Abstract
Cytokines play a critical role in the normal development and function of the immune system. On the other hand, many rheumatologic diseases are characterized by poorly controlled responses to or dysregulated production of these mediators. Over the past decade tremendous strides have been made in clarifying how cytokines transmit signals via pathways using the Janus kinase (Jak) protein tyrosine kinases and the Signal transducer and activator of transcription (Stat) proteins. More recently, research has focused on several distinct proteins responsible for inhibiting these pathways. It is hoped that further elucidation of cytokine signaling through these pathways will not only allow for a better comprehension of the etiopathogenesis of rheumatologic illnesses, but may also direct future treatment options.
Similar content being viewed by others
Introduction
Since their discovery and cloning, it has become abundantly clear that cytokines play critical roles in regulating immune and inflammatory cells. For instance, the development of lymphoid and myeloid cells is now known to be controlled to a major degree by cytokines such as interleukin (IL)-7, IL-3, granulocyte-monocyte colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor, among others. Similarly, numerous studies have documented the role of IL-6 in promoting inflammatory responses. Other cytokines can be classified as immunoregulatory cytokines. For example, IL-2 controls lymphoid homeostasis both positively and negatively; in addition, the differentiation of CD4+ T-helper (Th) cells into Th1 and Th2 subsets has been documented to be controlled in large measure by cytokines. For instance, IL-12 promotes the differentiation of naïve Th cells to those that produce interferon (IFN)-γ and lymphotoxin (Th1 cells), whereas IL-4 drives the differentiation of T cells to those that secrete IL-4, IL-5, and IL-10 (Th2 cells).
Not only do these processes contribute to normal host defence, but also to the pathogenesis of autoimmune disease. Much research has focused on the roles that cytokines play in diseases such as rheumatoid and psoriatic arthritis, systemic lupus erythematosus, and even such disparate illnesses as scleroderma and osteoarthritis. It is clear that both the pathogenesis and clinical manifestations of these debilitating diseases are at least in part due to aberrant immune and inflammatory responses, both of which are critically dependent on cytokines. In several animal models of rheumatoid arthritis, most notably collagen-induced arthritis in mice, disease susceptibility has been shown to be highly dependent on immunoregulatory cytokines. For instance, when susceptible mouse strains are rendered genetically deficient in either IL-12 or the IL-12 receptor, they develop a much milder manifestation of arthritis when immunized with collagen compared to normal animals. Even more strikingly, mice that are incapable of producing IL-6 become totally resistant to disease in response to collagen immunization.
Obviously, then, it is of great interest to understand the molecular basis of cytokine action. Fortunately, the mechanisms by which cytokines transmit signals from the cell membrane to the nucleus have been studied extensively, and knowledge of these pathways has increased tremendously over the past several years. In particular, analysis of mice and humans with cytokine receptor mutations or mutations of signaling molecules has provided important insights into the specific functions of these molecules; this information is emphasized in the present review.
The present review focuses on signaling by receptors that are members of two structurally related families, termed type I and type II cytokine receptors. Type I cytokine receptors include those for cytokines such as erythropoietin, prolactin, growth hormone, thrombopoietin, granulocyte colony-stimulating factor, and GM-CSF. In addition, many, but not all of the receptors for different interleukins are part of this family: IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-11, IL-12, IL-13, and IL-15. The type II cytokine receptors include those for the IFNs (IFN-α, IFN-β, and IFN-γ) and IL-10. Of note, the receptors for IL-1, IL-18, IL-8, transforming growth factor-β, and tumor necrosis factor are not part of this family; despite their importance to immune-mediated disease, the signaling pathways used by these cytokines are not discussed here.
Type I and II cytokine receptors lack intrinsic kinase activity and instead rely on Janus kinase (Jak) proteins to initiate signaling. Cytokine binding to these receptors can activate a variety of pathways within cells including mitogen-activated protein kinases (MAPKs) and phosphoinositide 3' kinase. However, the discovery of a new family of tyrosine phosphorylated transcription factors, the Signal transducer and activator of transcription (Stat) family, provided great insight into the action of cytokines. Recently, research has focused on molecules that attenuate cytokine signaling. Of considerable interest is the suppressor of cytokine signaling (SOCS) family of molecules [1,2,3,4,5,6,7,8,9,10,11,12].
