Abstract
Activated protein C (APC) has emerged as a novel therapeutic agent for use in selected patients with severe sepsis, even though the mechanism of its benefit is not well established. APC has anticoagulant, anti-inflammatory, antiapoptotic, and profibrinolytic properties, but it is not clear through which of these mechanisms APC exerts its benefit in severe sepsis. Focus has recently turned to the role of APC in maintaining endothelial barrier function, and in vitro and in vivo studies have examined this relationship. This article critically reviews these studies, with a focus on potential mechanisms of action.
Similar content being viewed by others
Introduction
A defining feature of sepsis and the related acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) is damage to the microvascular endothelium leading to altered blood flow, oxygen extraction, and increased permeability to protein and solutes [1–3]. Increased lung capillary permeability leads to flooding of the alveolus with protein-rich pulmonary edema fluid, with resulting hypoxemia and decreased lung compliance. Much effort over recent years has focused on elucidating the mechanisms responsible for maintaining the integrity of the endothelium in sepsis and in ALI/ARDS, and many potential mediators have been identified.
Activated protein C and sepsis
The major pathophysiologic processes involved in producing organ dysfunction in severe sepsis include exuberant inflammation, coagulation, and apoptosis. Over recent years much effort has been devoted to targeting specific mediators of the inflammatory cascade in sepsis and ALI/ARDS. Unfortunately, these anti-inflammatory strategies, whether based on anticytokine antibodies or systemic glucocorticoids, have been unsuccessful in ameliorating organ injury [3]. Recently, anticoagulants with anti-inflammatory properties have been tested in clinical trials of sepsis with variable results.
The protein C pathway has been appreciated to be important in experimental models of sepsis, and in a randomized clinical trial of patients with severe sepsis activated protein C (APC) significantly decreased mortality [4, 5]. Protein C is activated on the endothelial surface by the thrombin-thrombomodulin complex to yield APC, a natural anticoagulant that limits thrombin production [6]. The epithelial protein C receptor (EPCR) plays a role in accelerating the activation of protein C by binding protein C and moving it closer to the thrombin-thrombomodulin complex [7]. APC appears to have pleiotropic properties that may form the basis of its observed benefit in sepsis models. In addition to its anticoagulant properties, APC has anti-inflammatory effects through the inhibition of nuclear factor-κB (NF-κB) activation [8] and it inhibits neutrophil chemotaxis [9]. APC also has antiapoptotic properties and is neuroprotective in stroke models through this mechanism [10, 11]. Finally, APC binds plasminogen activator inhibitor-1, a potent antifibrinolytic factor, and is thus indirectly profibrinolytic. Other anticoagulants that have been successful in experimental models, but not clinical trials, may have a more limited profile of actions as compared with APC [12, 13].
Despite all of these potentially beneficial properties of APC in the context of sepsis, it is not clear through which mechanism(s) APC exerts its clinical effects. In studies conducted in humans, the procoagulant effects of intrapulmonary endotoxin were countered by pretreatment with APC, and there was also evidence of decreased neutrophil migration into the air spaces [14, 15]. However, in the human systemic endotoxin model, pretreatment with APC does not lead to an anti-inflammatory, anticoagulant, or profibrinolytic response, although in one study the systemic mean arterial blood pressure was better preserved in the APC treatment group [16, 17]. In the landmark PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis) study, patients with severe sepsis receiving APC infusion also had an improvement in cardiovascular outcomes with decreased vasopressor requirements [18].
Direct and indirect modulation of endothelium by activated protein C
Although sepsis often causes clinically apparent injury to multiple organs, the major common denominator of injury is the vascular endothelium. In the lung, this manifests as a permeability pulmonary edema, which is the hallmark of ALI/ARDS. Can APC protect against or help to repair injured endothelium, and if so then through which of its mechanisms? Evidence has been produced using in vitro models that address mechanisms and more limited evidence exists from in vivo models. We summarize the in vitro and in vivo evidence and concentrate on potential mechanisms of endothelial barrier preservation.
