Abstract
Introduction
Based on the hypothesis that failure of weaning from mechanical ventilation is caused by respiratory demand exceeding the capacity of the respiratory muscles, we evaluated whether extubation failure could be characterized by increased respiratory drive and impaired efficiency to generate inspiratory pressure and ventilation.
Methods
Airway pressure, flow, volume, breathing frequency, and diaphragm electrical activity were measured in a heterogeneous group of patients deemed ready for a spontaneous breathing trial. Efficiency to convert neuromuscular activity into inspiratory pressure was calculated as the ratio of negative airway pressure and diaphragm electrical activity during an inspiratory occlusion. Efficiency to convert neuromuscular activity into volume was calculated as the ratio of the tidal volume to diaphragm electrical activity. All variables were obtained during a 30-minute spontaneous breathing trial on continuous positive airway pressure (CPAP) of 5 cm H2O and compared between patients for whom extubation succeeded with those for whom either the spontaneous breathing trial failed or for those who passed, but then the extubation failed.
Results
Of 52 patients enrolled in the study, 35 (67.3%) were successfully extubated, and 17 (32.7%) were not. Patients for whom it failed had higher diaphragm electrical activity (48%; P < 0.001) and a lower efficiency to convert neuromuscular activity into inspiratory pressure and tidal volume (40% (P < 0.001) and 53% (P < 0.001)), respectively. Neuroventilatory efficiency demonstrated the greatest predictability for weaning success.
Conclusions
This study shows that a mixed group of critically ill patients for whom weaning fails have increased neural respiratory drive and impaired ability to convert neuromuscular activity into tidal ventilation, in part because of diaphragm weakness.
Trial Registration
Clinicaltrials.gov identifier NCT01065428. ©2012 Liu et al.; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Similar content being viewed by others
Introduction
Mechanical ventilation is essential for patients with acute respiratory failure, yet it is associated with risks and complications that prolong its duration and increase the risk of death [1]. Mechanically ventilated patients can develop rapid and severe diaphragm weakness [2–8]. Compared with successfully weaned patients, patients for whom weaning fails have increased respiratory loads [9] and demonstrate increased respiratory pressures during spontaneous breathing trials (SBTs) [9–11]. This scenario agrees well with the hypothesis that weaning failure is partly due to the patients' respiratory loads exceeding the capacity of their respiratory muscles [12–16]. If this is correct, then difficult-to-wean patients would be expected to demonstrate increased respiratory drive and compromised ventilatory breathing efficiency.
The introduction of Neurally Adjusted Ventilatory Assist (NAVA) [17] has made available a standardized and validated method to monitor and measure diaphragm electrical activity (EAdi) [18] during conventional modes of ventilation as well as during spontaneous breathing trials. The EAdi allows quantification of the neural respiratory drive to the diaphragm [19, 20]. If expressed in relation to airway pressure (Paw) during an inspiratory effort against an occluded respiratory circuit, the Paw/EAdi ratio provides an estimate of inspiratory pressure generation normalized to neural inspiratory effort (that is, the neuromechanical efficiency (NME)) [21]. If the tidal volume (Vt) is expressed in relation to EAdi, the Vt/EAdi ratio expresses the ability to generate volume normalized to neural drive (that is, the neuroventilatory efficiency (NVE)). A recent study suggests that these physiological indices (EAdi, NME, and NVE) may predict readiness for extubation in chronic obstructive pulmonary disease (COPD) patients [22].
The aim of this study was to examine whether a general patient population for whom extubation fails, when compared with successfully extubated patients, have increased EAdi, reflecting a higher neural respiratory drive, have lowered NME as an indication of more pronounced respiratory muscle weakness, and have a reduction in NVE due to a combination of weakness and increased load.
Materials and methods
The trial was conducted in a 20-bed general intensive care unit (ICU) of a teaching hospital affiliated with Southeast University in China. The protocol was approved by Jiangsu Institutional Ethics Committee (Approval Number: 2009ZDLL012.0), and written informed consent was obtained from the patients or next of kin. The trial was registered at clinicaltrials.gov (NCT01065428).
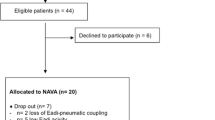
Intubated patients receiving controlled mechanical ventilation for >24 hours were screened and were enrolled if they did not meet exclusion criteria (Figure 1). The enrolled patients were switched to a Servo-i ventilator (Maquet, Solna, Sweden), and the conventional nasogastric tube was replaced by one capable of measuring the EAdi (Maquet). Patients were then ventilated with pressure support (PSV) or synchronized intermittent mandatory ventilation (SIMV) + PSV. The following morning, patients were screened by the clinical team, and an SBT was performed if (a) the cause of mechanical ventilation was resolved, (b) PaO2/FiO2 > 200; PEEP ≤5 cm H2O; FiO2 ≤50%; and respiratory frequency (f) <35 breath/min; (c) patients were hemodynamically stable (heart rate <140 beats/min, no vasopressors required, or <5 μg/kg/min dopamine); (d) no sedation or receiving minimal sedation with a low dose of morphine (<3 mg/h, by continuous intravenous infusion); and (e) patients were breathing spontaneously with adequate cough. Cough was evaluated by placing a white card about 1.5 cm away from the end of the endotracheal tube and asking the patient to cough (3 to 4 times). Adequate cough was considered if wetness appeared on the card [23]. If the criteria were not fulfilled, a new screening was performed the next morning.