Jaks
In contrast to other tyrosine kinase families, the Jak family is rather small. There are only four known mammalian Jaks — Jak1, Jak2, Jak3, and Tyk2 — which were identified in the early 1990s by techniques that capitalized on homology of their kinase domains to other tyrosine kinases [13,14,15,16].Since the discovery of these family members, no new mammalian members have been identified, suggesting that they may comprise the entire family. Teleost and avian Jaks have been identified, as has a single Drosophila Jak; thus, these critical signaling molecules are highly conserved throughout evolution [17,18,19]. Shortly after their discovery, their functional importance in IFN and cytokine signaling was established [1,20]. It was first shown that Jaks are essential for IFN signaling using a panel of cell lines that were resistant to IFNs [21,22,23], and subsequently, type I cytokines were also found to activate Jaks; in fact, all type I and II cytokines activate Jaks in some combination [6,24,25,26,27,28]. It was also shown that Jaks physically associate with cytokine receptors. For the IFN-α receptor, the presence of Tyk2 is required for appropriate receptor expression on the cell surface; this does not appear to be the case for other cytokines, however.
Jak function
Jak3
Jak3, in marked contrast to the relatively ubiquitous expressionof Jak1, Jak2, and Tyk2, has a much more regulated and specific tissueexpression. It is constitutively expressed at high levels in natural killercells and thymocytes, and is inducible in T cells, B cells, and myeloid cells[16,29,30,31,32]. Jak3is activated by a limited number of cytokines, only those receptors that usethe common γ chain (γc) (IL-2, IL-4, IL-7, IL-9, andIL-15) [26,27,33,34,35] (Table1). This is explained by the fact that Jak3 specificallyassociates with γc, and IL-2 and IL-4 signaling is markedlycompromised in cells lacking Jak3 [36,37].
The pivotal function of Jak3 was established when a form of humansevere combined immunodeficiency (SCID) was found to result from Jak3 mutations[38,39]. As was predicted on thebasis of the association of Jak3 with γc, thephenotype of thesepatients was quite similar to that seen in patients with X-linked SCID, whichresults from a mutation in the γc [40].These patients lack T cells and natural killer cells and have dysfunctional Bcells. Jak3 knockout mice were subsequently generated that also have defects inthe same cell lineages (T, B, and natural killer cells) [41,42,43,44].
Because Jak3 is activated by all of the γccytokines, thequestion arises as to how the deficient signaling by thesevarious cytokines relates to Jak3 and γc deficiency. Thephenotype of Jak3 SCID and X-linked SCID is most similar to IL-7 and IL-7receptor gene-targeted mice [45,46,47]. The lack of IL-7 signaling canclearly result in SCID in mice, and recently it was documented that a subset ofpatients with autosomal recessive SCID have IL-7 receptor mutations [48]. One notable feature is that these patients do havenatural killer cells, indicating that IL-7 signaling is not essential fornatural killer cell development. In this regard it is important to note thatIL-15 receptor α chain knockout mice lack natural killer cells [49]. Thus, the SCID phenotype associated with Jak3/deficiencylargely results γc from defective IL-7 and IL-15 signaling. Itshould be noted, however, that the phenotypes of Jak3 and deficientγc mice and humans differ somewhat. Whereas human SCID patientshave dysfunctional B cells and few, if any, T cells, deficient mice lack Bcells and have reduced T cell numbers. The explanation for this difference isunclear at present. It should be noted that the T cell defect in human Jak3SCID is not absolute; some patients do develop some T cells [50].
Somewhat surprisingly, IL-2, IL-2 receptor α chain, and IL-2receptor β chain knockout mice exhibit lymphoproliferative and autoimmunedisease [51,52,53,54], an abnormality that has beenattributed to defective apoptosis of activated T cells. T cells from humanswith Jak3 SCID and Jak3/γc-/- mice are abnormal inthat they express activation markers [41,55]; impaired negative thymic selection has been postulated asone mechanism, but an alternative explanation is that absence of IL-2 signalingresults in impaired apoptosis.
Although we and others previously showed that myeloid cellsexpress Jak3 upon stimulation with a variety of cytokines and proinflammatorystimuli, no abnormal function has been reported in this cell lineage due to thelack of Jak3 expression [56]; the function of Jak3 inthis lineage, therefore, remains unclear.
