Abstract
Introduction
Prognostic models, such as the Acute Physiology and Chronic Health Evaluation (APACHE) II or III, the Simplified Acute Physiology Score (SAPS) II, and the Mortality Probability Models (MPM) II were developed to quantify the severity of illness and the likelihood of hospital survival for a general intensive care unit (ICU) population. Little is known about the performance of these models in specific populations, such as patients with cancer. Recently, specific prognostic models have been developed to predict mortality for cancer patients who are admitted to the ICU. The present analysis reviews the performance of general prognostic models and specific models for cancer patients to predict in-hospital mortality after ICU admission.
Methods
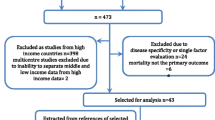
Studies were identified by searching the Medline databases from 1994 to 2004. We included studies evaluating the performance of mortality prediction models in critically ill cancer patients.
Results
Ten studies were identified that evaluated prognostic models in cancer patients. Discrimination between survivors and non-survivors was fair to good, but calibration was insufficient in most studies. General prognostic models uniformly underestimate the likelihood of hospital mortality in oncological patients. Two versions of a specific oncological scoring systems (Intensive Care Mortality Model (ICMM)) were evaluated in five studies and showed better discrimination and calibration than the general prognostic models.
Conclusion
General prognostic models generally underestimate the risk of mortality in critically ill cancer patients. Both general prognostic models and specific oncology models may reliably identify subgroups of patients with a very high risk of mortality.
Similar content being viewed by others
Introduction
Advances in oncological and supportive care have led to improved prognosis and extension of survival time in cancer patients. However, such advances have often been achieved through aggressive therapies and support, at high expense. Some of these patients require admission to the intensive care unit (ICU) for acute concurrent illness, postoperative care, or complications of their cancer or its therapy. Recent studies [1–6] suggest that mortality of cancer patients in the ICU is comparable with that of patient groups suffering from other severe diseases, but others reported a poor prognosis with much higher mortality rates [7]. Efforts have been made to identify parameters that are associated with poor prognosis and to develop scoring models for predicting hospital mortality at ICU admission of cancer patients.
Different prognostic systems, such as the Acute Physiology and Chronic Health Evaluation (APACHE) II or III [8, 9], the Simplified Acute Physiology Score (SAPS) II [10], and the Mortality Probability Models (MPM) II [11], have been developed to predict the outcome of critically ill patients admitted to the ICU. Although these models perform well in predicting the mortality of the general ICU patient population, they may well under- or overestimate mortality in selected patient subpopulations that were not well represented in the original cohort on which the model was developed. Therefore, new models were designed for specific populations, such as cancer patients [12, 13]. By including variables specific to oncology such as disease progression/recurrence, performance status and type of treatment, they were expected to perform better than the general models. The aim of this review is to evaluate the performance of the general severity-of-illness scores (APACHE II and III, SAPS II, MPM II) and the specific oncological scoring systems in cancer patients requiring admission to the ICU.
Methods and materials
Sources and selection criteria
The information in this review is based on results of a Medline search for recent studies published between 1994 and 2004. The key words used included "Severity of illness scores", "Acute Physiology and Chronic Health Evaluation (APACHE)", "Simplified Acute Physiology Score (SAPS)", "Mortality Probability Model II", "Cancer", "Oncology", "Critical care", "Prognosis and outcome" and "Hospital mortality". Based on the title and abstract of the publication, we selected English-language articles containing information on the performance of prognostic models in cancer patients admitted to ICUs. The references of all selected reports were cross-checked for other potentially relevant articles. It was envisaged that the studies would be too heterogeneous to combine for a formal meta-analysis and therefore a narrative synthesis was undertaken.
Results
Performance of the prognostic models
Although several measures exist for evaluating the performance of prognostic models, all identified studies used receiver operating characteristic (ROC) curves and the area under the curve (AUC) [14] to evaluate discrimination and the Hosmer-Lemeshow goodness-of-fit H- or Ĉ-statistics [15] to evaluate the calibration of the prognostic models.