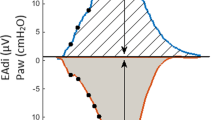
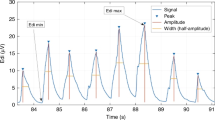
The protocol started with patients ventilated on a PSV of 10 cm H2O above a positive end-expiratory pressure (PEEP) of 5 cm H2O (PSV10) for 5 minutes. Then assist was removed, and a 30-minute SBT was performed with continuous positive airway pressure (CPAP) of 5 cm H2O at the prescribed level of FiO2. At 1, 5, 10, 15, and 30 minutes into the SBT, inspiratory occlusions (starting at end expiration) were performed for one or two breath cycles by using the internal Servo-i feature.
Patients who reached the criteria for failed SBT: (a) impaired gas exchange (SpO2 <90%; PaO2 <60 mm Hg; increase in PaCO2 >10 mm Hg); (b) hemodynamic instability (heart rate changed >20%; systolic blood pressure (BP) >180 or <90 mm Hg; BP changed >20%; vasopressors required); (c) unstable ventilatory pattern (>35 breaths/min; Vt <4 ml/kg); (d) change in mental status (somnolence, coma, agitation, anxiety), diaphoresis, and other onset or worsening of discomfort deemed by the clinical team, resumed mechanical ventilation. Patients who were able to tolerate the 30-minute SBT without reaching the exclusion criteria were extubated. Patients who were extubated from a completed SBT and remained extubated for more than 48 hours were considered to be successfully extubated [24].
Patients who resumed mechanical ventilation during the SBT, or required noninvasive ventilation (NIV) after extubation, or were reintubated, or deceased within 48 hours after extubation were considered to have failed extubation. The criteria for reintubation were (a) emergency status, such as respiratory or cardiac arrest, and gasping for air; (b) neurologic deterioration (coma or agitation requiring continuous intravenous sedation); (c) hemodynamic instability (that is, need for continuous infusion of epinephrine, norepinephrine, or dopamine (>5 µg/kg/min) to maintain systolic arterial pressure >90 mm Hg); (d) upper airway obstruction; (e) unmanageable tracheobronchial secretions; and (f) respiratory distress, as assessed by the combination of SpO2 <90% (SpO2 <85% for COPD patients), respiratory rate >35/min, and visible accessory muscle recruitment or thoracoabdominal paradox, despite administration of oxygen and noninvasive ventilation.
In case of extubation failure, NIV was administered through an oronasal mask when an FiO2 of 0.4 failed to maintain SpO2 ≥90% (SpO2 ≥85% for COPD patients), and the patient showed evidence of respiratory distress, as assessed by the combination of tachypnea (>30 breaths/min) and visible accessory muscle recruitment.
Throughout the protocol, EAdi, Paw, flow, VT, f, and minute ventilation (VE) were acquired from the Servo-I ventilator into a personal computer, by using "Servo-tracker" software (Maquet). Arterial blood gases were measured at the end of PSV10 and at 5 and 30 minutes (or at termination) of the SBT.
NME was calculated as the ratio of the (Paw-PEEP) divided by EAdi during inspiratory occlusion. NVE was calculated as the ratio of Vt and EAdi during inspiration. All parameters and indices (except NME) were calculated as the mean value of five inspirations at each time point. NVE was calculated from one inspiratory occlusion at each time point.
Statistics
Statistical analysis was performed with SPSS 13.0 (IBM, Minneapolis, MN, USA) and SigmaStat 3.5 (Systat Software Inc., Chicago, IL, USA). Results are presented as mean ± SD, and significant difference was defined as P < 0.05. A t test for independent variables and χ2 tests were used to compare group characteristics and causes of ARF. Two-way repeated measures ANOVA with Student-Newman-Keuls post hoc comparison test was used to compare variables between groups and time points. Spearman correlation and linear regression analysis was used to determine relations between variables. ROC (receiver operating characteristic) curves were generated, and the area under the curve (AUC) was calculated, as well as sensitivity, specificity, and the Youden index, to determine the utility of measured variables to predict extubation outcome. Generalized U statistics were used to compare areas under the ROC curves [25].
Results
In total, 404 patients were screened; 352 patients met exclusion criteria, and 52 patients were eligible (Figure 1). Of the 52 patients enrolled, 35 (67.3%) were successfully extubated, and for 17 (32.7%), extubation failed. Two extubation-failure patients died because of refusal to reintubate by the family.
All patients discontinued sedative drugs (propofol, midazolam) at least 1 hour before the SBT. Morphine delivery was discontinued in nine (25.7%) and six (35.3%) of the successfully extubated and failed-extubation groups (NS), respectively. In the remainder of the patients, low-dose morphine infusion was 1.1 ± 0.5 mg/h and 1.2 ± 0.5 mg/h for the successfully extubated and failed-extubation groups (NS), respectively. Clinical characteristics of the patients are presented in Table 1.
EAdi, NVE, and NME
The progression of EAdi, NVE, and NME from PSV10 and throughout the SBT are depicted in Figure 2 for both the successful and the failed-extubation groups.