Importantly, after treatment with bone marrow transplantation, nosignificant defects have been reported outside the immune system in Jak3 andγc deficient humans. This argues that the functions of Jak3 andγc are truly limited to the immune system, which is consistentwith the relative tissue specificity of this molecule. The specificity of thedefects suggests that Jak3 or the Jak3-γc interaction mayrepresent a useful target for the development of novel immunosuppressants[33,38,39].
Jak1, Jak2, and TyK 2
As stated previously, Jak1 and Jak2 have a wide tissue expressionand are activated by a variety of cytokines (Table 1).Specifically, IFN-α/β signaling requires Jak1 and Tyk2; the αsubunit of the IFN-α /β receptor associates with Tyk2, and the βsubunit with Jak 1 [57,58,59].In contrast, IFN-γ requires Jak1 and Jak2 [1,21,22,23,60]. In this circumstance, the IFN-γ receptor αsubunit associates solely with Jak1 and the β subunit only with Jak2[5]. Jak1 is also activated by γccytokines and associates with the ligand-specific receptor subunit. Hormonessuch as growth hormone and erythropoietin predominantly associate with andactivate Jak2 [24,25], but Jak2also associates with the common β chain, a shared subunit for IL-3, IL-5,and GM-CSF [61]. IL-6 and related cytokines can activateJak1, Jak2, or Tyk2; gp130, the shared subunit of this family of receptors, canbind each of these Jaks [62]. Finally, the IL-12receptor β 1 chain associates with Tyk2 and the β 2 chain associateswith Jak2 [63].
As might be expected on the basis of the cytokines that activatethem, mice that lack either Jak1 or Jak2 have more diverse abnormalities. LikeJak3 knockout mice, Jak1-/- mice have SCID. In contrast to Jak3knockouts, though, Jak1 deficient mice die perinatally as a result of anincompletely defined neurologic defect [64]. Inaddition, they fail to manifest biologic responses to all receptors thatutilize this kinase, including all type II cytokine receptors, cytokinereceptors that utilize the γc subunit for signaling, and thefamily of cytokine receptors that depend on the gp130 subunit forsignaling.
Jak2 deficiency is embryonically lethal, because these mice failto develop erythroid cells [65,66]. Interestingly, this phenotype is more severe than thatseen in erythropoietin receptor deficient mice, perhaps due to the necessity ofsignaling through other Jak2-requiring receptors such as IL-3 for efficienterythropoiesis. Enhanced signaling through Jak2 has also been implicated in thepathogenesis of leukemia. Chromosomal translocations in several patients withleukemia were characterized and shown to fuse the 3' portion of Jak2 tothe 5' region of TEL, a gene encoding a member of the ETS transcriptionfactor family. The TEL-Jak2 fusion protein includes the catalytic domain ofJak2 and the TEL-specific oligomerization domain. TEL-induced oligomerizationof TEL-Jak2 resulted in the constitutive activation of its tyrosine kinaseactivity and conferred cytokine-independent proliferation to theinterleukin-3-dependent Ba/F3 hematopoietic cell line [67,68]. These findings underscore theimportance of Jak2 mediated signaling in driving proliferation anddifferentiation in both myeloid and lymphoid cells.
Tyk2 knockout mice have not been reported as of yet, and no knownhuman disorders have been linked to a defect in this Jak, so the cytokines forwhich Tyk2 signaling is uniquely responsible have not been ascertained. Basedon the findings with deficient cell lines, the expectation is that IFN α/β actions will be impaired in such mice.
Jaks and noncytokine receptors
Since the discovery of the Jaks, occupancy of a vast array of receptors other than type I and II receptors have been shown to induce Jak phosphorylation and/or activation. To date, however, it has not been proved that Jaks are essential, nonredundant components of the signaling pathways of noncytokine receptors. For instance, although CD40 was found to associate with Jak3, no defect in CD40 signaling was apparent in Jak3 deficient cells [69,70]. At present, the only receptors documented to be absolutely dependent on Jaks for signaling are type I and II cytokine receptors. Whether this small family of tyrosine kinases is dedicated to signaling by this class of receptors or whether wider functions exist remains to be determined; this is one of the most interesting remaining questions that pertain to the biology of the Jaks.
