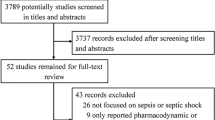
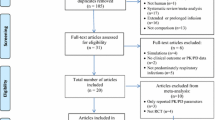
Abstract
Background
Administering β-lactam antibiotics via prolonged infusions for critically ill patients is mainly based on preclinical evidence. Preclinical data on this topic have not been systematically reviewed before.
Objectives
The aim of this study was to describe the pharmacokinetic/pharmacodynamic (PK/PD) indices and targets reported in preclinical models and to compare the bactericidal efficacy of intermittent and prolonged infusions of β-lactam antibiotics.
Methods
The MEDLINE and EMBASE databases were searched. To compare the bactericidal action of β-lactam antibiotics across different modes of infusion, the reported PK/PD outcomes, expressed as the percentage of time (T) that free (f) β-lactam antibiotic concentrations remain above the minimal inhibitory concentration (MIC) (%fT>MIC) or trough concentration (Cmin)/MIC of individual studies, were recomputed relative to the area under the curve of free drug to MIC ratio (fAUC24/MIC). A linear mixed-effects meta-regression was performed to evaluate the impact of the β-lactam class, initial inoculum, Gram stain, in vivo or in vitro experiment and mode of infusion on the reduction of bacterial cells (in colony-forming units/mL).
Results
Overall, 33 articles were included for review, 11 of which were eligible for meta-regression. For maximal bactericidal activity, intermittent experiments reported a PK/PD target of 40–70% fT>MIC, while continuous experiments reported a steady-state concentration to MIC ratio of 4–8. The adjusted effect of a prolonged as opposed to intermittent infusion on bacterial killing was small (coefficient 0.66, 95% confidence interval − 0.78 to 2.11).
Conclusions
Intermittent and prolonged infusions of β-lactam antibiotics require different PK/PD targets to obtain the same level of bacterial cell kill. The additional effect of a prolonged infusion for enhancing bacterial killing could not be demonstrated.



Similar content being viewed by others
References
Tabah A, Waele JD, Lipman J, Zahar JR, Cotta MO, Barton G, et al. The ADMIN-ICU survey: a survey on antimicrobial dosing and monitoring in ICUs. J Antimicrob Chemother. 2015;70:2671–7.
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care. 2019;23:104.
Roberts JA, Kirkpatrick C, Roberts MS, Dalley AJ, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. 2010;35:156–63.
Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, Randomized Controlled Trial. Clin Infect Dis. 2013;56:236–44.
Roberts JA, Abdul-Aziz M-H, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, et al. Continuous versus intermittent β-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194:681–91.
Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18:108–20.
Shiu J, Wang E, Tejani AM, Wasdell M. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst Rev. 2013;3:CD008481.
Abdul-Aziz MH, Sulaiman H, Mat-Nor M-B, Rai V, Wong KK, Hasan MS, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42:1535–45.
Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, et al. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am J Respir Crit Care Med. 2015;192:1298–305.
Lipman J. ClinicalTrials.gov (https://clinicaltrials.gov). Bethesda, MD: National Library of Medicine (US). Identifier NCT03213990, Beta-Lactam InfusioN Group Study (BLING III); 11 Jul 2017. https://clinicaltrials.gov/ct2/show/NCT03213990. Accessed 21 Mar 2020.
Dhaese S, Stove V, Waele DJ. Annual update in intensive care and emergency medicine. Berlin: Springer; 2018. p. 53–69.
Felton T, Goodwin J, O’Connor L, Sharp A, Gregson L, Livermore J, et al. Impact of bolus dosing versus continuous infusion of piperacillin and tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:5811–9.
Firsov A, Mattie H. Relationships between antimicrobial effect and area under the concentration-time curve as a basis for comparison of modes of antibiotic administration: meropenem bolus injections versus continuous infusions. Antimicrob Agents Chemother. 1997;41:352–6.
Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–47.
Roberts JA, Paul SK, Akova M, Bassetti M, Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34:467.
Bates D, Martin M, Ben B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Sabet M, Tarazi Z, Griffith DC. Pharmacodynamics of meropenem against Acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother. 2020;64(4):e02388–e2419.
Lamp KC, Vickers MK. Pharmacodynamics of ampicillin-sulbactam in an in vitro infection model against Escherichia coli strains with various levels of resistance. Antimicrob Agents Chemother. 1998;42:231–5.
Woodnutt G, Berry V. Efficacy of high-dose amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:35–40.
Sevillano D, Calvo A, Giménez M-JJ, Alou L, Aguilar L, Valero E, et al. Bactericidal activity of amoxicillin against non-susceptible Streptococcus pneumoniae in an in vitro pharmacodynamic model simulating the concentrations obtained with the 2000/125 mg sustained-release co-amoxiclav formulation. J Antimicrob Chemother. 2004;54:1148–51.