The development of (top to bottom) diaphragm electrical activity (EA di ), neuromechanical efficiency (NVE), and neuroventilatory efficiency (NME) during pressure support of 10 above 5 cm H 2 O of PEEP (PSV 10 ) and throughout the spontaneous breathing trial (SBT) on CPAP of 5 cm H 2 O. On the X-axis: T1, T5, T10, T15, T30, and Tend indicate minutes 1, 5, 10, 15, and 30, as well as the last data obtained during the SBT. In the successfully extubated group, n = 35 at all time points. In the group for whom extubation failed, n = 17 at PSV10, T1, and T5; n=16 at T10 and T15; n=14 at T30 (three for whom SBT failed at 30 minutes and 11 who were initially extubated but were later reintubated or provided with noninvasive ventilation or died). Data were obtained for the remaining three patients meeting SBT exclusion criteria at T30. At Tend, n = 17 in the group for whom extubation failed. *Difference (P < 0.05) between failure and successfully extubated groups at the same time point. †Differences (P < 0.05) between PSV10 and other time points in the group for whom extubation failed. ‡Differences (P < 0.05) between PSV10 and other time points in the group that was successfully extubated.
During PSV10, EAdi was approximately 10 μV and was not different between the success and failure groups. During the SBT, the EAdi increased significantly in both groups and reached a plateau during the first few minutes. The increase in EAdi in the extubation-failure group was twice that observed in the successfully extubated group.
The NVE values at PSV10 were about 80 and 50 ml/μV in the success and failure groups, respectively. At the onset of the SBT, the NVE decreased significantly in both groups; thereafter, the NVE for both groups reached a plateau at about 40 and 20 ml/μV in the success and failure patients, respectively.
NVE was inversely correlated with age, both for all subjects (r = 0.58 ± 0.04; P < 0.001; n = 52) and for the successfully extubated group (r = 0.53 ± 0.05; P = 0.007; n = 35). No relation was found between EAdi, NME, or NVE and duration of mechanical ventilation.
NME was not obtained during PSV10. During the SBT, NME was significantly higher in the success group (~1.5 cm H2O/μV) compared with the failure group (~1.0 cm H2O/μV); NME did not change significantly with time.
Subanalysis of the patients for whom extubation failed revealed no significant difference for EAdi, NVE, and NME between those for whom the SBT failed (n = 6) and those who were extubated but required reintubation, NIV, or died within 48 hours (n = 11). Nor was a difference noted at any time point for EAdi, NVE, and NME between COPD and non-COPD patients in either the success or failure groups.
Regression analysis showed that 40% (r = 0.67; P < 0.001) and 45% (r = 0.64; P < 0.001) of the changes in Vt and Vt/kg IBW (ideal body weight) from T1 to Tend were explained by simultaneous changes of the EAdi from T1 to Tend. EAdi was not correlated with f or f/Vt.
Breathing pattern
Figure 3 shows the timelines of Vt, f, VE, and f/Vt.
The development of (top to bottom) of tidal volume (V t ), breathing frequency (f), minute ventilation (V E ), and the ratio of breathing frequency and tidal volume (f/V t ) during pressure support of 10 above 5 cm H 2 O of PEEP (PSV 10 ) and throughout the spontaneous breathing trial (SBT) on CPAP of 5 cm H 2 O. See Figure 2 for more description.
During the PSV10 period, Vt was 474 ml (8.1 ml/kg IBW) in the group that was successfully extubated, and was significantly lower, 375 ml (7.0 ml/kg IBW), in the group for whom extubation failed. When the SBT started, and assist was removed, Vt decreased very quickly and reached a plateau at significantly lower values in both groups. Compared with that of the successfully extubated group, Vt remained lower at all times in the group for whom extubation failed.
During PSV10, f was 22 and 24 breaths/min in the groups of successful and failed extubation, respectively. Removal of PSV10 did not change f in the group of patients who were successfully extubated. In the extubation-failure group, f showed a tendency to increase during the early part of the SBT, such that f was significantly higher during T1 and T5, compared with the group with successful extubation. No significant differences in f were found between groups toward the end of the SBT.
During PSV10, VE was 9.9 L/min in the successfully extubated group, which was modestly but significantly higher than the VE of 8.4 L/min observed in the extubation-failure group. Except for a modest tendency for a decrease in VE during T1, VE remained stable throughout the SBT in both groups. The successfully extubated group maintained modest but significantly higher VE values throughout the majority of measurement points during the SBT.
The f/Vt was 51 and 75 during the PSV10 period and was not different between groups. During the SBT, f/Vt increased significantly in both groups and remained at values of about 60 to 70 (success) and 100 to 110 (failure) throughout the SBT.
Subanalysis of the patients for whom extubation failed revealed no significant differences for Vt, f, VE, and f/Vt between those whose SBT failed (n = 6) and those who were extubated but required reintubation, NIV, or died within 48 hours (n = 11).
Blood gases
During PSV10 and throughout the SBT, bicarbonate in the group for whom extubation failed was elevated and significantly higher than that in the group that was successfully extubated (Table 2).
During PSV10, PaCO2 values were normal and not significantly different between the groups. During the SBT, the PaCO2 increased in the extubation-failure group and reached higher values than those in the successfully extubated group (Table 2).
pH was stable and not different between groups at any time. The PaO2/FiO2 showed a consistent but nonsignificant tendency for higher values in the successfully extubated group.
Extubation predictability
NVE consistently demonstrated the highest values for predictability of extubation outcome throughout the SBT and could, according to the area under the ROC curve (AUC), be rated "good" for separating successful and failed extubation. Values for all time points and variables tested for extubation predictability are given in Table 3. Figure 4 shows the ROC curve for NVE predictability of extubation success at T5, having an AUC of 0.84 (P < 0.001) with a sensitivity of 0.69 and specificity of 0.88 at an NVE value of 24.1 ml/μV. Figure 4 also shows ROC curves obtained at T5 for EAdi (predicting extubation failure, AUC = 0.73; P = 0.009), and NME (predicting extubation success, AUC = 0.70; P = 0.02). As a reference, we also show the ROC curve for the f/Vt index (predicting extubation failure) having an AUC of 0.72 (P = 0.012). No statistically significant difference was found between the areas under the ROC curves for NVE, NME, EAdi, and f/Vt.