'Discrimination' refers to a model's ability to distinguish survivors from non-survivors. The AUC represents the probability that a patient who died had a higher predicted probability of dying than a patient who survived. An AUC of 0.5 indicates that the model does not predict better than chance. The discrimination of a prognostic model is considered perfect if AUC = 1, good if AUC >0.8, moderate if AUC is 0.6 to 0.8, and poor if AUC <0.6 [16]. The AUC of a model gives no indication of how close the predicted probabilities are to the observed outcome. To take this aspect of a model's performance into account, we have to look at the calibration and accuracy of the prognostic models.
'Calibration' refers to the agreement between predicted probabilities and the 'true probabilities'. Of course, the true probability of a patient's outcome is not known, otherwise there would be no need to develop prognostic models. However, the true probabilities can be approximated by taking the mean of the observed outcomes within predefined groups of patients. The selected studies used Hosmer-Lemeshow H- or Ĉ-statistics. Both H- and Ĉ-statistics compare the observed mortality in a group with the predicted mortality of that group. A disadvantage of the Hosmer-Lemeshow tests is that the value of the statistic is sensitive to the choice of the cut-off points that define the groups. The H- and Ĉ-statistics differ in the way the groups of patients are composed [15]. Grouping for the H-statistic is based on partitioning of the probability interval (0–1) into ten equally sized ranges. The Ĉ goodness-of-fit statistic sorts observations according to their expected probability and partitions the observations into ten groups of equal size. A high H or Ĉ relates to a small p value, implying significant difference between observed and predicted mortality, and thus indicates a lack of fit of the model. It is a generally known weakness of the Hosmer-Lemeshow goodness-of-fit statistics that the sample size has a major influence on the measured calibration. Using small samples will result in an apparently good fit, using large samples will result in an apparently poor fit [17, 18].
Discrimination and calibration describe the overall predictive power of a model. This is important when analyzing the mortality risk of a population, for example, to determine performance of an ICU by measuring the standardized mortality ratio (SMR: observed mortality divided by predicted mortality) as mortality adjusted for severity-of-illness. When caring for an patient, it is more important that a model can reliably predict the likelihood of an outcome of an individual patient; this is called 'accuracy'. Accuracy refers to the difference between predictions and observed outcomes at the level of individuals. The mean squared error (MSE), also called Brier score, is an example of an accuracy measure [19]. None of the selected studies evaluated accuracy measures. However, some studies notify that when caring for an individual patient, it is more important that a model can reliably identify patients with a very high risk of dying. Therefore, they evaluated the performance of prognostic models at specific cut-off points, dividing high-risk patients from low-risk patients.
Discrimination
Nine studies reported on discriminating ability of general prognostic models in ICU patients with cancer (Table 1) [12, 16, 20–26]. The APACHE II score was evaluated in six studies and showed poor to good discriminating value with areas under the ROC curve between 0.60 and 0.78 [16, 20–22, 25, 26]. Discrimination of the SAPS II model was fair to good with areas under the ROC curve between 0.67 and 0.83 [20–23, 25, 26]. The MPM II model showed poor discrimination in one study [12], but good discrimination in another [26]. Discrimination of all models differed importantly between studies. All models showed fair to good discrimination in the study by Soares [26] and poor discrimination in the study by Sculier [20]. This may be related to differences in casemix of patients; whereas most patients in the latter trial had metastatic or disseminated haematological disease, most patients in the study by Soares had locoregional cancer or cancer in remission only.
In 1998, Groeger and others developed a model specific for cancer patients [12]. It included physiological data, disease-related variables (allogeneic bone marrow transplantation and recurrent or progressive cancer), and performance status before hospitalization. Tested on an independent set of patients, the model showed good discriminating power with an area under the ROC curve of 0.81. Good discriminating ability was confirmed in two other studies [22, 26]. In 2003, Groeger et al. developed another specific model with good discriminating performance (AUC = 0.82) that predicts in-hospital mortality in cancer patients at 72 h after ICU admission [13].
