC for an entire dosing interval. For carbapenem antibiotics the required time above the MIC appears to be 20% of the dosing interval for bacteriostatic and 40% of the dosing interval for bactericidal activity in vivo [13–16]. Another strategy to optimize antibiotic use is to administer the same dose at the same frequency of administration but to extend the infusion time. This strategy overcomes the limited stability of carbapenem antibiotics at room temperature and increases the time above the MIC. Simulations predict that extended infusions can achieve maximal kill rates for organisms with high MICs [15, 17].
Pharmacokinetic/pharmacodynamic studies provide a rationale for extended infusion regimens. An extended infusion of carbapenem has a pharmacokinetic profile similar to that of a 0.5-hour infusion of the same dose, but it improves the pharmacodynamic profile (time above MIC; Figure 3) [18]. In a crossover study conducted to assess the pharmacodynamic profile of meropenem in healthy volunteers [18], the time above MIC (at the susceptibility breakpoint of 4 μg/ml) could be increased from 30% to 43% for the 0.5 g dose and from 58% to 73% for the 2 g dose when the infusion was extended from 0.5 to 3 hours. Surveillance data show that meropenem MICs are 4 μg/ml or less for most isolates of Pseudomonas aeruginosa [19, 20]. Although the time above MIC for more resistant strains (such as those with MICs of 8 or 16 μg/ml) is considerably reduced when conventional doses and/or administration techniques are used, drug concentrations can be maintained above these elevated MICs by both increasing the dose and using an extended infusion. This of course requires use of a carbapenem that can be safely dosed at these levels without fear of central nervous system toxicity. A patient-based simulation for the carbapenems demonstrated that utilization of extended infusions increased the probability of achieving bactericidal exposures for multidrug-resistant P. aeruginosa (Table 1) [17]. Moreover, the utilization of higher doses of meropenem administered as extended infusions further increased the probability of achieving bactericidal exposures for this resistant population of organisms. These data suggest that resistant organisms may successfully be treated by optimizing dosing regimens based on pharmacokinetic/pharmacodynamic parameters.
The extended infusion dosing strategy has resulted in good outcomes in the clinical setting [21, 22]. Sufficient antibiotic doses administered as an extended infusion with the same frequency as recommended for shorter infusions (for example, meropenem 2 g administered over 3 hours every 8 hours) not only maintained serum concentrations but also prolonged exposure at the site of infection (for example, lung) [21, 23]. At one institution, a patient-based simulation of piperacillin-tazobactam exposures versus the anticipated MIC profile was used to identify a dosing regimen of this compound that would optimize its pharmacodynamic profile (> 50% of a dosing interval spent above MIC) [22]. An institution-wide automatic substitution program was implemented based on the simulation results (piperacillin-tazobactam 3.375 g infused over 4 hours every 8 hours replaced piperacillin-tazobactam 3.375 g infused over 30 minutes every 4 or 6 hours), and outcomes for the two regimens were compared in a retrospective cohort study of critically ill patients with infections caused by P. aeruginosa. In patients at greatest mortality risk (Acute Physiology and Chronic Health Evaluation II score ≥ 17), the extended infusion regimen significantly reduced 14-day mortality compared with the intermittent infusion regimen (12.2% versus 31.6%; P = 0.04) as well as median length of stay (21 days versus 38 days; P = 0.02). In addition to improved clinical outcomes, the extended infusion regimen, which represents a 25% to 50% reduction in total daily piperacillin-tazobactam dose, resulted in an annual reduction in drug acquisition costs of $68,750 to $135,750.
An additional factor to consider in selecting extended or continuous infusion is that the total costs of intravenous antibiotic administration are not just the costs of the drugs themselves but also comprise costs resulting from the time expended by medical and nursing staff, costs of disposable materials, and overhead costs. These nonacquisition costs of antibiotic administration should be taken into account. Infusion with syringe pumps and volumetric pumps has been shown to be the most cost-effective strategy for administering antibiotics [24].
Extended infusion regimens therefore take full advantage of a drug's exposure potential within the context of in vivo potency without altering the dose or dosing schedule and with no increase in toxicity. Administering higher doses allows one to treat patients with severe illness and/or organisms with high MICs. Importantly, extended infusion regimens are a cost-effective strategy.
Conclusion
Optimizing the pharmacokinetics/pharmacodynamics of an antibiotic allows one to 'make good drugs better'. Extended infusion regimens improve the effectiveness of therapy with few limitations and provide a cost-effective strategy.
Abbreviations
- MIC :
-
MIC = minimum inhibitory concentration.
References
Chain E, Florey HW, Gardner AD, Heatley NG, Jennings MA, Orr-Ewing J, Sanders AG: Penicillin as a chemotherapeutic agent. Lancet 1940, 1: 226-228. 10.1016/S0140-6736(01)08728-1
Grant EM, Kuti JL, Nicolau DP, Nightingale C, Quintiliani R: Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 2002, 22: 471-483. 10.1592/phco.22.7.471.33665
Loewe AB: The combined use of penicillin and heparin in the treatment of subacute bacterial endocarditis. CMAJ 1945, 52: 1-14.
Priest WS, Smith JM, McGee CJ: The effect of anticoagulants on the penicillin therapy and the pathologic lesion of subacute bacterial endocarditis. N Engl J Med 1946, 235: 699-706.