Upper left panel shows the ROC curve (receiver operating characteristic) for neuroventilatory efficiency (NVE) to predict extubation success after 5 minutes of the spontaneous breathing trial (T 5 ). Area under the curve (AUC) was 0.84 (P < 0.001). Upper right panel shows the ROC curve for diaphragm electrical activity (EAdi) to predict extubation failure at T5. AUC was 0.73 (P < 0.009). Lower left panel shows ROC curve for neuromuscular efficiency (NME) to predict extubation success at T5. AUC = 0.70 (P < 0.02).The lower right panel shows the ROC curve for the ratio of breathing frequency and tidal volume (f/Vt) to predict extubation failure at T5. AUC was 0.72 (P = 0.012).
Discussion
The major finding of this study is that a mixed population of critically ill patients for whom extubation fails are characterized by increased neural activation of the diaphragm as well as a reduced ability to generate inspiratory pressure and tidal volume for a given diaphragmatic neural activation. The present study also suggests that NVE has good predictive power to determine the outcome of extubation.
In the present study, the lower Vt, higher f, and f/Vt, as well as increased PaCO2 in the failure group, are all "classic" clinical signs associated with weaning failure, when compared with successful weaning [15, 26]. Compared with the classic approach, the new measurement and indices (that is, EAdi, NME, and NVE) were found to have more power to discriminate between groups for successful extubation and failed extubation for physiological reasons that are discussed later.
EAdi
EAdi reflects the neural respiratory drive, and transesophageal measurements of EAdi have been validated for reliability in healthy subjects and in patients with respiratory dysfunction [18–21, 27–36].
In the present study, EAdi was not different between the success and failure extubation groups at PSV10, suggesting that PSV provided sufficient assist to decrease neural respiratory drive in the failure group, despite a large difference in NVE. The higher bicarbonate level in the extubation failure group likely played a role in the lower VE in the failure group.
Our findings of a rapid increase in EAdi reaching a plateau in both groups, with a higher plateau in the failure group, confirms previous work, demonstrating that inspiratory mechanical effort increases during SBT and increases more in patients for whom extubation fails [9–11]. This finding is in accord with previous studies demonstrating that 100 ms occlusion pressure (P0.1) was higher in patients for whom weaning failed [37, 38].
The time until the EAdi reached a plateau was shorter in the present study than previously reported for increases of mechanical effort when using T-piece [9, 11]. This difference was likely due to differences in how the SBT was performed (T-piece or CPAP) [39, 40].
Thus, our findings, along with previous findings, highlight that the neural respiratory drive is higher in patients for whom extubation fails.
NME
A limitation of EAdi is that it describes only neural respiratory drive, and it is not possible to ascertain whether an increase in EAdi is due to diaphragm weakness, overcoming increased respiratory load, or to a response to increased metabolic demand.
Beck et al. [21] showed, in healthy subjects, that the EAdi is linearly related to the diaphragm pressure generation up to 75% of maximum force. Finucane and Singh [41] showed that EAdi is closely related to the diaphragm power output. Beck et al. [20] described that changes in transdiaphragmatic pressure closely resembled changes in EAdi in patients with acute respiratory failure receiving mechanical ventilation. Given a relatively linear relation between EAdi and diaphragm pressure generation, normalization of the inspiratory occlusion pressure to EAdi provides a means to compare pressure-generating efficiency without the need to standardize effort.
In the present study, we measured the inspiratory Paw deflection during an inspiratory occlusion to reflect the inspiratory pressure generation. Previous studies have shown that this maneuver accurately reflects esophageal pressure deflections during both voluntary [42] and phrenic nerve-stimulated diaphragm contractions in intubated subjects [5, 8].
It has been well established that the diaphragm contractile function decreases rapidly [4] to about 25% of normal values [3, 5–8] in critically ill and mechanically ventilated patients. However, controversies still exist as to whether the inspiratory force is different between those for whom weaning fails and those who are successfully extubated [6, 8]. In the present study, we found that the NME was significantly lower in the failure group, indicating a weaker diaphragm. This agrees with findings of Buscher [8], who compared twitch diaphragm force in patients successfully weaned shortly after surgery to patients for whom weaning failed and found lower twitch pressure in the failure patients. In contrast, Laghi et al. [6], who studied patients with similar duration of mechanical ventilation, did not find any difference in twitch diaphragm force between patients who were successfully extubated and for whom extubation failed.
Changes in FRC due to dynamic hyperinflation could have affected NME in the present study. Beck et al. [21] showed that an increase in volume from FRC to TLC reduces the transdiaphragmatic pressure by 60% for a given EAdi. Sinderby et al. [43] showed that progressive dynamic hyperinflation in exercising COPD patients resulted in continuous increase of EAdi, whereas the slope of the increase of transdiaphragmatic pressure decreased, and eventually plateau was reached, despite increased neural drive. In the present study, the higher number of patients with COPD in the extubation-failure group, prone to dynamic hyperinflation, may thus have been one contributing factor to the lower NME and NVE in the extubation-failure group.
Our findings of a lower NME in the extubation-failure group supports the concept that impaired pressure-generating capacity of the diaphragm, regardless of its origin, plays a role in the outcome of extubation.
