Abstract
Introduction
Respiratory viruses are a major cause of respiratory tract infections. The prevalence of a virus-positive respiratory sample and its significance in patients requiring mechanical ventilation remain unknown.
Methods
We conducted a cohort study in all consecutive adults ventilated for more than 48 hours admitted to a 22-bed medical intensive care unit during a 12-month period. Respiratory samples at the time of intubation were assessed by culture, by indirect immunofluorescence assay or by molecular methods in systematic tracheobronchial aspirates. Patients with a virus-negative respiratory sample at the time of intubation were considered unexposed and served as the control group.
Results
Forty-five viruses were isolated in 41/187 (22%) patients. Rhinovirus was the most commonly isolated virus (42%), followed byherpes simplex virus type 1 (22%) and virus influenza A (16%). In multivariate analysis controlling for the Acute Pathophysiology and Chronic Health Evaluation II score, patients with respiratory disorder at admission (adjusted odds ratio, 2.1; 95% confidence interval, 0.8–5.1; P = 0.12), with chronic obstructive pulmonary disease/asthma patients (adjusted odds ratio, 3.0; 95% confidence interval, 1.3–6.7; P = 0.01) and with admission between 21 November and 21 March (adjusted odds ratio, 2.8; 95% confidence interval, 1.3–5.9; P = 0.008) were independently associated with a virus-positive sample. Among the 122 patients admitted with respiratory disorder, a tracheobronchial aspirate positive for respiratory viruses at the time of intubation (adjusted hazard ratio, 0.273; 95% confidence interval, 0.096–0.777; P < 0.006) was independently associated with better survival, controlling for the Simplified Acute Physiology Score II and admission for cardiogenic shock or cardiac arrest. Among the remaining 65 patients, a virus-positive sample on intubation did not predict survival.
Conclusion
We confirmed the pathogenic role of respiratory viruses in the intensive care unit, particularly rhinovirus. We suggest, however, that the prognostic value of virus-associated respiratory disorder is better than that of other causes of respiratory disorder.
Similar content being viewed by others
Introduction
Respiratory viruses represent an important role in the etiology of community-acquired pneumonia in adults [1–3]. Respiratory viruses are also the leading cause of acute exacerbations of chronic obstructive pulmonary disease (COPD)/asthma patients [4, 5], resulting in frequent consultations with a general practitioner and hospitalisations. In some cases, invasive ventilation is required [3, 5, 6]. The number of studies that document the presence of viruses in respiratory samples of critically ill patients is currently growing in the literature [7–9]. What is really needed, however, are more data on the clinical significance of these findings, particularly as regards morbidity and mortality.
In a previous work we investigated the incidence of nosocomial viral ventilator-associated pneumonia [10]. The aims of the present study were to determine the epidemiology of and risk factors for virus-positive respiratory samples taken at the time of intubation in acutely ill patients, and to compare clinical outcome (survival and time to ventilated acquired pneumonia) with and without respiratory viruses, according to the presence (group 1) or the absence (group 2) of respiratory disorder at admission.
Methods
Patients
All consecutively intubated adults admitted to the intensive care unit (ICU) in the University Hospital of Caen between September 2003 and September 2004 were screened, as previously reported [10].
Data collection
Patient characteristics recorded at the time of intubation included age, sex, main reason for ICU admission, scoring of disease severity within the first day in the ICU – assessed by the admission Simplified Acute Physiology Score type II [11], the Acute Physiology and Chronic Health Evaluation II score [12] and the admission logistic organ dysfunction system [13] – and concomitant diseases such as immunocompromised status defined as HIV infection, neoplasia, innate immunity deficit, cystic fibrosis, chronic use of steroids or immunosuppressive drugs. Other comorbidities such as diabetes, COPD/asthma or cardiovascular diseases were also recorded at admission.
The main reasons for ICU admission (defined on enrolment without the knowledge of viral assessment) included cardiac arrest, septic shock, cardiac shock, mixed shock, hemorrhagic shock, respiratory distress alone (without other associated organ failure), acute renal failure, coma, intoxication, surgery and other. In addition, clinical outcomes assessed by the occurrence of ventilator-associated pneumonia and death were recorded.
According to French legislation at the time of the study and given the observational nature of our study, no ethical committee approval was requested and thus no informed consent was obtained from the patients.