Louie A, Liu W, VanGuilder M, Neely MN, Schumitzky A, Jelliffe R, et al. Combination treatment with meropenem plus levofloxacin is synergistic against Pseudomonas aeruginosa infection in a murine model of pneumonia. J Infect Dis. 2015;211:1326–33.
MacGowan AP, Noel AR, Rogers CA, Bowker KE. Antibacterial effects of amoxicillin-clavulanate against Streptococcus pneumoniae and Haemophilus influenzae strains for which MICs are high, in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 2004;48:2599–603.
Gustafsson I, Löwdin E, Odenholt I, Cars O. Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob Agents Chemother. 2001;45:2436–40.
Chavanet P, Dalle F, Delisle P, Duong M, Pechinot A, Buisson M, et al. Experimental efficacy of combined ceftriaxone and amoxycillin on penicillin-resistant and broad-spectrum cephalosporin-resistant Streptococcus pneumoniae infection. J Antimicrob Chemother. 1998;41:237–46.
Andes D, Craig WA. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother. 1998;42:2375–9.
Bowker KE, Noel AR, Tomaselli SG, Elliott H, MacGowan AP. Pharmacodynamics of the antibacterial effect of and emergence of resistance to doripenem in Pseudomonas aeruginosa and Acinetobacter baumannii in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 2012;56:5009–155.
Zhang W, Guo Y, Li J, Zhang Y, Yang Y, Dong D, et al. In vitro and in vivo bactericidal activity of ceftazidime-avibactam against Carbapenemase-producing Klebsiella pneumoniae. Antimicrob Resist Infect Control. 2018;7:142.
Soontornpas C, Saraya S, Chulasiri M, Chindavijak B, Mootsikapun P. Time-kill curves as a tool for targeting ceftazidime serum concentration during continuous infusion for treatment of septicaemic melioidosis. Int J Antimicrob Agents. 2005;26:403–7.
Manduru M, Mihm LB, White RL, Friedrich LV, Flume PA, Bosso JA. In vitro pharmacodynamics of ceftazidime against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 1997;41:2053–6.
Piccoli L, Larosa M, Marchetti F. Time-kill curves as a tool for targeting ceftazidime serum concentration during continuous infusion. J Antimicrob Chemother. 2003;52:1047–8.
Mouton J, de Hollander J. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1994;38:931–6.
Cappelletty D, Kang S, Palmer S, Rybak M. Pharmacodynamics of ceftazidime administered as continuous infusion or intermittent bolus alone and in combination with single daily-dose amikacin against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob Agents Chemother. 1995;39:1797–801.
Bakker-Woudenberg IA, ten Kate MT, Goessens WH, Mouton JW. Effect of treatment duration on pharmacokinetic/pharmacodynamic indices correlating with therapeutic efficacy of ceftazidime in experimental Klebsiella pneumoniae lung infection. Antimicrob Agents Chemother. 2006;50:2919–25.
Alou L, Aguilar L, Sevillano D, Giménez M-JJ, Echeverría O, Gómez-Lus M-LL, et al. Is there a pharmacodynamic need for the use of continuous versus intermittent infusion with ceftazidime against Pseudomonas aeruginosa? An in vitro pharmacodynamic model. J Antimicrob Chemother. 2005;55:209–13.
Rodriguez CA, Agudelo M, Zuluaga AF, Vesga O. In vivo pharmacodynamics of piperacillin/tazobactam: implications for antimicrobial efficacy and resistance suppression with innovator and generic products. Int J Antimicrob Agents. 2017;49:189–97.
Bergen PJ, Bulitta JBB, Kirkpatrick CM, Rogers KE, McGregor MJ, Wallis SC, et al. Effect of different renal function on antibacterial effects of piperacillin against Pseudomonas aeruginosa evaluated via the hollow-fibre infection model and mechanism-based modelling. J Antimicrob Chemother. 2016;71:2509–20.
Kim A, Banevicius M, Nicolau DP. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob Agents Chemother. 2008;52:2497–502.
Katsube T, Yano Y, Yamano Y, Munekage T, Kuroda N, Takano M. Pharmacokinetic–pharmacodynamic modeling and simulation for bactericidal effect in an in vitro dynamic model. J Pharm Sci. 2008;97:4108–17.
Katsube T, Yamano Y, Yano Y. Pharmacokinetic–pharmacodynamic modeling and simulation for in vivo bactericidal effect in murine infection model. J Pharm Sci. 2008;97:1606–14.
