Abstract
Introduction
Macrophages in the mammary gland are essential for morphogenesis of the ductal epithelial tree and have been implicated in promoting breast tumor metastasis. Although it is well established that macrophages influence normal mammopoiesis, the mammary cell types that these accessory cells influence have not been determined. Here we have explored a role for macrophages in regulating mammary stem cell (MaSC) activity, by assessing the ability of MaSCs to reconstitute a mammary gland in a macrophage-depleted fat pad.
Methods
Two different in vivo models were used to deplete macrophages from the mouse mammary fat pad, allowing us to examine the effect of macrophage deficiency on the mammary repopulating activity of MaSCs. Both the Csf1op/opmice and clodronate liposome-mediated ablation models entailed transplantation studies using the MaSC-enriched population.
Results
We show that mammary repopulating ability is severely compromised when the wild-type MaSC-enriched subpopulation is transplanted into Csf1op/opfat pads. In reciprocal experiments, the MaSC-enriched subpopulation from Csf1op/opglands had reduced regenerative capacity in a wild-type environment. Utilizing an alternative strategy for selective depletion of macrophages from the mammary gland, we demonstrate that co-implantation of the MaSC-enriched subpopulation with clodronate-liposomes leads to a marked decrease in repopulating frequency and outgrowth potential.
Conclusions
Our data reveal a key role for mammary gland macrophages in supporting stem/progenitor cell function and suggest that MaSCs require macrophage-derived factors to be fully functional. Macrophages may therefore constitute part of the mammary stem cell niche.
Similar content being viewed by others
Introduction
Increasing evidence suggests that macrophages play a role in the normal development of specific organs. In the pubertal mammary gland, macrophages are recruited to the highly mitotic terminal end buds (TEBs) from which ducts elongate and branch to give rise to a mature ductal tree. Macrophages are presumed to play a role in tissue remodeling by engulfing apoptotic epithelial cells during ductal morphogenesis [1, 2]. Colony stimulating factor-1 (Csf1; also referred to as macrophage-Csf) is a key growth factor that regulates the proliferation and survival of macrophages [3]. Csf1op/opmice, homozygous for a null mutation in Csf1, exhibit multiple defects and have reduced macrophage numbers in most tissues including the mammary gland. These mice are severely runted, toothless and osteopetrotic, owing to an osteoclast deficiency that impairs bone resorption. The mammary glands of Csf1op/opmice display lower numbers of terminal end buds as well as reduced ductal branching and elongation [4]. During pregnancy, Csf1op/opglands develop precocious alveolar units but fail to switch to the lactational state resulting in impaired lactation [5]. Mammary-specific rescue has shown that the mammary gland phenotype is not due to secondary effects induced by a systemic Csf1 deficiency [6]. Targeted ablation of the receptor for Csf1 (Csf1r or c-fms), recapitulated all the phenotypes observed in Csf1op/opmice, although the receptor-null mice exhibited slightly more severe defects [7].
The nature of the mammary stem cell niche is yet to be defined but candidate cell types that may constitute the niche include epithelial cells, macrophages and eosinophils, among other cells in the mammary stroma (fibroblasts and adipocytes). One mechanism that may underlie the requirement of macrophages for normal mammopoiesis could be their interaction with mammary stem cells (MaSCs) [8, 9]. To explore this possibility, we have utilized two in vivo approaches to determine whether macrophages are required for the function of MaSCs. We report that stem/progenitor cell activity is markedly attenuated by depletion of macrophages from the mammary gland, indicating that macrophages may influence stem cell function.
Materials and methods
Mouse strains
Csf1op/opmice were a kind gift from Dr Robin Anderson (Peter MacCallum Cancer Centre, Melbourne, Australia) and were on a pure BALB/c background. These mice were housed in a clean facility to optimise the viability of the homozygous mutant mice. Rosa26 mice were on a pure FVB/N background. All experiments were approved by the WEHI Animal Ethics Committee, and the care of animals was in accordance with institutional guidelines.
Mammary stem cell preparation and cell culture
A single cell suspension of mammary cells was prepared from freshly harvested mammary glands and sorted by flow cytometry as previously described [8]. For transplantation assays, freshly harvested cells from the Lin-CD29hiCD24+ fraction were transplanted in a volume of 10 μl into the de-epithelialized fourth mammary fat pads of three week-old syngeneic mice. Limiting dilution and statistical analyses were carried out as described by Shackleton et al [8]. For experiments using liposomes, the injection solution contained approximately 42 μg of clodronate liposomes per injection volume. Wholemount and histology analyses, as well as staining of mammary glands for LacZ activity using X-Gal (Sigma), were performed as described [8]. Clodronate liposomes were synthesized as previously described [10].