Bodey GP, Ketchel SJ, Rodriguez V: A randomized study of carbenicillin plus cefandamole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med 1979, 67: 608-616. 10.1016/0002-9343(79)90242-0
Lau W, Mercer D, Itani K, Nicolau D, Kuti J, Mansfield D, Dana A: A randomized, open-label, comparative study of piperacillin/tazobactam administered by continuous infusion vs. intermittent infusion for the treatment of hospitalized patients with complicated intra-abdominal infection. Antimicrob Agents Chemother 2006, 50: 3556-3561. 10.1128/AAC.00329-06
Ambrose PG, Quintiliani R, Nightingale CH, Nicolau DP: Continuous vs. intermittent infusion of cefuroxime for the therapy of community-acquired pneumonia. Infect Dis Clin Practice 1998, 7: 463-470. 10.1097/00019048-199812000-00007
Nicolau DP, McNabb JC, Lacy MK, Quintiliani R, Nightingale CH: Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int J Antimicrob Agent 2001, 17: 497-504. 10.1016/S0924-8579(01)00329-6
van Zanten AR, Oudijk M, Nohlmans-Paulssen MK, van der Meer YG, Girbes AR, Polderman KH: Continuous vs. intermittent cefotaxime administration in patients with chronic obstructive pulmonary disease and respiratory tract infections: pharmacokinetics/pharmacodynamics, bacterial susceptibility and clinical efficacy. Br J Clin Pharmacol 2007, 63: 100-109. 10.1111/j.1365-2125.2006.02730.x
Thalhammer F, Traunmüller F, El Menyawi I, Frass M, Hollenstein UM, Locker GJ, Stoiser B, Staudinger T, Thalhammer-Scherrer R, Burgmann H: Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother 1999, 43: 523-527. 10.1093/jac/43.4.523
Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML: Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to Gram-negative bacilli. Ann Pharmacother 2006, 40: 219-223. 10.1345/aph.1G467
Kuti JL, Nightingale CH, Knauft RF, Nicolau DP: Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin Ther 2004, 26: 493-501. 10.1016/S0149-2918(04)90051-3
Craig WA: The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis 1997,24(suppl 2):S266-S275.
DeRyke CA, Banevicius MA, Fan HW, Nicolau DP: Evaluation of the bactericidal activity of meropenem and ertapenem against extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob Agents Chemother 2007, 51: 1481-1486. 10.1128/AAC.00752-06
Drusano GL: Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis 2003,36(suppl 1):S42-S50. 10.1086/344653
Ong CT, Tessier PR, Li C, Nightingale CH, Nicolau DP: Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diag Microbiol Infect Dis 2007, 57: 153-161. 10.1016/j.diagmicrobio.2006.06.014
Ludwig E, Konkoly-Thege M, Kuti JL, Nicolau DP: Optimising antibiotic dosing regimens based on pharmacodynamic target attainment against Pseudomonas aeruginosa collected in Hungarian hospitals. Int J Antimicrob Agents 2006, 28: 433-438. 10.1016/j.ijantimicag.2006.07.014
Dandekar PK, Maglio D, Sutherland CA, Nightingale CH, Nicolau DP: Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy 2003, 23: 988-991. 10.1592/phco.23.8.988.32878
Jones RN, Stilwell M, Sader H, Fritsche T: Uniformly enhanced activity of doripenem compared to other carbapenems (imipenem, meropenem) when testing P. aeruginosa isolates: results from three continents. Int J Infect Dis 2006,10(suppl 1):S127-S128.
Santos Filho L, Eagye KJ, Kuti JL, Nicolau DP: Addressing resistance evolution in Pseudomonas aeruginosa using pharmacodynamic modelling: application to meropenem dosage and combination therapy. Clin Microbiol Infect 2007, 13: 579-585. 10.1111/j.1469-0691.2007.01693.x
Kuti JL, Moss KM, Nicolau DP, Knauft RF: Empiric treatment of multi-drug resistant Burkholderia cepacia lung exacerbation in a patient with cystic fibrosis: application of pharmacodynamic concepts to meropenem therapy. Pharmacotherapy 2004, 24: 1641-1645. 10.1592/phco.24.16.1641.50960
Lodise TP, Lomaestro B, Drusano GL: Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 2007, 44: 357-363. 10.1086/510590
Drusano GL, Sorgel F, Ma L, Kinzig M, Mason B, Melnick D: Pharmacokinetics (PK) and penetration of meropenem (M) into epithelial lining fluid (ELF) in patients with ventilator-associated pneumonia (VAP) [abstract A-15]. In Program and Abstracts of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 30 October to 2 November 2004; Washington, DC. Washington, DC: American Society for Microbiology; 2004.
van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH: Importance of nondrug costs of intravenous antibiotic therapy. Crit Care 2003, 7: R184-R190. 10.1186/cc2388
Acknowledgements
The author acknowledges the assistance of Phase Five Communications Inc. in the preparation of this manuscript. This work was supported by Johnson & Johnson.
This article is published as part of Critical Care Volume 12 Supplement 4, 2008: Optimizing the use of carbapenems in the face of increasing Gram-negative resistance. The full contents of the supplement are available online at http://ccforum.com/supplements/12/S4
Publication of this supplement has been sponsored by Ortho-McNeil, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr Nicolau has received research grants and has acted as a consultant for AstraZeneca, Johnson & Johnson, Merck, and Wyeth.
Rights and permissions
About this article
Cite this article
Nicolau, D.P. Pharmacodynamic optimization of β-lactams in the patient care setting. Crit Care 12 (Suppl 4), S2 (2008). https://doi.org/10.1186/cc6818
Published:
DOI: https://doi.org/10.1186/cc6818