Calibration
As shown in Table 1, most studies showed poor calibration for APACHE II and III, SAPS II and MPM II [12, 20, 22, 26]. The studies that have good H- or Ĉ-statistics (p>0.05) are very small. Poor calibration resulted in a uniform underestimation of the mortality risk using the general prognostic models. Hence, the observed mortality was uniformly higher than the predicted mortality (SMR>1). In contrast with the general prognostic models, the specific models for cancer patients showed good calibration with SMR of 1.0 to 1.05 [12, 13, 21] or poor calibration resulting in a uniform overestimation of mortality risk (SMR 0.75 [26]).
Identification of subgroups at (very) high risk
It may be particularly important to identify patients at very high risk of dying. Patients with limited life expectancy do not necessarily prefer life-extending treatment over care focused on relieving pain and discomfort. The willingness to receive life-sustaining treatment depends on the burden of treatment, the outcome and the likelihood of the outcome [27]. In patients aged 65 years and older, the willingness to receive cardiopulmonary resuscitation if they suffered a cardiac arrest decreased from 41 to 22% after learning the probability of survival (10 to 17%) [28].
Although none of the selected studies report on accuracy measures, some studies report on the performance of prognostic models at specific cut-off points for predicted mortality. Results are summarized in Table 2. High predicted mortality by the general prognostic models as well as the specific ICU Cancer Mortality Model (ICCM) was associated with very high observed mortality rates. For example, in a study by Staudinger and others, 7% of the studied population had more than 79 APACHE III points, and all of these patients died before hospital discharge [24]. In another study by Sculier and others, 5.4% of patients had a predicted mortality of >70% according to the APACHE II model. In these patients, the actual observed mortality was 86% [20]. Thus, in a limited number of cancer patients, a very high mortality chance after ICU admission may be predicted. It may be speculated that some patients would prefer not to undergo intensive care treatment if their predicted mortality is very high, for example, more than 80 or 90%. Providing prognostic information to patients, their relatives and physicians could help to provide intensive care that is more in accordance to patients' own preferences.
Discussion
The general prognostic models for ICU patients generally underestimate the risk of dying for cancer patients admitted to ICUs. This is important when interpreting SMRs of different ICUs, since ICUs with relatively more cancer patients will have a higher SMR. However, these models are able to identify subgroups of patients with a very high mortality risk. Thus, they may have a role in giving information about the prognosis to patients and their relatives.
Only a few models exist that were specifically designed for cancer patients and which include data about type and stage of cancer, and functional status of patients. These models showed better discrimination and calibration than the general models. Thus, they may have a role in comparing SMRs of cancer patient populations in the ICU. However, they have been validated in relatively few patients and new larger studies are required to confirm the value of these models. Most studies had a retrospective design and limited number of patients, and the moderate differences among the scoring systems do not allow conclusion of the superiority of one of them. Because of large variations in their design (type of patients, observed mortality (33 to 60%), mix of H- and Ĉ-statistics), it is difficult to perform meaningful comparisons between them. Different casemixes, national or regional patient populations and critical care management can lead to different outcomes.
A limitation of all models is the fact that they do not take into account that better treatments may become available and that prognosis may improve over time. Indeed, it has been shown that survival of patients after haematopoietic stem cell transplantation who received mechanical ventilation, improved from lower than 10% in the period before 1990 to 25 to 50% in the period 1994 to 2000 [16]. Thus, prognostic information should be interpreted cautiously. Nevertheless, patients and physicians need optimal information about the likelihood of a beneficial outcome of intensive care treatment. Prognostic models do at least contribute to this information.
