Abstract
Reduced microvascular perfusion has been implicated in organ dysfunction and multiple organ failure associated with severe sepsis. The precise mechanisms underlying microvascular dysfunction remain unclear, but there are considerable experimental data showing reduced microcirculatory flow, particularly of small vessels, and increased heterogeneity. With the development of newer imaging techniques, human studies have also been conducted and have given rise to similar findings. Importantly, the degree of microvascular disturbance and its persistence is associated with poorer outcomes. The ability to influence these changes may result in better outcomes and bedside systems, enabling direct visualization of the microcirculation, which will help in the assessment of ongoing microcirculatory dysfunction and its response to established and new therapeutic interventions.
Similar content being viewed by others
Introduction
Organ dysfunction and multiple organ failure (MOF) occur frequently in patients with sepsis, even after apparent restoration of stable systemic hemodynamics. The mechanisms underlying these effects are unclear but, increasingly, alterations in microvascular blood flow and oxygenation are being implicated. An intact and correctly functioning microcirculatory system is essential for efficient tissue oxygen delivery, yet, in sepsis, mediators of the inflammatory response impair microcirculatory function. The precise mechanisms involved are unclear but include a reduction in the number of perfused capillaries (so-called reduced functional capillary density), reduced red blood cell deformability, endothelial cell dysfunction with increased permeability and apoptosis, altered vasomotor tone, increased numbers of activated neutrophils with more neutrophil–endothelial interactions due to increased endothelial expression of surface adhesion molecules, and activation of the clotting cascade with fibrin deposition.
Supported by considerable experimental data, these changes are now beginning to be quantified clinically using newer monitoring techniques, including orthogonal polarization spectral (OPS) imaging. The ability to visualize the microcirculatory changes in sepsis brings about the possibility of assessing the impact of therapeutic strategies directly on this critical organ system, and perhaps of being able to target the microcirculation as a resuscitation endpoint.
Experimental studies
Alterations in microvascular blood flow have been described in various experimental models of sepsis [1–5]. In a normodynamic model using cecal ligation and perforation in rats, Lam and colleagues [2] reported a 36% reduction in perfused capillary density and a 265% increase in stopped-flow capillaries in striated muscles. They also reported that the spatial distribution of perfused capillaries was 72% more heterogeneous in the septic rats compared with sham-operated rats. Other groups using the same model have reported similar findings of reduced perfused capillary density and increased heterogeneity in intestinal mucosa [5–7]. In dogs, Drazenovic and colleagues [4] reported decreased capillary density in the small intestine after endotoxin administration. Nakajima and colleagues [8] reported that mice given endotoxin developed a significant decrease in intestinal mucosal perfusion characterized by a diminished red blood cell velocity and flux in villi, and a significant decrease in the number of perfused villi. Interestingly, these authors [8] reported that, for the same level of hypotension, mucosal perfusion disorders were considerably larger in endotoxin-induced hypotension than in hemorrhagic hypotension. The heterogeneity of microvascular blood flow may help explain some of the alterations in oxygen extraction capabilities that are seen in sepsis. Walley [9] indicated, using a mathematical model, that an increase in blood flow heterogeneity was associated with an increase in critical delivery of oxygen, and animal models have shown that gut and muscle blood flow heterogeneity increases together with impaired oxygen extraction after endotoxin administration or fecal peritonitis [10, 11].
Clinical studies
Extrapolating from animal models to the clinical situation is difficult and, until relatively recently, human studies have been hampered by a lack of adequate techniques to investigate the microcirculation. Visualizing the microcirculation in humans has relied on the use of large microscopes, thus limiting investigation to the nailfold area. Nevertheless, even with such limitations, as long ago as 1922 Freedlander and Lenhart [12] noted capillary constriction and decreased flow in the nailfold capillaries in patients with septic shock. More recently, Weinberg and colleagues [13] reported that nailfold capillary blood cell velocity was decreased in normotensive febrile patients. Using venous air plethysmography, Astiz and colleagues [14] and Kirschenbaum and colleagues [15] observed that the forearm skin blood flow response to transient ischemia was decreased in patients with septic shock compared with controls, suggesting impaired microvascular blood flow. Neviere and colleagues [16] reported similar findings for leg blood flow during reactive hyperemia. However, laser Doppler and plethysmography do not take into account the heterogeneity of blood flow, an important and consistent finding in animal studies.