Experimental evidence supports a role for APC in maintaining the integrity of the endothelium through both direct and indirect mechanisms. APC can potentially limit the elaboration of proinflammatory cytokines, such as tumor necrosis factor-α [19], which can indirectly protect the endothelium from cytokine-mediated apoptosis or upregulation of endothelial adhesion molecules that could facilitate neutrophil-endothelial interaction [20–22]. Also, via its anticoagulant properties, APC inhibits thrombin generation, which can reduce the protease-activated receptor (PAR)-mediated proinflammatory effects of thrombin [23]. In addition to indirect mechanisms through which APC maintains endothelial integrity, there has been considerable work done on the potential direct effects of APC on the endothelium. Direct effects of APC on the vascular endothelium are biologically plausible because this is the site of protein C activation, the endothelium contains the receptor for APC (EPCR), and the endothelium contains the PARs, which may also mediate APC signaling [24].
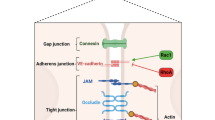
Evidence for direct modulation of endothelial function has been reported through a variety of experimental techniques. Using a gene expression approach, Joyce and colleagues [25] identified modulation of proinflammatory and cell survival pathways in primary cultured human umbilical vein endothelial cells (HUVECs) exposed to APC. Human APC directly suppressed the expression of NF-κB subunits and blocked the expression of NF-κB regulated genes following TNF-α challenge. Antiapoptotic transcripts, such as survivin (inhibitor of apoptosis protein) and BCL-2, were upregulated by APC, whereas there was suppression of the apoptotic genes calreticulin and TRMP-2. Furthermore, when endothelial cells were challenged with a potent inducer of apoptosis, the APC-treated cells were protected in a dose-dependent manner. The potential direct anti-inflammatory and antiapoptotic effects of APC are summarized in Figure 1.
The role of the protein C pathway in the endothelial cell. APC modulates endothelial phenotype by inhibiting thrombin production, direct antiapoptotic effects, and suppression of NF-κB subunits and therefore decreased inflammatory cell adhesion. APC, activated protein C; ICAM, intercellular adhesion molecule; NF-κB, nuclear factor-κB; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule. Reprinted with permission from the American Society for Biochemistry and Molecular Biology [25].
Other investigators have also documented a direct antiapoptotic effect of APC. Using human brain endothelium in a stroke model, Cheng and coworkers [10] reported that APC had a direct antiapoptotic effect on hypoxic brain endothelium that required binding to EPCR and PAR1 activation. The mechanism of neuroprotection in this model was attributed to inhibition of the proapoptotic transcription factor p53, normalization of the proapoptotic Bax/Bcl-2 ratio, and reduction of caspase-3 signaling, all of which decreased apoptosis. Using an in vivo murine model of focal ischemic stroke, administration of mouse APC significantly decreased brain infarct size and edema, and was dependent on EPCR and PAR1. Furthermore, low-dose mouse APC produced in vivo neuroprotection, independent of its anticoagulant activity.
Activated protein C and endothelial barrier protection
Another direct mechanism of action of APC on the endothelium is modulation of the endothelial monolayer, leading to increased cell-cell contact and decreased permeability. Two investigations have documented this phenomenon and explored its mechanisms. Feistritzer and Riewald [26] used HUVECs grown in a transwell with a dual chamber liquid interface to explore the permeability effects of APC and other agents. Thrombin and the PAR1 agonist peptide both greatly increased the permeability of the HUVECs to Evans blue labeled albumin. The thrombin-mediated hyperpermeability was reduced by pretreatment with human APC. Also, when subconfluent endothelial monolayers were incubated with control or APC, there was less permeability in the APC-treated cells, implying that APC somehow sealed cell-cell contacts. Using a cleavage site specific antibody to PAR1, the endothelial protective effects of APC and the endothelial disruptive effects of thrombin could both be blocked, which suggests that the opposing effects of the two proteases were operating through the same receptor.
It seems paradoxical that thrombin and APC, both operating through PAR1, can have opposing biologic effects on endothelial permeability. A potential explanation for this paradox was explored by targeting the sphingosine 1-phosphate (S1P) pathway, which is known to enhance endothelial barrier integrity via cytoskeletal rearrangement [27]. Transfection of the endothelial cells with small interfering RNA (siRNA) targeting the enzyme responsible for S1P production, sphingosine kinase-1, blocked the barrier-enhancing signaling of APC. In addition, siRNA targeting the S1P receptor S1P1 also blocked barrier enhancement by APC. Feistritzer and Riewald [26] concluded that the endothelial barrier protection produced by APC is mediated through PAR1 and by crosstalk with the S1P pathway.