Conclusion
The general prognostic models for ICU patients generally underestimate the risk of dying for cancer patients admitted to ICUs. Models specific for cancer patients show better calibration and discrimination than the general models. Both general models and specific oncology models may reliably identify subgroups of patients with a very high mortality risk and thus may be useful to inform patients and their relatives about the likelihood of a beneficial outcome.
Key messages
-
General prognostic models for ICU patients, such as APACHE II or SAPS II, tend to underestimate the risk of dying for patients with cancer admitted to ICUs.
-
Prognostic models specifically designed for ICU patients with cancer show better calibration and discrimination than the general models.
-
Both general models and specific oncology models reliably identify subgroups of patients with a very high risk of dying.
Abbreviations
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- AUC:
-
area under the curve
- ICCM:
-
ICU Cancer Mortality Model
- ICU:
-
intensive care unit
- MPM:
-
Mortality Probability Model
- ROC:
-
receiver operating curve
- SAPS:
-
Simplified Acute Physiology Score
- SMR:
-
standardized mortality rate.
References
Baumann WR, Jung RC, Koss M, Boylen CT, Navarro L, Sharma OP: Incidence and mortality of adult respiratory distress syndrome: a prospective analysis from a metropolitan hospital. Crit Care Med 1986, 14: 1-4.
Sussman NL, Lake JR: Treatment of hepatic failure 1996: current concepts and progress toward liver dialysis. Am J Kidney Dis 1996, 27: 605-621.
Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, Hammerle AF, Steltzer H: The acute respiratory distress syndrome: definitions, severity and clinical outcome. An analysis of 101 clinical investigations. Intensive Care Med 1996, 22: 519-529.
Marik PE, Craft M: An outcomes analysis of in-hospital cardiopulmonary resuscitation: the futility rationale for do not resuscitate orders. J Crit Care 1997, 12: 142-146. 10.1016/S0883-9441(97)90044-7
The British Thoracic Society Research Committee and The Public Health Laboratory Service: The aetiology, management and outcome of severe community-acquired pneumonia on the intensive care unit. Respir Med 1992, 86: 7-13.
Massion PB, Dive AM, Doyen C, Bulpa P, Jamart J, Bosly A, Installe E: Prognosis of hematologic malignancies does not predict intensive care mortality. Crit Care Med 2002, 30: 2260-2270. 10.1097/00003246-200210000-00014
Sculier JP, Markiewicz E: Medical cancer patients and intensive care. Anticancer Res 1991, 11: 2171-2174.
Knaus W, Draper E, Wagner D, Zimmerman J: APACHE II: a severity of disease classification system. Crit Care Med 1985, 13: 818-829.
Knaus W, Wagner D, Draper E, Zimmerman J, Bergner M, Bastos P, Sirio C, Murphy D, Lotring T, Damiano A, Harrel F: The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalised adults. Chest 1991, 100: 1619-1636.
Le Gall J-R, Lemeshow SS, Saulnier F: A new Simplified Acute Physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270: 2957-2963. 10.1001/jama.270.24.2957
Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J: Mortality Probability Models (MPMII) based on an international cohort of intensive care unit patients. JAMA 1993, 270: 2478-2486. 10.1001/jama.270.20.2478
Groeger JS, Lemeshow S, Price K, Nierman DM, White P Jr, Klar J, Granovsky S, Horak D, Kish SK: Multicenter outcome study of cancer patients admitted to the intensive care unit: a probability of mortality model. J Clin Oncol 1998, 16: 761-770.
Groeger JS, Glassman J, Nierman DM, Wallace SK, Price K, Horak D, Landsberg D: Probability of mortality of critically ill cancer patients at 72 h of intensive care unit (ICU) management. Support Care Cancer 2003, 11: 686-695. 10.1007/s00520-003-0498-9
Hanley JA, McNeil BJ: The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143: 29-36.
Hosmer DW, Lemeshow S: Applied logistic regression. New York: John Wiley & Sons; 2000.