Virologic assessment
Details of the virologic methods for virus detection are published elsewhere [10]. Briefly, tracheobronchial aspirates performed at the time of intubation were assessed by culture, by indirect immunofluorescence assay or by molecular methods (PCR or RT-PCR) using previously described procedures [14–17].
The following viruses were tested: parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3 and parainfluenza virus 4, influenza virus A, influenza virus B and influenza virus C, respiratory syncytial virus, metapneumovirus, rhinovirus, coronavirus 229E and coronavirus OC43, adenovirus, cytomegalovirus and herpes simplex virus. Chlamydia pneumoniae and Mycoplasma pneumoniae were also detected by PCR assay.
Respiratory specimens were processed for PCR or RT-PCR at the end of the study period. One positive sample and several negative control samples were included for each infectious agent, which were treated identically to the virus samples throughout. Results of conventional methods for viral isolation, routinely performed (in case of respiratory disorder), were transmitted weekly to the clinicians. Antiviral drugs could be used during the study period for proven herpes simplex virus or cytomegalovirus infection in immunocompromised patients.
Definitions
Pneumonia was defined as any acute septic episode with respiratory symptoms (cough, sputum production, dyspnea, pleuretic chest pain or altered mental status) and a radiographic infiltrate that was neither preexisting or of other known cause [18]. Pneumonia occurring after 48 hours of hospitalisation was considered nosocomial. Ventilator-associated pneumonia was defined as described elsewhere [10]. Acute exacerbation of COPD was defined according to the NHLBI/WHO Workshop Summary [19]. Respiratory disorder was defined as respiratory distress alone or any other reasons for admission with associated respiratory symptoms.
Statistical analysis
Quantitative data and qualitative data were expressed as the mean ± standard deviation or as the median (range) and percentage (95% confidence interval), respectively. Categorical variables were compared using the chi-square test or Fischer's exact test, when appropriate. Quantitative variables were compared using the Student t test or the Mann-Whitney nonparametric test, when appropriate. The confidence intervals of percentages were based on normal approximation.
We modeled the probability of a positive virus respiratory sample using a logistic regression model. Because we hypothesised that the pathogenic role of respiratory viruses in the respiratory tract may differ when associated with respiratory symptoms or not, we examined outcome according to the presence (group 1) or the absence (group 2) of respiratory disorder at admission. To assess the impact of the virus respiratory sample on time to death and time to ventilated acquired pneumonia, we constructed Kaplan-Meier curves and Cox models.
A stepwise selection of variables associated with outcome at P < 0.1 in the univariate analysis was chosen for multivariate modeling in both the logistic and the proportional hazards models. Multivariable modeling is a tradeoff between model complexity and parcimony. Because of our relatively small sample size, we selected a level of alpha risk <0.25 to remain in the multivariable model [20] to avoid partial confusion bias. The level of significance was set at P < 0.05, and all tests were two-sided.
We used EPI-INFO version 6.04dfr software (EPI-INFO; CDC, Atlanta, GA, USA) for data collection, and used EPI-INFO and SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA) for data analysis.
Results
Prevalence and baseline characteristics
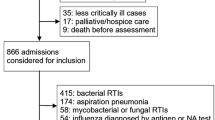
Among 653 patients admitted to our ICU during the study period, a tracheobronchial aspirate was taken for viral studies in 187 patients, as shown in Figure 1. The prevalence of admitted patients with at least one virus-positive respiratory sample was 41/187 (22%; 95% confidence interval, 16–28) at the time of intubation. Baseline characteristics of patients with or without a virus-positive respiratory sample are presented in Table 1. The main reason for admission was respiratory distress alone (77/187), including 46 cases of pneumonia, nine cases of acute COPD/asthma exacerbations, 11 cases of pulmonary edema, five aspirations, two cases of intraalveolar bleedings, one case of atelectasia, one pneumothorax, one pulmonary embolism and one case of myasthenia. Forty-five out of 187 additional patients had respiratory disorders as associated symptoms at admission.