NVE
The NVE index (Vt/EAdi), reflects determinants of the volume generated (that is, the respiratory drive, diaphragm function, and respiratory load). In agreement with the notion that weaning failure is caused by respiratory demand exceeding the capacity of the respiratory muscles [12–16], NVE was the variable that demonstrated the largest difference between groups (50% lower NVE in the extubation-failure group) and best predictability for extubation outcome with the largest AUC (0.84). These findings agree with previous observations in COPD patients (22). Given the limitations of post hoc analysis, future prospective trials are required to show the predictive power of the NVE to determine extubation readiness.
The present study also showed that NVE is inversely correlated to age. This "negative" influence of age on the NVE was not surprising, given that respiratory mechanics, and lung and inspiratory muscle function decrease with age [44].
Does NME or NVE discriminate fatigue during SBT?
Neither NME nor NVE changed throughout the SBT in either group. This result suggests that diaphragm contractility did not decrease and/or that load increased throughout the SBT. Previous studies suggested that the work of the diaphragm in nonassisted patients for whom extubation failed is such that diaphragm fatigue could develop [6, 9, 13]. This has been supported by early predictors of fatigue, such as muscle relaxation rate [14] and power spectrum shift of the EAdi power spectrum [12]. Other studies, however, suggest that respiratory muscle contractile fatigue [6] and reduced muscle contractility [45] do not occur in weaning-failure patients. A possible explanation for these discrepancies is that the development of diaphragm fatigue would have a disastrous effect on the patient's survival and is therefore preceded by many changes in breathing strategies (for example, rapid shallow breathing and thoracoabdominal paradox) to prevent fatigue [6, 11, 14, 21]. A dissociation between NVE and fatigue during strenuous breathing is also possible, as Luo et al. [28] showed, in healthy subjects, that diaphragm fatigue, expressed as >10% loss of diaphragm contractility, did not alter the NVE.
Hence, although not supportive of significant diaphragm fatigue development, our finding of constant NME and NVE over time throughout the SBT does not preclude the development of diaphragm contractile fatigue.
Limitations of the method
The general population of critically ill patients in this study is skewed toward COPD and pneumonia (33 of 52) patients and does not represent specific disease states causing ventilatory failure. Future studies should focus on specific disease states.
The EAdi in absolute units (µV) is affected by anatomic differences between subjects, where an increased distance between the electrodes in the esophagus and the crural diaphragm lowers the EAdi amplitude [46]. Thus, unless this distance filtering is corrected for, some individual differences in EAdi, NME, and NVE may pertain to anatomic deviations. However, given the size of the present study, occasional individual deviations should not affect the group-mean data.
Changes in recruitment of respiratory muscles other than the diaphragm may affect the NVE and NME. In healthy subjects who have a large respiratory muscle reserve and therefore the possibility to alternate between diaphragm and other inspiratory muscles, the errors of the NME and NVE indices to predict changes accurately in the diaphragm force-generating or ventilatory capacity can be expected to be of some magnitude unless the breathing pattern is controlled. In contrast, patients who have or are recovering from acute respiratory failure are characterized by very weak inspiratory muscles [47] and are not likely have enough reserve to alternate their inspiratory-pressure generation between different inspiratory muscle groups. Parthasarathy et al. [11] showed that the electrical activity of the rib cage and accessory inspiratory muscles increases early and more during a failing SBT. Although increased extradiaphragmatic muscle recruitment increases the amount of pressure and volume that will be delivered for a given EAdi, it is unlikely that the diaphragm activity remains constant. It has been shown that the motor unit firing rate in healthy subjects increased more for the diaphragm than for nondiaphragmatic inspiratory muscles with increasing respiratory drive [48]. Sinderby et al. [27] provided evidence that changes in electrical activation of the diaphragm and intercostal muscles during near-maximal efforts are related in severe COPD patients. In the present study, nearly half of the changes in Vt from T1 to Tend were explained by changes of the EAdi from T1 to Tend, suggesting that the diaphragm is the major contributor to pressure and volume generation. In other words, it is not likely that diaphragm activation would remain constant when other extradiaphragmatic muscle activity increases.
It has been demonstrated that measures representative of breathing pattern and muscle efforts during weaning pertain to the method applied [39, 40]. Numeric data from the present study should be interpreted with caution when compared with other approaches for SBT (for example, T-piece trial or PSV with or without PEEP). It should also be noted that differences in strategies to wean (CPAP, T-piece, PSV) may affect the results because they can have an impact on pressure/time product and hemodynamics differently [49, 50].
Because of methodologic variability, methods to obtain and calculate EAdi usually differ between investigators. Hence, comparison of numeric data from the present study may pertain only to other studies using the same standardized commercially available equipment. Compared with studies using the same equipment, the peak EAdi observed during PSV in the present study agrees with previously reported data [33, 51, 52], suggesting that EAdi values in patients with acute respiratory failure are in the same range. Values for the NME and NVE indices in the present study do not relate to infants. Future work on normalizing these indices to body weight is required to create a reliable index for all ages.