In another investigation, Finigan and colleagues [28] also explored the endothelial barrier enhancement properties of APC. Those investigators used human pulmonary artery endothelial cells and measured transendothelial electrical resistance in response to thrombin in the presence or absence of APC. Using this in vitro system, APC attenuated thrombin-induced endothelial cell disruption at concentrations as low as 0.1 to 1.0 μg/ml. Additionally, APC reversed the formation of transcellular actin stress fibers by thrombin and produced peripheral cortical actin distribution, which promotes cell-cell tethering and barrier protection. This peripheral cytoskeletal arrangement is similar to the effects of S1P, and indeed using siRNA against S1P1 this effect of APC was also S1P dependent. Using immunoprecipitation studies the APC-mediated phosphorylation of S1P1 was also documented, as was the co-immunoprecipitation of EPCR and S1P1. The proposed schema for endothelial barrier protection by APC and its involvement with the S1P pathway is summarized in Figure 2. In summary, in two different in vitro investigations, APC promoted endothelial barrier protection in a PAR1- and S1P1-dependent mechanism.
Proposed schema for APC signaling in the endothelial cell. APC binds to EPCR, which then interacts with the S1P1 receptor leading to its phosphorylation by PI3-kinase. S1P1 signaling through Rac1 leads to cortical cytoskeletal rearrangement and endothelial barrier protection. APC, activated protein C; EPCR, epithelial protein C receptor; PI3-kinase, phosphatidyl-inositol-3 kinase; S1P, sphingosine 1-phosphate. Reprinted with permission from the American Society for Biochemistry and Molecular Biology [28].
Very low (picomolar) concentrations of thrombin and PAR1 agonist peptide can actually be barrier protective, analogous to the effects of APC. Also, supraphysiologic concentrations of APC can be barrier disruptive, which suggests that the level of PAR1 activation may determine the cellular response [29]. Thrombin is an excellent activator of PAR1, and picomolar concentrations of thrombin may produce similar PAR1 activation as pharmacologic concentrations of APC, which is a poor activator of PAR1. Furthermore, thrombin can locally generate APC that may potentially exert its own barrier enhancing effects [30].
In vivo endothelial barrier protection by activated protein C
The in vivo significance of APC signaling through PAR1 is not entirely clear. It is clear, however, that thrombin is much more potent (approximately 104-fold) at cleaving PAR1 than is APC [31]. The concentrations of APC used in the in vitro studies showing endothelial barrier protection were within the pharmacologic range of APC in the PROWESS study in one investigation [26], but another investigation failed to show significant PAR1 cleavage at concentrations of APC that were approximately 10-fold higher than the plasma concentrations in the PROWESS study [31]. Also, PAR1-/- mice have the same rate of death as wild-type mice in a model of endotoxemia, arguing that PAR1 activation by endogenous mediators in vivo does not play a role in a standard model of sepsis [32, 33]. Methodologic differences between in vitro models and the inherent limitations of in vitro modeling may explain the discordant results on the significance of APC signaling through PAR1.
Other in vivo models have yielded conflicting results that may have tempered the enthusiasm surrounding an endothelial protective effect of APC. Robriquet and colleagues [34] reported their experience with a rat model of Pseudomonas aeruginosa induced lung injury and continuous intravenous human APC. Rats that received APC exhibited trends toward increased vascular permeability to radiolabeled albumin and increased lung edema. The authors postulated that early fibrin formation in this pneumonia model was potentially beneficial, and that disruption of this fibrin response by intravenous APC was possibly deleterious. Of note, human APC was used in this investigation at a dose of 300 μg/kg per hour, which is a much higher dose than used in humans but may be appropriate given the activity of human APC in rats. In another investigation of systemic endotoxin in rats, Murakami and coworkers [35] showed that APC prevented lipopolysaccharide-induced pulmonary vascular permeability.
We have preliminary data from a noninfectious model of ALI (intratracheal acid) on the potential role of APC in endothelial permeability. Acid-induced lung injury produces damage to the alveolar epithelium and prominent lung vascular permeability to protein [36]. This model of lung injury is also very neutrophil dependent and is therefore a good choice for testing the direct and indirect effects of APC on the lung microvasculature. Mice were given acid intratracheally and were then treated with murine APC. In the APC-treated mice lung injury was worsened, with increased pulmonary edema and lung vascular permeability to protein (unpublished data). The reason for the conflicting results of endothelial barrier protection in the in vivo studies is not clear, but these findings reinforce the need to cautiously interpret cell culture experiments and their relationship to in vivo experimental or human conditions.