Jak structure
The three-dimensional structure of the Jaks is presently unknown. This is no doubt partly because they are relatively large kinases of more than 1100 amino acids with apparent molecular weights of 120–130 kDa. Their messenger RNA transcripts range from 4.4 to 5.4 kilobases in length. Multiple spliced forms of Jak3 have been identified, including a variant that lacks a segment of the catalytic domain [29,71,72]. It is intriguing to speculate that a naturally occurring dominant negative form of Jak3 may have regulatory function, but this has yet to be proved.
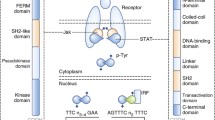
Jaks have seven regions of homology termed Janus homology (JH) domains 1-7 (Fig. 1), and the carboxy-terminal tyrosine kinase, or JH1 domain, shares the features of other tyrosine kinase domains. For example, phosphorylation of tyrosine residues in the activation loop of kinases such as the insulin receptor play an important role in regulating phosphotransferase activity [73]. A number of autophosphorylated sites are being identified in Jaks, two of which reside within the putative activation loop. Depending upon the specfic Jak, however, mutations at these sites appear to have slightly different functional consequences. That is, mutation of tyrosine 1007 abrogated any signaling capacity of Jak2, whereas mutations in both the corresponding tyrosine residues and the adenosine triphosphate binding site were required to abolish activity of Tyk2 completely [74,75].In contrast, mutations of Y981 in Jak3 actually increased activity [76]. Thus, there may be subtle differences in the regulation of catalytic activity of each Jak. The molecule src homology (SH)2Bβ, an SH-2 domain containing protein, associates with Jak2 and increases its catalytic activity in response to growth hormone, but the mechanism of this regulation has not been determined [77,78].
The hallmark of the Jak family of protein tyrosine kinases is the existence of tandem kinase and pseudokinase domains; it is this feature that gives the Jaks their name, and among mammalian tyrosine kinases only the Jaks have this domain. Like the Roman god of gates and doorways, the Jaks are 'two-faced'. The pseudokinase domain is also termed the JH2 domain. Although it has overall similarity to kinase domains, the JH2 domain lacks critical residues that are required for phosphotransferase activity; rather, the function of this domain appears to be to regulate catalytic activity. Mutations or deletions of this region have complicated effects that either inhibit or enhance catalytic function, depending upon the exact mutation generated [79,80] (Chen M, et al, unpublished data). Importantly, Jak3 SCID patients have been identified with mutations in this region, underscoring its critical function [50]. Another function suggested for the JH2 domain is as a docking site for Stats [81].
Although it has not been well characterized for all of the Jaks, the amino-terminus appears to confer binding to the appropriate cytokine receptor [79,82,83,84]. For Jak3, at least, the amino-terminal JH6 and JH7 domains are sufficient to confer binding specificity to γc [85,86]. For otherJaks, the amino-terminus is clearly involved in receptor interactions, but may extend beyond the JH6 and JH7 domains [87,88]. The region of the cytokine receptor to which Jaks bind has been much better characterized and is found in the membrane proximal region (reviewed in Ihle, 1995 [28]). Signal transducing adaptor molecule (STAM), a 70-kDa adaptor molecule that is phosphorylated in response to IL-2, IL-4, GM-CSF, epidermal growth factor and platelet-derived growth factor, binds to both Jak3 and Jak2, and couples cytokine stimulation to DNA synthesis [89]. STAM is a molecule that associates with Jaks and may enhance the formation of Jak-receptor complexes (authors' unpublished observations). Two STAM-associated molecules, Hrs and AMSH, have been hypothesized to act downstream of Jaks in cytokine signaling pathways [90,91].
Many tyrosine kinases have intrinsic SH2 and SH3 domains that mediate protein-protein interactions. Although the JH4 domain has overall homology to SH2 domains, mutation of the critical Arg residue, which would be expected to bind phosphotyrosine, had no effect on signaling. Thus, the function of this segment remains unclear at present [15,84].
Binding of cytokines to type I and II receptors has been suggested to initiate signaling by effecting homodimerization or heterodimerization of the receptor subunits, which in turn leads to the apposition of Jaks. This may allow transphosphorylation of the Jaks at sites within their activation loops, thus enhancing catalytic activity. A more recently proposed mechanism [92,93] is that ligand-induced allosteric alteration of the receptor itself leads to Jak activation. For receptors that heterodimerize (most of the IL and IFN receptors), heterodimerization of different Jaks also occurs, and the Jaks are interdependent for activation. For instance, in cells that lack Jak1, no phosphorylation of Tyk2 or Jak2 was observed upon stimulation with IFN [60], and conversely no phosphorylation of Jak1 was seen in cells lacking Jak2 or Tyk2. In Jak3 deficient cells, no phosphorylation of Jak1 occurs in response to IL-2 [36].