It can be argued that during conditions in which ventilatory drive is impaired or the diaphragm is weak, as are common in critical illness, EAdi (also known as diaphragmatic EMG) may not reflect drive or strength. Theoretic descriptions of the relation between activation and EMG signal strength, as well as theoretic explanations for the relation between transdiaphragmatic pressure and global diaphragm activation, support that EAdi reflects respiratory drive and its link to force generation [21]. Studies in animals have shown that the diaphragm EMG is related to phrenic nerve activity [53]. Studies in healthy subjects, patients with respiratory disorders, and mechanically ventilated critically ill patients show that EAdi represents respiratory drive and that its amplitude and coupling to diaphragm force (transdiaphragmatic pressure) may vary because of, for example, anatomic variations and pathology [18–21, 27–32, 54–56]. Human motor-unit discharge rates are strongly associated with neural respiratory drive in both health and disease [57, 58]. Rare conditions that rapidly affect neuromuscular transmission (for example, myasthenia gravis) can alter the ratio between central respiratory drive to the diaphragm and EAdi [59]. Changes in muscle fiber action-potential velocity vary with diaphragm fatigue and temperature and alter the measured amplitude of the EAdi relative to that of the respiratory drive [60]; however, these changes are not of a magnitude to explain the differences in EAdi observed in the present study. Finally, data from studies using Neurally Adjusted Ventilatory Assist (NAVA) support that EAdi has a close integration with chemoreceptors and vagal lung afferents during muscle unloading in acute respiratory failure [61]. Despite the evidence supporting that EAdi is a reliable measure of respiratory drive, this research area is vast and requires further advancement.
In the present study, we applied a post hoc analysis to validate prediction of weaning success, but prospective trials are required to evaluate truly the accuracy of using EAdi-based indices for prediction of weaning success.
Conclusions
This study shows that a mixed group of critically ill patients for whom weaning fails are characterized by increased neural respiratory drive and impaired ability to convert neuromuscular activity into tidal ventilation, and this is at least in part due to inspiratory muscle weakness. Measurement of neuroventilatory efficiency (quantifying the imbalance between increased neural effort, respiratory load, and diaphragm weakness) can be valuable in clinical decision making about extubation readiness. Future studies should focus on specific disease states.
Key messages
-
Weaning failure is associated with increased neural respiratory drive and impaired ability to convert neuromuscular activity into tidal ventilation, and this is at least in part due to inspiratory muscle weakness.
-
Measurement of neuroventilatory efficiency, quantifying the imbalance between increased neural effort, respiratory load, and diaphragm weakness, can be valuable in clinical decision making about extubation readiness.
Abbreviations
- AUC:
-
area under the (ROC) curve
- BP:
-
blood pressure
- COPD:
-
chronic obstructive pulmonary disease
- CPAP:
-
continuous positive airway pressure
- EAdi:
-
diaphragm electrical activity
- f:
-
respiratory frequency
- f/Vt:
-
Weaning index based on ratio of breathing frequency and tidal volume
- IBW:
-
Ideal body weight
- ICU:
-
intensive care unit
- NAVA:
-
neurally adjusted ventilatory assist
- NME:
-
neuromechanical efficiency
- NS:
-
nonsignificant
- NVE:
-
neuroventilatory efficiency
- Paw:
-
airway pressure
- PEEP:
-
positive end-expiratory pressure
- PSV:
-
pressure support ventilation
- PSV10:
-
PSV of 10 cm H2O above a positive end-expiratory pressure (PEEP) of 5 cm H2O
- ROC:
-
receiver operating characteristic
- SBT:
-
spontaneous breathing trial
- SIMV:
-
synchronized intermittent mandatory ventilation
- T1:
-
minute 1
- T5:
-
minute 5
- T10:
-
minute 10
- T15:
-
minute 15
- T30:
-
minute 30
- Tend:
-
the last data obtained during the SBT
- VE:
-
minute ventilation
- Vt:
-
tidal volume.
References
Epstein SK: Weaning from ventilatory support. Curr Opin Crit Care. 2009, 15: 36-43. 10.1097/MCC.0b013e3283220e07.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrage JB: Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008, 358: 1327-1335. 10.1056/NEJMoa070447.
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Mateck S: Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011, 183: 364-371. 10.1164/rccm.201004-0670OC.
Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G: Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010, 14: R127-10.1186/cc9094.
Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J: Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001, 29: 1325-1331. 10.1097/00003246-200107000-00005.
Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YSA, Tobin MJ: Is weaning failure caused by low-frequency fatigue of the diaphragm?. Am J Respir Crit Care Med. 2003, 167: 120-127. 10.1164/rccm.200210-1246OC.
Pontoppidan H, Wilson RS, Rie MA, Schneider RC: Respiratory intensive care. Anesthesiology. 1977, 47: 96-116. 10.1097/00000542-197708000-00003.
Buscher H, Valta P, Boie T, Hinz J, Moerer O, Sydow M, Mudaliar MY, Burchardi H: Assessment of diaphragmatic function with cervical magnetic stimulation in critically ill patients. Anaesth Intensive Care. 2005, 33: 483-491.
Jubran A, Grant BJ, Laghi F, Parthasarathy S, Tobin MJ: Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med. 2005, 171: 1252-1259. 10.1164/rccm.200503-356OC.
Teixeira C, Teixeira PJ, de Leon PP, Oliveira ES: Work of breathing during successful spontaneous breathing trial. J Crit Care. 2009, 24: 508-514. 10.1016/j.jcrc.2008.10.013.
Parthasarathy S, Jubran A, Laghi F, Tobin MJ: Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol. 2007, 103: 140-147. 10.1152/japplphysiol.00904.2006.
Cohen CA, Zagelbaum G, Gross D, Roussos C, Macklem PT: Clinical manifestations of inspiratory muscle fatigue. Am J Med. 1982, 73: 308-316. 10.1016/0002-9343(82)90711-2.