Potential additional clinical applications beyond sepsis
The PROWESS trial showed a 6% mortality benefit in severe sepsis from APC in a large, multicenter, placebo-controlled trial of 1640 patients [4]. Most of the patients had a pulmonary source of sepsis and 75% were intubated and ventilated. Because patients were not required to have a chest radiograph and arterial blood gas assessment at the time of study enrollment, we do not know how many of these severe sepsis patients had ALI. Thus, it is plausible that APC was beneficial in sepsis-induced lung injury, although the data cannot be obtained from the PROWESS study. The pathogenesis of organ injury in ALI/ARDS is similar to the proposed mechanisms for septic-induced injury, and so it is conceivable that APC may exert anticoagulant, anti-inflammatory, antiapoptotic, or barrier-enhancing effects that might benefit patients with ALI from a variety of risk factors besides sepsis. Also, some studies in patients with ALI from nonseptic causes demonstrated reduced plasma protein C and elevated plasminogen activator inhibitor-1 levels, which correlate with worse clinical outcomes [37, 38]. Therefore, we hypothesized that APC may be of therapeutic value in patients with ALI. Accordingly, we are currently conducting a randomized, double blind phase II clinical trial of APC for early ALI. This multicenter trial is supported by the US National Heart, Lung, and Blood Institute and will enroll 90 patients to test for several biologic and clinical end-points. If the results are encouraging, then a phase III randomized trial could be conducted to test the potential value of APC in ALI in a large number of patients.
Conclusion
APC has important indirect effects on the integrity of the vascular endothelium that are both thrombin dependent and independent, but it also has emerging direct effects on endothelial function. Apoptosis appears to be a significant mechanism contributing to endothelial dysfunction in sepsis, and APC has well described direct antiapoptotic properties that are independent of its anticoagulant activity. APC also has a direct effect on endothelial cytoskeletal rearrangement that strengthens endothelial tight junctions. This mechanism appears to operate in a PAR1 and SIP1 dependent manner. The lack of significant anticoagulant or anti-inflammatory responses in the human systemic endotoxin-APC model lends credence to the benefits of APC in sepsis operating through alternative mechanisms, such as antiapoptosis and SIP-mediated endothelial protection. APC remains an important therapy for patients with severe sepsis with major organ dysfunction, and the mechanism of its benefit in these patients appears to be in part through direct interactions with the endothelium.
Abbreviations
- ALI:
-
acute lung injury
- APC:
-
activated protein C
- ARDS:
-
acute respiratory distress syndrome
- EPCR:
-
epithelial protein C receptor
- HUVEC:
-
human umbilical vein endothelial cell
- NF-κB:
-
nuclear factor-κB
- PAR:
-
protease-activated receptor
- S1P:
-
sphingosine 1-phosphate
- siRNA:
-
small interfering RNA.
References
Bateman RM, Walley KR: Microvascular resuscitation as a therapeutic goal in severe sepsis. Crit Care 2005, (Suppl 4):S27-S32. 10.1186/cc3756
Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 2000, 342: 1334-1349. 10.1056/NEJM200005043421806
Hotchkiss RS, Karl IE: The pathophysiology and treatment of sepsis. N Engl J Med 2003, 348: 138-150. 10.1056/NEJMra021333
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al.: Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001, 344: 699-709. 10.1056/NEJM200103083441001
Looney MR, Matthay MA: The role of protein C in sepsis. Curr Infect Dis Rep 2001, 3: 413-418.