Virus finding
Forty-five viruses were isolated from the respiratory specimens of 41 patients (Table 2). Rhinovirus was the most commonly isolated virus (19/45), followed by herpes simplex virus type 1 (10/45) and virus influenza A (7/45). Rhinovirus detected in the lower respiratory tract was associated with clinical signs of acute COPD exacerbation, of pneumonia or of pulmonary edema in all cases except in four patients, and virus influenza was associated with acute respiratory or cardiac failure in all cases but one. Viral coinfection was detected in four patients: one case of rhinovirus and virus parainfluenza 3, one case of rhinovirus and cytomegalovirus, one case of herpes simplex virus type 1 and virus influenza A, and one case of herpes simplex virus type 1 and coronavirus. We reported two seasonal peaks: December for virus influenza A and March for rhinovirus (Figure 2). Other viral detection details are presented in Table 2.
Risk factors associated with a virus-positive sample
In univariate analysis (Table 1), COPD/asthma patients (P = 0.0012), admission between 21 November and 21 March (P = 0.003) and admission with respiratory disorder (P = 0.03) were significantly more prevalent in patients with a virus-positive sample. In addition, patients with a virus-positive sample had a nonsignificant lower Acute Physiology and Chronic Health Evaluation II score compared with patients with a virus-negative sample (18.0 versus 20.9, respectively; P = 0.057). In multivariate analysis controlling for Acute Physiology and Chronic Health Evaluation II score and respiratory disorder, the COPD/asthma patients and admission between 21 November and 21 March remained significantly associated with a virus-positive sample, as shown in Table 3.
Clinical outcome by virus respiratory sample
The Kaplan-Meier curves of survival according to the presence of respiratory viruses in group 1 and in group 2 are shown in Figure 3. A tracheobronchial aspirate positive for respiratory viruses at the time of intubation was independently associated with better survival in group 1 (P < 0.006) but not in group 2 (P = 0.94), where only a higher Simplified Acute Physiology Score II (P < 0.002) and admission for cardiac arrest or cardiogenic shock (P = 0.07) predicted time to death, as shown in Table 4 and Table 5, respectively. A virus-positive sample did not predict the time to ventilator-associated pneumonia in group 1, in group 2 or overall (data not shown).
Discussion
The present study reports that respiratory viruses, as systematically screened with sensitive methods at the time of intubation, are common (22%) among adults ventilated for more than 48 hours, regardless of the reason for admission to the ICU. Rhinovirus was the most commonly isolated virus. We have identified, for the first time in this setting, three risks factors associated with a virus-positive sample – namely, admission with respiratory disorder, COPD/asthma and admission during the winter endemic viral season. These factors highlight that the diagnosis of respiratory viral infection should be focused for patients with a respiratory disease, and support the hypothesis of the clinical impact and pathogenic role of viral infection. In addition, we suggest that the ICU mortality might be lower in viral-associated respiratory disorder than in nonviral-associated respiratory disorder. A virus-positive sample had no impact on the time to ventilator-associated pneumonia, as previously reported in a smaller sample of this cohort [10].
Our finding differs from previous studies assessing the microbiologic pattern of severe pneumonia [18, 21, 22] or acute exacerbation of COPD [7], which reported a lower prevalence of respiratory tract viral infection, varying from 0% [23] to 16% [7]. Differences in the diagnosis tests, the lack of a PCR assay and the limited range of viruses sought may explain this differential. Our rates of virus-positive respiratory samples were consistent with the prevalence of respiratory tract viral infections of 17–48% [8, 9, 24, 25] observed in recent prospective studies using molecular methods for viral detection and focusing on COPD patients [9, 24, 25] or patients admitted to the ICU for cardiorespiratory failure [8]. As previously reported [9, 26], the prevalence of virus-positive respiratory samples was increased in the endemic viral period.
The molecular method used in this study for viral detection is recognised as the most sensitive technique [27, 28]. Nonetheless, the clinical relevance of a positive respiratory virus PCR test needs to be appraised. This topic has been discussed in specific populations that differed from our ventilated ICU patients; however, no chronic shedding or carriage of respiratory virus RNA was found in children [29] and no chronic shedding or carriage of respiratory syncytial virus was found in COPD patients [30]. Rhinovirus RNA could be detected up to 2–3 weeks after infection [31], without exceeding five weeks and virus influenza RNA could be detected up to seven days after infection [32]. These findings suggest that PCR-positive patients had been infected recently in our study, most of them within the two weeks before admission.
According to previous studies focusing on patients at high risk for viral disease [4, 6, 8, 9, 24, 25, 28, 33], rhinovirus and virus influenza were the most frequently recovered viruses. These epidemiological data underscore the potential pathogenic role of rhinovirus and of influenza virus as the cause of severe respiratory disorder.