Vassilakopoulos T, Zakynthinos S, Roussos C: The Tension-Time Index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998, 158: 378-385.
Goldstone JC, Green M, Moxham J: Maximum relaxation rate of the diaphragm during weaning from mechanical ventilation. Thorax. 1994, 49: 54-60. 10.1136/thx.49.1.54.
Jubran A, Tobin MJ: Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997, 155: 906-915.
Carlucci A, Ceriana P, Prinianakis G, Fanfulla F, Colombo R, Nava S: Determinants of weaning success in patients with prolonged mechanical ventilation. Crit Care. 2009, 13: R97-10.1186/cc7927.
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindström L: Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999, 5: 1433-1436. 10.1038/71012.
Aldrich TK, Sinderby C, McKenzie DK, Estenne M, Gandevia SC: ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002, 166: 518-624.
Beck J, Sinderby C, Lindström L, Grassino A: Crural diaphragm activation during dynamic contractions at various inspiratory flow rates. J Appl Physiol. 1998, 85: 451-458.
Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, Sinderby C: Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001, 164: 419-424.
Beck J, Sinderby C, Lindström L, Grassino A: Effects of lung volume on diaphragm EMG signal strength during voluntary contractions. J Appl Physiol. 1998, 85: 1123-1134.
Liu HG, Liu L, Tang R, Guo WG, Huang YZ, Yang Y, Liu SQ, Wu AP, Huang DY, Wu XY, Qiu HB: A pilot study of diaphragmatic function evaluated as predictors of weaning in chronic obstructive pulmonary disease patients. Zhonghua Nei Ke Za Zhi. 2011, 50: 459-464.
Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA: Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001, 120: 1262-1270. 10.1378/chest.120.4.1262.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T: Weaning from mechanical ventilation. Eur Respir J. 2007, 29: 1033-1056. 10.1183/09031936.00010206.
DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988, 44: 837-845. 10.2307/2531595.
Yang KL, Tobin MJ: A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991, 324: 1445-1450. 10.1056/NEJM199105233242101.
Sinderby C, Beck J, Spahija J, Weinberg J, Grassino A: Voluntary activation of the human diaphragm in health and disease. J Appl Physiol. 1998, 85: 2146-2158.
Luo YM, Hart N, Mustfa N, Lyall RA, Polkey MI, Moxham J: Effect of diaphragm fatigue on neural respiratory drive. J Appl Physiol. 2001, 90: 1691-1699.
Singh B, Panizza JA, Finucane KE: Diaphragm electromyogram root mean square response to hypercapnia and its intersubject and day-to-day variation. J Appl Physiol. 2005, 98: 274-281.
Finucane KE, Panizza JA, Singh B: Efficiency of the normal human diaphragm with hyperinflation. J Appl Physiol. 2005, 99: 1402-1411. 10.1152/japplphysiol.01165.2004.
Luo YM, Moxham J: Measurement of neural respiratory drive in patients with COPD. Respir Physiol Neurobiol. 2005, 146: 165-174. 10.1016/j.resp.2004.12.014.
Jolley CJ, Luo YM, Steier J, Reilly C, Seymour J, Lunt A, Ward K, Rafferty GF, Polkey MI, Moxham J: Neural respiratory drive in healthy subjects and in COPD. Eur Respir J. 2009, 33: 289-297.
Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte FD, Navalesi P: Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med. 2008, 34: 2010-2018. 10.1007/s00134-008-1208-3.
Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, Sinderby C: Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010, 38: 518-526. 10.1097/CCM.0b013e3181cb0d7b.
Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, Sinderby C: Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest. 2009, 135: 695-703. 10.1378/chest.08-1747.
Sinderby C, Beck J, Spahija J, Marchie M, Lacroix J, Navalesi P, Slutsky AS: Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest. 2007, 131: 711-717. 10.1378/chest.06-1909.
Capdevila XJ, Perrigault PF, Perey PJ, Roustan JPA, d'Athis F: Occlusion pressure and its ratio to maximum inspiratory pressure are useful predictors for successful extubation following T-piece weaning trial. Chest. 1995, 108: 482-489. 10.1378/chest.108.2.482.
Vallverdú I, Calaf N, Subirana M, Net A, Benito S, Mancebo J: Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998, 158: 1855-1862.
Sassoon CS: Positive pressure ventilation: alternate modes. Chest. 1991, 100: 1421-1429. 10.1378/chest.100.5.1421.
Cabello B, Thille AW, Roche-Campo F, Brochard L, Gomez FJ, Mancebo J: Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med. 2010, 36: 1171-1179. 10.1007/s00134-010-1870-0.
Finucane KE, Singh B: Human diaphragm efficiency estimated as power output relative to activation increases with hypercapnic hyperpnea. J Appl Physiol. 2009, 107: 1397-1405. 10.1152/japplphysiol.91465.2008.
Baydur A, Cha EJ, Sassoon CS: Validation of esophageal balloon technique at different lung volumes and postures. J Appl Physiol. 1987, 62: 315-321. 10.1063/1.339151.
Sinderby C, Spahija J, Beck J, Kaminski D, Yan S, Comtois N, Sliwinski P: Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001, 163: 1637-1641.
Janssens JP, Pache JC, Nicod LP: Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999, 13: 197-205. 10.1183/09031936.99.14614549.
Swartz MA, Marino PL: Diaphragmatic strength during weaning from mechanical ventilation. Chest. 1985, 88: 736-739. 10.1378/chest.88.5.736.
Beck J, Sinderby C, Weinberg J, Grassino A: Effects of muscle-to-electrode distance on the human diaphragm electromyogram. J Appl Physiol. 1995, 79: 975-985.