For in vitro cell culture assays, 100 freshly sorted epithelial cells (56 cells/cm2) were cultured for one week on 25,000 irradiated NIH-3T3 fibroblasts (14,000 cells/cm2) in 24-well culture dishes as described [8]. Colony size was measured using ImageJ [11].
Results
To evaluate whether the impaired ductal morphogenesis that occurs in Csf1op/opmice (Figure 1a) reflects a perturbation in MaSC activity, freshly sorted CD29hiCD24+ mammary epithelial cells were isolated from BALB/c Csf1op/opand wild-type glands. They were then transplanted into the de-epithelialized fat pads of three-week-old BALB/c mice at limiting dilution. The CD29hiCD24+ subpopulation has previously been shown to be enriched for MaSCs but also contains mature myoepithelial cells and possibly basal progenitor cells [8]. Cells were purified from 12 week-old Csf1op/opmice and eight-week-old wild-type littermates, in order to compensate for the recognised delay in filling of the fat pad that occurs in Csf1op/opmutant mice [4]. The glands were harvested for wholemount analysis eight weeks post-transplantation.
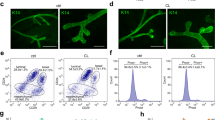
The MaSC-enriched population from Csf1op/opmice yields smaller outgrowths in vivo and has lower clonogenicity in vitro. (a) Wholemounts of fourth mammary glands from eight-week-old wild-type and Csf1op/opmice showing delayed filling of the fat pad and persistence of terminal end buds in mutant animals. (b) Wholemounts of outgrowths following transplantation of CD29hiCD24+ cells from wild-type or Csf1op/opmammary glands into the cleared fat pads of syngeneic wild-type recipients. (c) CD29hiCD24+ cells from Csf1op/opmammary glands exhibit lower clonogenicity and colony size than those from control glands. Histogram (upper panel) showing colony forming capacity of 100 CD29hiCD24+ cells for each genotype. Error bars show standard error of the mean for three independent experiments. Lower panel shows cultures of CD29hiCD24+ cells grown on fibroblast feeder layers. Scale bars = 1 mm. Csf1 = colony stimulating factor 1; MaSC = mammary stem cell.
Limiting dilution analysis of four independent experiments revealed that the stem cell frequency was reduced in Csf1op/opmice. The mammary repopulating frequency was 1 in 261 (95% confidence interval (CI) = 1/166 to 1/408) versus 1 in 97 (95% CI = 1/66 to 1/141) for Csf1op/opand wild-type mammary glands, respectively (P < 0.001; Table 1). Estimation of the absolute number of mammary repopulating cells revealed an 18-fold decrease in mutant glands (mean of 702 versus 40 cells per gland in wild-type and Csf1op/opmice, respectively). In addition to the decrease in repopulating frequency by CD29hiCD24+ cells in the Csf1op/opgroup, only 5 of the 45 transplanted fat pads (11 ± 4%; mean ± standard error of the mean (SEM)) yielded outgrowths filling more than 50% of the fat pad, whereas 22 of the 45 transplanted fat pads (49 ± 4%) were filled by outgrowths from wild-type stem cells. Thus, despite being transplanted into a normal microenvironment, the MaSC-enriched population from Csf1op/opmice did not give rise to fully developed mammary outgrowths (Figure 1b), indicating that the function of mammary stem/progenitor cells had been attenuated in the macrophage-depleted environment.
Consistent with the transplantation data, in vitro colony forming assays on fibroblast feeder layers revealed a two-fold reduction in the clonogenic potential of CD29hiCD24+ cells from Csf1op/opmice relative to those from control mice (Figure 1c). The absolute numbers of clonogenic cells in the MaSC-enriched population per mammary gland from either wild-type or Csf1op/opmice were estimated to be 14,666 ± 1530 and 1306 ± 348, respectively (mean ± SEM, n = 3). Notably, a 2.4-fold decrease in colony size was also apparent on comparison of colonies derived from Csf1op/opCD29hiCD24+ cells relative to those from wild-type mice. The addition of F4/80-positive mouse mammary macrophages to the clonogenic assays had no discernible effect on colony size or morphology, but may reflect inadequate culture conditions for macrophages in the epithelial cell assays (data not shown).