Afessa B, Tefferi A, Dunn WF, Litzow MR, Peters SG: Intensive care unit support and Acute Physiology and Chronic Health Evaluation III performance in haematopoietic stem cell transplant recipients. Crit Care Med 2003, 31: 1715-1721. 10.1097/01.CCM.0000065761.51367.2D
Zhu BP, Lemeshow S, Hosmer DW, Klar J, Avrunin J, Teres D: Factors affecting the performance of the models in the Mortality Probability Model II system and strategies of customization: a simulation study. Crit Care Med 1996, 24: 57-63. 10.1097/00003246-199601000-00011
Vergouwe Y, Steyerberg EW, Eijkemans R, Habbema D: Sample size considerations for the performance assessment of predictive models: A simulation study. Control Clin Trials 2003, S43-S44.
Brier G: Verification of forecasts expressed in terms of probability. Monthly Weather Rev 1950, 78: 1-3.
Sculier JP, Paesmans M, Markiewicz E, Berghmans T: Scoring systems in cancer patients admitted for an acute complication in a medical intensive care unit. Crit Care Med 2000, 28: 2786-2792. 10.1097/00003246-200008000-00018
Schellongowski P, Benesch M, Lang T, Traunmuller T, Zauner C, Laczika K, Locker GJ, Frass M, Staudinger T: Comparison of three severity scores for critically ill cancer patients. Intensive Care Med 2004, 30: 430-436. 10.1007/s00134-003-2043-1
Berghmans T, Paesmans M, Sculier JP: Is a specific oncological scoring system better at predicting the prognosis of cancer patients admitted for an acute medical complication in an intensive care unit than general gravity scores? Support Care Cancer 2004, 12: 234-239. 10.1007/s00520-003-0580-3
Guiguet M, Blot F, Escudier B, Antoun S, Leclercq B, Nitenberg G: Severity-of-illness scores for neutropenic cancer patients in an intensive care unit: Which is the best predictor? Do multiple assessment times improve the predictive value? Crit Care Med 1998, 26: 488-493. 10.1097/00003246-199803000-00020
Staudinger T, Stoiser B, Mullner M, Locker GJ, Laczika K, Knapp S, Burgmann H, Wilfing A, Kofler J, Thalhammer F, Frass M: Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med 2000, 28: 1322-1328. 10.1097/00003246-200005000-00011
Benoit D, Vandewoude K, Decruyenaere J, Hoste E, Colardyn F: Outcome and early prognostic indicators in patients with a haematological malignancy admitted to the intensive care unit for a life-threatening complication. Crit Care Med 2003, 31: 104-112. 10.1097/00003246-200301000-00017
Soares M, Fontes F, Dantas J, Gadelha D, Cariello P, Nardes F, Amorim C, Toscano L, Rocco J: Performance of six severity-of-illness scores in cancer patients requiring admission to the intensive care unit: a prospective observational study. Crit Care 2004, 8: R194-R203. 10.1186/cc2870
Fried T, Bradley E, Towle V, Allore H: Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002, 346: 1061-1066. 10.1056/NEJMsa012528
Murphy D, Burrows D, Santilli S, Kemp A, Tenner S, Kreling B, Teno J: The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation. N Engl J Med 1994, 330: 545-549. 10.1056/NEJM199402243300807
Kroschinsky F, Weise M, Illmer T, Haenel M, Bornhaeuser M, Hoeffken G, Ehninger G, Schuler U: Outcome and prognostic features of intensive care unit treatment in patients with haematological malignancies. Intensive Care Med 2002, 28: 1294-1300. 10.1007/s00134-002-1420-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they do not have competing interests.
Authors' contributions
SdB and EdJ acquired and interpreted the data. NFdK interpreted the data. All authors contributed in preparing the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
den Boer, S., de Keizer, N.F. & de Jonge, E. Performance of prognostic models in critically ill cancer patients – a review. Crit Care 9, R458 (2005). https://doi.org/10.1186/cc3765
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc3765