Esmon C: The protein C pathway. Crit Care Med 2000, (Suppl):S44-S48. 10.1097/00003246-200009001-00010
Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT: The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA 1996, 93: 10212-10216. 10.1073/pnas.93.19.10212
White B, Schmidt M, Murphy C, Livingstone W, O'Toole D, Lawler M, O'Neill L, Kelleher D, Schwarz HP, Smith OP: Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol 2000, 110: 130-134. 10.1046/j.1365-2141.2000.02128.x
Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ: Expression and function of the endothelial protein Creceptor in human neutrophils. Blood 2003, 102: 1499-1505. 10.1182/blood-2002-12-3880
Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV: Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med 2003, 9: 338-342. 10.1038/nm826
Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV: Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 2004, 41: 563-572. 10.1016/S0896-6273(04)00019-4
Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, et al.: Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 2003, 290: 238-247. 10.1001/jama.290.2.238
Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, et al.: Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001, 286: 1869-1878. 10.1001/jama.286.15.1869
Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O'Brien J, Gruber M, Zarini S, Murphy RC, Kuhn K, et al.: Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 2004, 104: 3878-3885. 10.1182/blood-2004-06-2140
van der Poll T, Levi M, Nick JA, Abraham E: Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med 2005, 171: 1125-1128. 10.1164/rccm.200411-1483OC
Derhaschnig U, Reiter R, Knobl P, Baumgartner M, Keen P, Jilma B: Recombinant human activated protein C (rhAPC; drotrecogin alfa [activated]) has minimal effect on markers of coagulation, fibrinolysis, and inflammation in acute human endotoxemia. Blood 2003, 102: 2093-2098. 10.1182/blood-2003-02-0416
Kalil AC, Coyle SM, Um JY, LaRosa SP, Turlo MA, Calvano SE, Sundin DP, Nelson DR, Lowry SF: Effects of drotrecogin alfa (activated) in human endotoxemia. Shock 2004, 21: 222-229. 10.1097/01.shk.0000116778.27924.79
Vincent JL, Angus DC, Artigas A, Kalil A, Basson BR, Jamal HH, Johnson G III, Bernard GR: Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med 2003, 31: 834-840. 10.1097/01.CCM.0000051515.56179.E1
Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW: Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol 1994, 153: 3664-3672.
Joyce DE, Nelson DR, Grinnell BW: Leukocyte andendothelial cell interactions in sepsis: relevance of the protein C pathway. Crit Care Med 2004, (Suppl):S280-S286. 10.1097/01.CCM.0000128037.72072.22
Joyce DE, Grinnell BW: Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med 2002, (Suppl):S288-S293. 10.1097/00003246-200205001-00019
Iba T, Kidokoro A, Fukunaga M, Nagakari K, Shirahama A, Ida Y: Activated protein C improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat lipopolysaccharide model. Crit Care Med 2005, 33: 368-372. 10.1097/01.CCM.0000153415.04995.88
Coughlin SR: Thrombin signalling and protease-activated receptors. Nature 2000, 407: 258-264. 10.1038/35025229
Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W: Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002, 296: 1880-1882. 10.1126/science.1071699
Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW: Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem 2001, 276: 11199-11203. 10.1074/jbc.C100017200
Feistritzer C, Riewald M: Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 2005, 105: 3178-3184. 10.1182/blood-2004-10-3985
Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D: Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001, 108: 689-701. 10.1172/JCI200112450
Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG: Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 2005, 280: 17286-17293. 10.1074/jbc.M412427200
Camerer E, Coughlin SR: APC signaling: tickling PAR1 for barrier protection? Blood 2005, 105: 3004-3005. 10.1182/blood-2005-01-0334
Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E, Griffin JH, Riewald M: Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem 2006, 281: 20077-20084. 10.1074/jbc.M600506200
Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR: PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem 2005, 280: 13122-13128. 10.1074/jbc.M410381200
Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade-Gordon P, Frank RD, Mackman N: Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood 2004, 103: 1342-1347. 10.1182/blood-2003-09-3051
Camerer E, Cornelissen I, Kataoka H, Duong DN, Zheng YW, Coughlin SR: Roles of protease-activated receptors in a mouse model of endotoxemia. Blood 2006, 107: 3912-3921. 10.1182/blood-2005-08-3130
Robriquet L, Collet F, Tournoys A, Prangere T, Neviere R, Fourrier F, Guery BP: Intravenous administration of activated protein C in Pseudomonas-induced lung injury: impact on lung fluid balance and the inflammatory response. Respir Res 2006, 7: 41. 10.1186/1465-9921-7-41
Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K: Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood 1996, 87: 642-647.
Folkesson HG, Matthay MA, Hebert CA, Broaddus VC: Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 1995, 96: 107-116.
Ware LB, Fang X, Matthay MA: Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003, 285: L514-L521.
Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA: Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003, 285: L20-L28.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Looney, M.R., Matthay, M.A. Bench-to-bedside review: The role of activated protein C in maintaining endothelial tight junction function and its relationship to organ injury. Crit Care 10, 239 (2006). https://doi.org/10.1186/cc5099
Published:
DOI: https://doi.org/10.1186/cc5099