To investigate a potential role for macrophages in the mammary stem cell microenvironment, the wild-type MaSC-enriched population was next transplanted into the macrophage-deficient fat pads of Csf1op/opmice. Two hundred CD29hiCD24+ cells isolated from the mammary glands of young wild-type female mice (BALB/c) were transplanted into either three-week-old wild-type or six-week-old Csf1op/opfat pads that had been cleared of endogenous epithelium. Older mutant recipient mice were used to compensate for the marked developmental delay that occurs in Csf1op/opmice. Substantial outgrowths were observed in wild-type recipients (18 of 24), whereas only a single small outgrowth (1 of 18) comprising two ducts was evident in Csf1op/oprecipients (P < 0.00001; Figures 2a, b). These data suggest that mammary repopulating cells require the presence of resident macrophages in the mouse mammary gland.
Wild-type mammary stem cells are unable to efficiently repopulate macrophage-deficient fat pads. (a) Wild-type CD29hiCD24+ cells transplanted into Csf1op/opfat pads resulted in only a single small outgrowth whereas those transplanted into wild-type recipients resulted in extensive filling of the fat pad, all harvested at eight weeks post-transplantation. (b) Histogram showing percent outgrowth and the extent of fat pad filling following transplantation of normal CD29hiCD24+ cells into wild-type or Csf1op/opfat pads. Error bars represent standard error of the mean for three independent experiments. (c) Clodronate liposomes inhibit mammary stem cell function. CD29hiCD24+ cells (200) from Rosa26 mice were co-transplanted with liposomes containing either saline or clodronate into the cleared mammary fat pads of syngeneic wild-type female mice. The cells co-transplanted with clodronate liposomes resulted in no outgrowths or rudimentary structures, an example of which is shown. Scale bars = 1 mm. Csf1 = colony stimulating factor 1.
To further investigate the effect of macrophage depletion from the mammary fat pad on stem cell activity, an alternative model of macrophage deficiency was sought. Clodronate is a bisphosphonate that can be delivered in a targeted manner when packaged into liposomes [10]. The liposomes are non-toxic until ingestion by macrophages, in which they are broken down by liposomal phospholipases to reveal the drug. Sufficient clodronate uptake by macrophages causes cell death by apoptosis. In co-transplantation experiments, CD29hiCD24+ cells (200 cells) from the mammary glands of Rosa26 mice were coinjected into the cleared mammary fat pads with either clodronate-containing or saline-containing (control) liposomes. Six weeks after transplantation, the glands were harvested and the outgrowths analysed by staining for LacZ activity (see Materials and methods). Three independent experiments revealed outgrowths in 22 of 28 (77 ± 5%) fat pads coinjected with saline liposomes compared with only 4 of 28 (18 ± 10%) fat pads co-injected with clodronate-containing liposomes (P < 0.000001). Of the four outgrowths evident in the clodronate group, only one branching outgrowth was observed, while the remaining three were very rudimentary structures (Figure 2c).
Discussion
Our data reveal that mammary macrophages contribute to normal stem/progenitor cell function in the developing mouse mammary gland. In a macrophage-deficient environment, the MaSC-enriched subpopulation had a markedly diminished capacity to reconstitute mammary outgrowths. The role of macrophages in this organ, however, appears to be supportive rather than integral to MaSC function, because these mice harbor some MaSCs and limited development can occur in Csf1op/opmice. Interestingly, even in a macrophage-replete system, the function of MaSCs from Csf1op/opglands was compromised. Indeed, both the absolute number and repopulating capability of MaSCs appear to have been altered by the macrophage-deficient environment, possibly reflecting downregulation of growth factor-mediated signal transduction pathways required for stem cell function.
Here we demonstrate the efficacy of clodronate-containing liposomes for macrophage depletion in the mammary gland. Clodronate liposomes have been successfully utilized for macrophage depletion from liver, spleen, lung, lymph nodes, joints, peritoneal cavity, and testis [10]. Local depletion of resident macrophages from the developing mammary gland by clodronate liposomes led to a marked decrease in repopulation by the MaSC-enriched fraction. The depletion of macrophages is likely to be temporary as the liposomes are phagocytosed within the first few days and macrophages presumably return to normal levels by the time of tissue harvest at six weeks post-transplantation. Therefore, mammary macrophages appear to be required for the early stages of repopulation by MaSCs and their progeny.