In the present study, the proportion of rhinovirus (42%) was higher than reported in ICU patients [8, 9]. While its role as an important respiratory pathogen remains the subject of debate, several experimental studies with nasal inoculation demonstrated that rhinovirus could reach, penetrate and replicate in the lower airway epithelium and could induce a proinflammatory response [34, 35]. Rhinovirus was also associated with severe lower respiratory tract illness [36].
In contrast, influenza virus is recognised to play a major pathogenic role during flu outbreaks in the winter-spring season. A causal relationship between influenza virus infection and hospitalisation for respiratory or cardiac failure has been shown in vaccine effectiveness studies [37, 38].
We failed to demonstrate that patient exposure to respiratory viruses significantly increased the risk of ventilator-associated pneumonia. It is commonly reported that respiratory viruses could facilitate bacterial infection of the airways, by damaging the respiratory epithelium [39]. Some experimental studies have reported that respiratory viruses may promote bacterial adhesion to respiratory epithelial cells, a process that may increase bacterial colonisation [40, 41], and that rhinovirus may increase the ability of Staphylococcus aureus to internalise into pneumocytes with a mechanism that involves the virus-induced release of IL-6 and IL-8 and the overexpression of ICAM-1 [42]. Finally, an epidemiological association has been described between viral pneumonia and nosocomial infection [43, 44] or respiratory sepsis [26].
In the subgroup of patients with respiratory disorder, those with virus-positive samples surprisingly had a better survival. This result should be interpreted cautiously because it relies on the control group (that is to say, patients with a virus-negative sample). This finding does not question the severity of virus-associated respiratory disorder, but simply suggests that the prognostic may well differ from other causes of respiratory disorder. It has been reported that the clinical severity and inflammatory responses in COPD exacerbations could be modulated by the nature of the infecting organism [24, 25].
We are aware of limitations. The monocenter design of the study, the relatively small number of included patients, patients' underlying disease heterogeneity as well as the fact that 18 patients (8.7%) were eligible but not screened may limit the interpretation and relevance of our data. Because the systematic search for bacteria was not obtained at the time of intubation, 'virus-associated respiratory disorder' does not necessarily mean virus-induced respiratory disorder. In addition, the PCR might be too sensitive and we cannot exclude an asymptomatic carriage of respiratory viruses in the airways in some cases. In the future, a quantifying viral load might be another approach to improve the diagnostic accuracy.
The results reported here may have important implications for the design and power analysis of a randomised controlled trial using antiviral drugs. With a 12% mortality rate in the control group (that is to say, the rate we observed in virus-associated respiratory disorder), the room for improvement in patients with viral pneumonia would be lower than that for respiratory disorder overall (34%). An appropriate sample size would consequently be necessary to demonstrate the clinical impact, if any, of antiviral drugs.
Conclusion
We have reported and described the prevalence of virus-positive respiratory samples taken at the time of intubation in ventilated adults, contributing to improving epidemiological knowledge in the critical care setting. Using the most sensitive methods for viral detection, we were able to identify that 22% of our patients had viruses in their airways. The detection of respiratory viruses in the respiratory tract, however, was not always associated with respiratory symptoms, as demonstrated by the 12% asymptomatic carriage in group 2 (Figure 3, group 2). Finally, we suggest that patients with viruses in the respiratory tract and respiratory symptoms (suggesting a virus-associated respiratory disorder) had a better prognosis in the ICU than patients without viruses and respiratory symptoms (suggesting other causes of respiratory disorder), as shown in Figure 3 (group 1). Further studies are necessary: first, to confirm the importance of viral infections as a cause of acute respiratory failure in patients admitted to the ICU; and, second, to address the role of antiviral therapy in this population.
Key messages
-
Respiratory viruses screened at the time of intubation are common among adults ventilated for more than 48 hours, especially in patients admitted for respiratory disorder.
-
Rhinovirus is the most commonly isolated virus.
-
We suggest that virus-associated respiratory disorder may be associated with a lower clinical severity and better prognosis, as compared with other causes of respiratory disorder.
-
Further studies are necessary to confirm the importance of viral infections as a cause of acute respiratory failure in patients admitted to the ICU, and to address the role of antiviral therapy in this patient population.