Bergen PJ, Bulitta JB, Kirkpatrick CM, Rogers KE, McGregor MJ, Wallis SC, et al. Substantial impact of altered pharmacokinetics in critically ill patients on the antibacterial effects of meropenem evaluated via the dynamic hollow-fiber infection model. Antimicrob Agents Chemother. 2017;61:e02642–e2716.
DeRyke C, Banevicius MA, Fan HW, Nicolau DP. Bactericidal activities of meropenem and ertapenem against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob Agents Chemother. 2007;51:1481–6.
Macvane SH, Crandon JL, Nicolau DP. Characterizing in vivo pharmacodynamics of carbapenems against Acinetobacter baumannii in a murine thigh infection model to support breakpoint determinations. Antimicrob Agents Chemother. 2014;58:599–601.
Tsala M, Vourli S, Kotsakis S, Daikos GL, Tzouvelekis L, Zerva L, et al. Pharmacokinetic–pharmacodynamic modelling of meropenem against VIM-producing Klebsiella pneumoniae isolates: clinical implications. J Med Microbiol. 2016;65:211–8.
Vourli S, Tsala M, Kotsakis S, Daikos GL, Tzouvelekis L, Miriagou V, et al. Comparison of short versus prolonged infusion of standard dose of meropenem against carbapenemase-producing Klebsiella pneumoniae isolates in different patient groups: a pharmacokinetic–pharmacodynamic approach. J Pharm Sci. 2016;105:1513–8.
Tam VH, Chang K-TT, Zhou J, Ledesma KR, Phe K, Gao S, et al. Determining β-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J Antimicrob Chemother. 2017;72:1421–8.
Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. Optimization of meropenem minimum concentration/mic ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:4920–7.
White RL, Friedrich LV, Manduru M, Mihm LB, Bosso JA. Comparative in vitro pharmacodynamics of imipenem and meropenem against ATCC strains of Escherichia coli, Staphylococcus aureus and Bacteroides fragilis. Diagn Microbiol Infect Dis. 2001;39:39–47.
Abdul Aziz A-A, Staatz CE, Kirkpatrick CM, Lipman J, Roberts JA. Continuous infusion vs. bolus dosing: implications for beta-lactam antibiotics. Minerva Anestesiol. 2012;78:94–104.
Kristoffersson AN, David-Pierson P, Parrott NJ, Kuhlmann O, Lave T, Friberg LE, et al. Simulation-based evaluation of PK/PD indices for meropenem across patient groups and experimental designs. Pharm Res. 2016;33:1115–25.
Nielsen EI, Cars O, Friberg LE. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother. 2011;55:4619–30.
Louie A, Vanscoy B, Liu W, Kulawy R, Drusano GL. Hollow-fiber pharmacodynamic studies and mathematical modeling to predict the efficacy of amoxicillin for anthrax postexposure prophylaxis in pregnant women and children. Antimicrob Agents Chemother. 2013;57:5946–60.
Woodnutt G, Berry V. Two pharmacodynamic models for assessing the efficacy of amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:29–34.
Author information
Authors and Affiliations
Contributions
SD, AH, DL, JR and JDW conceived and designed the study. SD designed the search strategy and wrote the first draft. SD and MHA-A performed the literature search. SD extracted the data for the review and AH extracted the data for the meta-analysis and meta-regression. SD performed the meta-regression. SD, AH, DL, MHA-A, VS, VT, JL, JR and JDW contributed to the final draft and revision of the manuscript.
Corresponding author
Ethics declarations
Funding
There was no funding source for this study.
Conflict of interest
DL has previously received conference travel funding from MSD. JL has been a consultant for MSD, Australia. JR has been a consultant for Accelerate Diagnostics, Astellas, Bayer, bioMerieux and MSD, and has received investigator-initiated grants from MSD, The Medicines Company and Cardeas Pharma. JDW is supported by a grant from the Research Foundation Flanders (Senior Clinical Investigator Grant FWO, Ref. 1881020N), and has consulted for Accelerate, Bayer Healthcare, Cubist, Grifols, MSD and Pfizer (honoraria paid to institution). Sofie Dhaese, Aaron Heffernan, Mohd Hafiz Abdul-Aziz, Veronique Stove and Vincent H. Tam declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Raw data available upon request.
Code Availability
Code written in R. R packages used are described in the manuscript. Code available in ESM 2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhaese, S., Heffernan, A., Liu, D. et al. Prolonged Versus Intermittent Infusion of β-Lactam Antibiotics: A Systematic Review and Meta-Regression of Bacterial Killing in Preclinical Infection Models. Clin Pharmacokinet 59, 1237–1250 (2020). https://doi.org/10.1007/s40262-020-00919-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00919-6