It is tempting to speculate that mammary macrophages constitute part of the normal MaSC microenvironment and facilitate the maintenance and/or proliferation of stem cells. Gene expression analysis has shown that the MaSC-enriched subpopulation expresses significantly higher levels of Csf1 (approximately five-fold by quantitiative RT-PCR) relative to the luminal epithelial subset. Only macrophages in the mammary gland appear to express the receptor (c-fms or Csf1R) for the Csf1 ligand under normal physiologic conditions, based on immunostaining for the receptor [4] as well as expression in c-fms reporter mice (data not shown). Csf1 may therefore be a chemoattractant for macrophages to the niche in which MaSCs are thought to reside in a basal location. Consistent with this notion, macrophages can be found around the cap cell region of TEBs [4], which has been proposed to be enriched for stem cells [12]. The factors released by macrophages that support stem cell activity are yet to be determined but may include macrophage-secreted cytokines such as CXCL12. Evidence for the direct participation of macrophages in the MaSC niche should eventually be possible through the prospective identification of a highly specific MaSC marker. The finding that the colonic niche was located at the base of the crypts allowed the localization of macrophages to this region, while electron microscopy identified a direct physical relation between colonic epithelial progenitor cells and macrophages [13].
Conclusions
Our data demonstrate that depletion of mammary gland macrophages either by chemical ablation or the use of Csf-1op/opmutant mice alters mammary stem/progenitor cell activity, reflected in a substantially reduced repopulating frequency. The diminished outgrowth potential indicates a continued requirement for normal macrophages during ductal morphogenesis and may be mediated by effects on the putative basal progenitor cell. Overall, these findings indicate that macrophages play a role in supporting normal mammary stem cell function in vivo and suggest that they may constitute part of the normal MaSC microenvironment.
Abbreviations
- CI:
-
confidence interval
- CSF1:
-
colony stimulating factor 1
- MaSC:
-
mammary stem cell
- RT-PCR:
-
reverse transcription polymerase chain reaction
- SEM:
-
standard error of the mean
- TEB:
-
terminal endbud.
References
Gouon-Evans V, Lin EY, Pollard JW: Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002, 4: 155-164. 10.1186/bcr441.
Schwertfeger KL, Rosen JM, Cohen DA: Mammary gland macrophages: pleiotropic functions in mammary development. J Mammary Gland Biol Neoplasia. 2006, 11: 229-238. 10.1007/s10911-006-9028-y.
Pixley FJ, Stanley ER: CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004, 14: 628-638. 10.1016/j.tcb.2004.09.016.
Gouon-Evans V, Rothenberg ME, Pollard JW: Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000, 127: 2269-2282.
Pollard JW, Hennighausen L: Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci USA. 1994, 91: 9312-9316. 10.1073/pnas.91.20.9312.
Van Nguyen A, Pollard JW: Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol. 2002, 247: 11-25. 10.1006/dbio.2002.0669.
Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER: Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002, 99: 111-120. 10.1182/blood.V99.1.111.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE: Generation of a functional mammary gland from a single stem cell. Nature. 2006, 439: 84-88. 10.1038/nature04372.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ: Purification and unique properties of mammary epithelial stem cells. Nature. 2006, 439: 993-997.
Van Rooijen N, Sanders A: Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994, 174: 83-93. 10.1016/0022-1759(94)90012-4.
ImageJ. [http://rsb.info.nih.gov/ij/]
Kenney NJ, Smith GH, Lawrence E, Barrett JC, Salomon DS: Identification of stem cell units in the terminal end bud and duct of the mouse mammary gland. J Biomed Biotechnol. 2001, 1: 133-143. 10.1155/S1110724301000304.
Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS: Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005, 102: 99-104. 10.1073/pnas.0405979102.
Acknowledgements
We are grateful to S. Holroyd and K. Haunholter for expert technical assistance, to K. Stoev and K. Johnson for excellent animal husbandry, and S. Mihajlovic and E. Tsui for expert assistance with histology. R. Anderson kindly provided the BALB/c Csf1op/opmice. We thank John Hamilton for discussions. This work was supported by the Victorian Breast Cancer Research Consortium, National Health and Medical Research Council (NHMRC, Australia). D. Gyorki received support from the National Health and Medical Research Council, Royal Australasian College of Surgeons and the National Breast Cancer Foundation. M-L Asselin-Labat was the recipient of an ARC Fellowship (Australia).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DG contributed to the conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing. MA contributed to the collection and assembly of data. NvR provided the liposomal reagents. GJL contributed to the study conception and design, provision of study materials, data analysis and interpretation. JEV contributed to the conception and design, provision of study materials, data analysis and interpretation and manuscript writing. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gyorki, D.E., Asselin-Labat, ML., van Rooijen, N. et al. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res 11, R62 (2009). https://doi.org/10.1186/bcr2353
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr2353