Abbreviations
- COPD:
-
COPD = chronic obstructive pulmonary disease
- ICU:
-
ICU = intensive care unit
- IL:
-
IL = interleukin
- PCR:
-
PCR = polymerase chain reaction
- RT:
-
RT = reverse transcriptase.
References
Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Kleemola M, Koskela M, Leinonen M, Ronnberg PR, Saikku P, Sten M, et al.: Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis 2001, 32: 1141-1154. 10.1086/319746
de Roux A, Marcos MA, Garcia E, Mensa J, Ewig S, Lode H, Torres A: Viral community-acquired pneumonia in nonimmunocompromised adults. Chest 2004, 125: 1343-1351. 10.1378/chest.125.4.1343
Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE: Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005, 352: 1749-1759. 10.1056/NEJMoa043951
Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB: Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med 1998, 158: 2453-2459. 10.1001/archinte.158.22.2453
Lieberman D, Gelfer Y, Varshavsky R, Dvoskin B, Leinonen M, Friedman MG: Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest 2002, 122: 1264-1270. 10.1378/chest.122.4.1264
Falsey AR, Walsh EE, Hayden FG: Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis 2002, 185: 1338-1341. 10.1086/339881
Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, Hernandez C, Rodriguez-Roisin R: Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998, 157: 1498-1505.
Carrat F, Leruez-Ville M, Tonnellier M, Baudel JL, Deshayes J, Meyer P, Maury E, Galimand J, Rouzioux C, Offenstadt G: A virologic survey of patients admitted to a critical care unit for acute cardiorespiratory failure. Intensive Care Med 2006, 32: 156-159. 10.1007/s00134-005-2861-4
Cameron RJ, de Wit D, Welsh TN, Ferguson J, Grissell TV, Rye PJ: Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med 2006, 32: 1022-1029. 10.1007/s00134-006-0202-x
Daubin C, Vincent S, Vabret A, du Cheyron D, Parienti JJ, Ramakers M, Freymuth F, Charbonneau P: Nosocomial viral ventilator-associated pneumonia in the intensive care unit: a prospective cohort study. Intensive Care Med 2005, 31: 1116-1122. 10.1007/s00134-005-2706-1
Le Gall JR, Lemeshow S, Saulnier F: A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270: 2957-2963. 10.1001/jama.270.24.2957
Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med 1985, 13: 818-829.
Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, Teres D: The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA 1996, 276: 802-810. 10.1001/jama.276.10.802
Freymuth F, Vabret A, Galateau-Salle F, Ferey J, Eugene G, Petitjean J, Gennetay E, Brouard J, Jokik M, Duhamel JF, Guillois B: Detection of respiratory syncytial virus, parainfluenzavirus 3, adenovirus and rhinovirus sequences in respiratory tract of infants by polymerase chain reaction and hybridization. Clin Diagn Virol 1997, 8: 31-40. 10.1016/S0928-0197(97)00060-3
Freymuth F, Vabret A, Brouard J, Toutain F, Verdon R, Petitjean J, Gouarin S, Duhamel JF, Guillois B: Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol 1999, 13: 131-139. 10.1016/S1386-6532(99)00030-X
Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F: Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J Virol Methods 2001, 97: 59-66. 10.1016/S0166-0934(01)00343-3
Bellau-Pujol S, Vabret A, Legrand L, Dina J, Gouarin S, Petitjean-Lecherbonnier J, Pozzetto B, Ginevra C, Freymuth F: Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods 2005, 126: 53-63. 10.1016/j.jviromet.2005.01.020
Moine P, Vercken JB, Chevret S, Chastang C, Gajdos P: Severe community-acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community-Acquired Pneumonia in the Intensive Care Unit. Chest 1994, 105: 1487-1495.
Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001, 163: 1256-1276.
Steyerberg EW, Eijkemans MJ, Harrell FE Jr, Habbema JD: Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med 2000, 19: 1059-1079. PublisherFullText10.1002/(SICI)1097-0258(20000430)19:81059::AID-SIM4123.0.CO;2-0
Pascual FE, Matthay MA, Bacchetti P, Wachter RM: Assessment of prognosis in patients with community-acquired pneumonia who require mechanical ventilation. Chest 2000, 117: 503-512. 10.1378/chest.117.2.503
Rello J, Bodi M, Mariscal D, Navarro M, Diaz E, Gallego M, Valles J: Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest 2003, 123: 174-180. 10.1378/chest.123.1.174
Almirall J, Mesalles E, Klamburg J, Parra O, Agudo A: Prognostic factors of pneumonia requiring admission to the intensive care unit. Chest 1995, 107: 511-516.
Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA: Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006, 129: 317-324. 10.1378/chest.129.2.317
Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL: Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006, 173: 1114-1121. 10.1164/rccm.200506-859OC
Danai PA, Sinha S, Moss M, Haber MJ, Martin GS: Seasonal variation in the epidemiology of sepsis. Crit Care Med, in press.
Pregliasco F, Mensi C, Camorali L, Anselmi G: Comparison of RT-PCR with other diagnostic assays for rapid detection of influenza viruses. J Med Virol 1998, 56: 168-173. PublisherFullText 10.1002/(SICI)1096-9071(199810)56:2168::AID-JMV113.0.CO;2-C
Garbino J, Gerbase MW, Wunderli W, Deffernez C, Thomas Y, Rochat T, Ninet B, Schrenzel J, Yerly S, Perrin L, et al.: Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med 2004, 170: 1197-1203. 10.1164/rccm.200406-781OC
Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkaranta A: Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol 2002, 66: 417-420. 10.1002/jmv.2161
Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE: Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006, 173: 639-643. 10.1164/rccm.200510-1681OC
Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O: Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol 2004, 72: 695-699. 10.1002/jmv.20027
van Elden LJ, Nijhuis M, Schipper P, Schuurman R, van Loon AM: Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol 2001, 39: 196-200. 10.1128/JCM.39.1.196-200.2001
Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA: Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J 2000, 16: 677-683. 10.1034/j.1399-3003.2000.16d19.x
Halperin SA, Eggleston PA, Hendley JO, Suratt PM, Groschel DH, Gwaltney JM Jr: Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis 1983, 128: 806-810.
Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, Johnston SL: Rhinoviruses infect the lower airways. J Infect Dis 2000, 181: 1875-1884. 10.1086/315513
Malcolm E, Arruda E, Hayden FG, Kaiser L: Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol 2001, 21: 9-16. 10.1016/S1386-6532(00)00180-3
Nichol KL, Baken L, Nelson A: Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Ann Intern Med 1999, 130: 397-403.
Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M: Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 2003, 348: 1322-1332. 10.1056/NEJMoa025028
Hament JM, Kimpen JL, Fleer A, Wolfs TF: Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol 1999, 26: 189-195. 10.1111/j.1574-695X.1999.tb01389.x
Ishizuka S, Yamaya M, Suzuki T, Takahashi H, Ida S, Sasaki T, Inoue D, Sekizawa K, Nishimura H, Sasaki H: Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 2003, 188: 1928-1939. 10.1086/379833
Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE: Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 2006, 80: 1629-1636. 10.1128/JVI.80.4.1629-1636.2006
Passariello C, Schippa S, Conti C, Russo P, Poggiali F, Garaci E, Palamara AT: Rhinoviruses promote internalisation of Staphylococcus aureus into non-fully permissive cultured pneumocytes. Microbes Infect 2006, 8: 758-766. 10.1016/j.micinf.2005.09.013
Price TM: Letter: Fulminating bacterial pneumonia complicating influenza [letter]. BMJ 1976, 1: 520.
Yap FH, Gomersall CD, Fung KS, Ho PL, Ho OM, Lam PK, Lam DT, Lyon DJ, Joynt GM: Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis 2004, 39: 511-516. 10.1086/422641
Acknowledgements
The authors want to thank the nursing staff of the Lemière and Babinsky units for their important contribution during the conduct of this study, and Fabien Chaillot and Jean-Jacques Duteil, for their expert data management. This study was funded by an academic unrestricted grant 'Appel d'Offre Interne' from the Caen Côte de Nacre University hospital (CD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CD and SV wrote the experimental protocol and initiated the study. AV and FF performed the virologic assessments. J-JP and CD computed the statistical analysis and were involved in the interpretation of the results. CD drafted the manuscript, which was critically revised by J-JP, SV, FF, AV, DdC, MR and PC. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Daubin, C., Parienti, JJ., Vincent, S. et al. Epidemiology and clinical outcome of virus-positive respiratory samples in ventilated patients: a prospective cohort study. Crit Care 10, R142 (2006). https://doi.org/10.1186/cc5059
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc5059