Roussos C, Koutsoukou A: Respiratory failure. Eur Respir J. 2003, 22: 3s-14s. 10.1183/09031936.03.00038503.
Gandevia SC, Gorman RB, McKenzie DK, De Troyer A: Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med. 1999, 160: 1598-1603.
Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK: Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis. 1991, 143: 469-475.
Frazier SK, Stone KS, Schertel ER, Moser DK, Pratt JW: A comparison of hemodynamic changes during the transition from mechanical ventilation to T-piece, pressure support, and continuous positive airway pressure in canines. Biol Res Nurs. 2000, 1: 253-264. 10.1177/109980040000100402.
Schmidt M, Demoule A, Cracco C, Gharbi A, Fiamma MN, Straus C, Duguet A, Gottfried SB, Similowski T: Neurally adjusted ventilatory assist increases respiratory variability and complexity in acute respiratory failure. Anesthesiology. 2010, 112: 670-681. 10.1097/ALN.0b013e3181cea375.
Terzi N, Pelieu I, Guittet L, Ramakers M, Seguin A, Daubin C, Charbonneau P, du Cheyron D, Lofaso F: Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med. 2010, 38: 1830-1837. 10.1097/CCM.0b013e3181eb3c51.
Lourenço RV, Cherniack NS, Malm JR, Fishman AP: Nervous output from the respiratory center during obstructed breathing. J Appl Physiol. 1966, 21: 527-533.
Sinderby CA, Comtois AS, Thomson RG, Grassino AE: Influence of the bipolar electrode transfer function on the electromyogram power spectrum. Muscle Nerve. 1996, 19: 290-301. 10.1002/(SICI)1097-4598(199603)19:3<290::AID-MUS3>3.0.CO;2-8.
Beck J, Weinberg J, Hamnegård CH, Spahija J, Olofson J, Grimby G, Sinderby C: Diaphragmatic function in advanced Duchenne muscular dystrophy. Neuromusc Disord. 2006, 16: 161-167. 10.1016/j.nmd.2006.01.003.
Tuchscherer D, Z'graggen WJ, Passath C, Takala J, Sinderby C, Brander L: Neurally adjusted ventilatory assist in patients with critical illness-associated polyneuromyopathy. Intensive Care Med. 2011, 37: 1951-1961. 10.1007/s00134-011-2376-0.
Gandevia SC, Leeper JB, McKenzie DK, De Troyer A: Discharge frequencies of parasternal intercostal and scalene motor units during breathing in normal and COPD subjects. Am J Respir Crit Care Med. 1996, 153: 622-628.
De Troyer A, Leeper JB, McKenzie DK, Gandevia SC: Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med. 1997, 155: 1335-1340.
Mier A, Brophy C, Moxham J, Green M: Repetitive stimulation of phrenic nerves in myasthenia gravis. Thorax. 1992, 47: 640-644. 10.1136/thx.47.8.640.
Sinderby C, Lindström L, Comtois N, Grassino AE: Effects of diaphragm shortening on the mean action potential conduction velocity in canines. J Physiol. 1996, 490: 207-214.
Sinderby C, Beck J: Neurally adjusted ventilatory assist (NAVA). Principles and Practice of Mechanical Ventilation. Edited by: Tobin MJ. 2012, New York: McGraw-Hill, 3
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province (Project BK2008298), and National Natural Science Foundation of China (Project 81000828). CS was supported by the R. S. McLaughlin Foundation. We are indebted to Mr. Norman Comtois for technical assistance and assisting with analysis of data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Arthur Slutsky is a consultant for Maquet Medical, the company that makes NAVA and is paid for this work. AS is Vice President, Research, at St. Michael's Hospital (SMH). SMH receives royalty payments from Maquet for NAVA. JB and CS have patented inventions related to neural control of mechanical ventilation. The license for these patents belongs to Maquet Critical Care. Commercial use of this technology provides financial benefit to JB and CS through royalties. JB and CS each owns 50% of Neurovent Research, Inc. (NVR), a research and development company that builds the equipment and catheters for research studies. NVR has a consulting agreement with Maquet Critical Care. JB and CS are inventors of NAVA and work for SMH. SMH receives royalty payments from Maquet for NAVA. The remaining authors have no competing interests to declare.
Authors' contributions
LL was responsible for conception and design of the study, the acquisition, analysis, and interpretation of data, and drafting and revising the article for final approval of the version to be published. HL was responsible for the design of the study, acquisition and analysis of data, revising the article, and final approval of the version to be published. YY participated in the design of the study, acquisition and analysis of data, and revising the article for final approval of the version to be published. YH participated in the design of study, acquisition and analysis of data, and revising the article for final approval of the version to be published. SL participated in the design of study, acquisition and analysis of data, revising the article, and final approval for the version to be published. AS was involved with the conception and design of the study, interpretation of the data, revising the article for important intellectual content, and final approval of the version to be published. JB was involved with the conception and design of the study, analysis and interpretation of data, drafting and revising the article for important intellectual content, and final approval of the version to be published. CS was responsible for the conception and design of the study, analysis and interpretation of data, drafting and revising the article for important intellectual content, and final approval of the version to be published. HQ was involved with the conception and design of study, analysis and interpretation of the data, drafting and revising the article for important intellectual content, and final approval of the version to be published.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liu, L., Liu, H., Yang, Y. et al. Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit Care 16, R143 (2012). https://doi.org/10.1186/cc11451
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc11451