The last decade or so has witnessed the advent of OPS imaging, and using this technique we are beginning to gather considerable human data which largely agree with the information provided by earlier experimental studies. OPS imaging techniques allow the investigation of the microvascular circulation in tissues covered by a thin epithelial layer, for example sublingual mucosa, ileostomy or colostomy, and rectal mucosa or vaginal mucosa. Of these, the sublingual area has been most extensively studied. Importantly, OPS imaging technology can be incorporated easily into a small hand-held device making it ideal for monitoring at the bedside. The technique has been described in detail elsewhere [17] but, briefly, the area of interest is illuminated by polarized light, which is reflected by the background and absorbed by hemoglobin. Specific optical filtration allows light reflected at the surface of the tissue to be eliminated from the image, so producing high-contrast reflected-light images of the microcirculation. Using a second polarizer oriented orthogonal to the first, the image appears to be back-illuminated and small blood vessels and individual red and white blood cells can be clearly seen (of note, white blood cells can be visualized only at the highest magnification). By definition, only vessels containing red blood cells will be visualized, whatever the type of flow, while vessels containing no red blood cells (total collapse or containing plasma only) will not be visualized. Hence, both vessel density and perfusion of the visualized vessels are important.
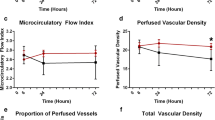
In a study using OPS on the sublingual circulation in 10 healthy volunteers, 16 patients before cardiac surgery, 10 acutely ill patients without sepsis, and 50 patients with severe sepsis, De Backer and colleagues [18] reported that, while there were no significant differences in microvascular blood flow among healthy volunteers, patients before cardiac surgery and patients without sepsis, the density of all vessels and the proportion of perfused small (< 20 μm) vessels were significantly reduced in patients with severe sepsis compared with volunteers (Fig. 1). These changes in microvascular flow were more severe in nonsurvivors (median proportion of perfused vessels = 48% [25th–75th percentile, 32–60%] versus 62% [48–70%]; P < 0.01). This decrease in the proportion of well perfused small vessels was due to a combined increase in nonperfused and intermittently perfused vessels. Similar findings were later reported by Spronk and colleagues [19] in a small series of patients with septic shock. In a more recent study by our group, the OPS technique was used to follow microvascular alterations in 49 patients with septic shock [20]; 20 of the patients died, 13 due to unresolved shock, and seven due to persistent MOF after resolution of shock. OPS measurements were obtained within 24 hours of the onset of shock and then daily up to the resolution of shock or death, with a maximum of 7 days. At the onset of shock, survivors and nonsurvivors had similar vascular density (5.6/mm [4.7–7.0/mm] versus 6.2/mm [5.4–7.0/mm]) and percentage of perfused small vessels (65.0% [53.1–68.9%] versus 58.4% [47.5–69.1%]). However, small-vessel perfusion improved over time in survivors but not in nonsurvivors. Additionally, changes in microvascular perfusion from day 1 to day 2 were more predictive of outcome than changes in global hemodynamics or lactate levels during the same period of time. Finally, despite similar hemodynamic and oxygenation profiles and use of vasopressors at the end of shock, patients dying after the resolution of shock in MOF had a lower percentage of perfused small vessels than survivors (57.4% [46.6–64.9%] versus 79.3% [67.2–83.2%]; P = 0.02) (Fig. 2). These findings support the concept that microvascular alterations are indeed associated with organ dysfunction and that their persistence is indicative of poor outcome.
Box plot demonstrating the time course of small vessel perfusion in survivors, patients dying in shock, and patients dying after resolution of shock due to persistent multiple organ failure (MOF). The numbers above the boxes show the numbers of patients at each time point. The evolution was significantly different between survivors and patients dying in shock or dying after the resolution of shock due to persistent MOF (*analysis of variance, P < 0.05). Small vessel perfusion increased only in survivors (P < 0.05). Reproduced with permission from [20].
The future
The exciting images of the microcirculation now being obtained, and evidence that survivors show improved microcirculatory flow compared with nonsurvivors, have given impetus to attempts to target the microcirculation with therapeutic interventions. Microvascular recruitment may indeed be a valid target for resuscitation in patients with septic shock [21, 22]. Local sublingual application of acetylcholine has been shown to reverse microcirculatory alterations in patients with septic shock treated with high doses of vasoactive agents, even in nonsurvivors [18], and Spronk and colleagues [19] reported that intravenous nitroglycerin administration increased sublingual microcirculatory flow in patients with septic shock. These findings suggest that microvascular dysfunction may be amenable to the correct therapy administered at the right time, although further studies are needed to identify which interventions may benefit the microcirculation. Resuscitation of the patient with severe sepsis has so far relied on surrogate targets of regional oxygenation, including blood lactate levels and mixed venous oxygen saturation (SvO2). Being able to directly visualize and quantify microcirculatory changes at the bedside, and to follow the course of such alterations during treatment, may enable us to optimize therapies better at an individual patient level.
Conclusion
The microcirculation is a vast system responsible for the transport and delivery of oxygen to tissues throughout the body. Microvascular dysfunction with reduced perfusion and oxygenation results in tissue hypoxia and, ultimately, in organ failure. In sepsis, microcirculatory alterations are more complex and, as new techniques for monitoring this difficult-to-access "organ" become available, the extent of microvascular dysfunction and the role it may have in promoting sepsis-related organ dysfunction are only now beginning to be evaluated. Whatever the underlying etiology, many believe that impaired microcirculatory perfusion is the final common pathway in the development of MOF, and ultimately death, and recent studies using OPS have indeed suggested that there is a connection between microcirculatory alterations and MOF in sepsis.
If microvascular dysfunction is the key to the development of MOF in sepsis, the microcirculation should be a key therapeutic target. Adequate and early fluid resuscitation and cardiovascular support are known to improve outcomes from septic shock [23], presumably by improving microcirculatory flow and tissue perfusion, and the use of newer techniques to visualize the microcirculation may soon provide some answers to the many questions that remain surrounding the mechanisms underlying microvascular dysfunction and its links to organ dysfunction.
Abbreviations
- OPS:
-
OPS = orthogonal polarization spectral
- SvO:
-
SvO2 = venous oxygen saturation
- MOF:
-
MOF = multiple organ failure.
References
Cryer HM, Garrison RN, Kaebnick HW, Harris PD, Flint LM: Skeletal microcirculatory responses to hyperdynamic Escherichia coli sepsis in unanesthetized rats. Arch Surg 1987, 122: 86-92.
Lam C, Tyml K, Martin C, Sibbald W: Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest 1994, 94: 2077-2083.
Piper RD, Pitt-Hyde M, Li F, Sibbald WJ, Potter RF: Microcirculatory changes in rat skeletal muscle in sepsis. Am J Respir Crit Care Med 1996, 154: 931-937.
Drazenovic R, Samsel RW, Wylam ME, Doerschuk CM, Schumacker PT: Regulation of perfused capillary density in canine intestinal mucosa during endotoxemia. J Appl Physiol 1992, 72: 259-265. 10.1063/1.352124
Farquhar I, Martin CM, Lam C, Potter R, Ellis CG, Sibbald WJ: Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J Surg Res 1996, 61: 190-196. 10.1006/jsre.1996.0103
Madorin WS, Martin CM, Sibbald WJ: Dopexamine attenuates flow motion in ileal mucosal arterioles in normotensive sepsis. Crit Care Med 1999, 27: 394-400. 10.1097/00003246-199902000-00048
Sielenkamper AW, Meyer J, Kloppenburg H, Eicker K, Van Aken H: The effects of sepsis on gut mucosal blood flow in rats. Eur J Anaesthesiol 2001, 18: 673-678. 10.1046/j.1365-2346.2001.00905.x
Nakajima Y, Baudry N, Duranteau J, Vicaut E: Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med 2001, 164: 1526-1530.
Walley KR: Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: theory. J Appl Physiol 1996, 81: 885-894.
Humer MF, Phang PT, Friesen BP, Allard MF, Goddard CM, Walley KR: Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J Appl Physiol 1996, 81: 895-904.
Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R: Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. Am J Physiol Heart Circ Physiol 2002, 282: H156-H164.
Freedlander SO, Lenhart CH: Clinical observations on the capillary circulation. Arch Intern Med 1922, 29: 12-32.
Weinberg JR, Boyle P, Thomas K, Murphy K, Tooke JE, Guz A: Capillary blood cell velocity is reduced in fever without hypotension. Int J Microcirc Clin Exp 1991, 10: 13-19.
Astiz ME, DeGent GE, Lin RY, Rackow EC: Microvascular function and rheologic changes in hyperdynamic sepsis. Crit Care Med 1995, 23: 265-271. 10.1097/00003246-199502000-00011
Kirschenbaum LA, Astiz ME, Rackow EC, Saha DC, Lin R: Microvascular response in patients with cardiogenic shock. Crit Care Med 2000, 28: 1290-1294. 10.1097/00003246-200005000-00005
Neviere R, Mathieu D, Chagnon JL, Lebleu N, Millien JP, Wattel F: Skeletal muscle microvascular blood flow and oxygen transport in patients with severe sepsis. Am J Respir Crit Care Med 1996, 153: 191-195.
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG: Orthogonal polarisation spectral imaging: a new method for study of the microcirculation. Nat Med 1999, 5: 1209-1212. 10.1038/13529
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL: Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002, 166: 98-104. 10.1164/rccm.200109-016OC
Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF: Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002, 360: 1395-1396. 10.1016/S0140-6736(02)11393-6
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL: Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004, 32: 1825-1831. 10.1097/01.CCM.0000138558.16257.3F
Buwalda M, Ince C: Opening the microcirculation: can vasodilators be useful in sepsis? Intensive Care Med 2002, 28: 1208-1217. 10.1007/s00134-002-1407-2
Spronk PE, Zandstra DF, Ince C: Bench-to-bedside review: sepsis is a disease of the microcirculation. Crit Care 2004, 8: 462-468. 10.1186/cc2894
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001, 345: 1368-1377. 10.1056/NEJMoa010307
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors received reimbursements, institutional funds and honoraria from Eli Lilly and Company.
Rights and permissions
About this article
Cite this article
Vincent, JL., De Backer, D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit Care 9 (Suppl 4), S9 (2005). https://doi.org/10.1186/cc3748
Published:
DOI: https://doi.org/10.1186/cc3748