Regardless of the precise mechanism, after activation the Jaks phosphorylate receptor subunits on tyrosine residues, enabling the recruitment of proteins with SH2 or phosphotyrosine binding domains. These proteins are then phosphorylated by Jaks. A number of signaling pathways, such as the Ras-Raf-MAPK pathway and the phosphoinositide 3' kinase pathways are activated in response to cytokines; the significance of these pathways in immune function has been extensively reviewed elsewhere and is not discussed here [94,95,96,97,98,99]. However, the function of another class of SH2-containing molecules, the Stats, is discussed in detail.
Structure of Janus kinases (Jaks), signal transducers andactivators of transcription (Stats), and suppressors of cytokine signaling(SOCS). Regions of homology shared by Jaks have been termed Jak homology (JH)domains. JH1 is a kinase domain and JH2 is a pseudo-kinase domain. Theamino-terminus of the Jaks appears to be important for association withcytokine receptors subunits. Stats have a conserved tyrosine residue,phosphorylation of which allows Stat dimerization; a src homology(SH2) domain that mediates the dimerization; and an amino-terminal region thatis known to play a role in the dimerization of Stats dimer. The amino-terminal,carboxy-terminal and coiled-coil regions of Stats can interact with othertranscription factors. SOCS proteins share a similar strucuture with a centralSH2 domain, a region at the amino-terminus that is variable in both length andin amino acid sequence, and a region of homology at the carboxy-terminus termedthe 'SOCS box'.
Stats
By purifying factors bound to promoters of IFN-inducible genes, Darnell and coworkers [100] cloned the first members of the Stat family. The IFN-α induced complex comprised a 91-kDa polypeptide, which later became known as Stat1; a 113-kDa protein (Stat2); and p48, a member of the IFN regulatory factor family. An IFN-γ induced complex, γ activated factor, turned out to be composed of Stat1 only [101].
Following the discovery of Stat1 and Stat2, the cloning of the remaining family members, Stat3, Stat4, Stat5a, Stat5b, and Stat6, quickly ensued [9,102,103,104,105,106,107,108,109,110]. Most Stats are approximately 750 amino acids long, but Stat2 and Stat 6 are larger (850 amino acids). These transcription factors were immediately recognized as a novel family, in that they had SH2 domains and were themselves tyrosine phosphorylated. Thus, a new signaling paradigm emerged (Fig. 2). Stats are latent cytosolic transcription factors that are recruited to phosphorylated cytokine receptors via their SH2 domains [111,112,113,114]. The Stats are then phosphorylated themselves by Jaks, they heterodimerize or homodimerize via reciprocal SH2-phosphotyrosine interactions, and translocate to the nucleus to regulate gene transcription.
Stat structure
In contrast to the Jaks, the structure of the Stat molecules has been reasonably well characterized [115,116,117] (Figs 1 and 3). Overall, the structure of the Stats is similar to that of other transcription factors such as nuclear factor-κ B and p53. The dimeric molecule forms a C-clamp structure around the DNA, but, unlike nuclear factor-κ B and p53, there are fewer direct contact sites with the DNA backbone. Rather, the nutcracker-like structure of the Stats is largely dependent upon SH2-phosphotyrosine interactions. Stats have a conserved amino-terminal protein-protein interaction domain, followed by a segment (the coiled-coil domain) with multiple protruding α -helices. This is followed by the actual DNA binding domain, a linker domain, the SH2 domain, a conserved site of tyrosine phosphorylation, and a variable carboxy-termini transcriptional activation domain.
Amino-terminal dimer-dimer interaction domain
With the exception of Stat6, Stats bind somewhat indiscriminatelyto the same consensus sequences; it is notable that clustered imperfect Statbinding sites are found in a number of relevant cytokine inducible promoters.Even though Stats bind poorly to these sites, cooperative dimer–dimerinteractions can occur [118]. This is mediated by theconserved amino-termini of Stats, which consists of eight helices that formhook-like structures, facilitating these interactions. Perhaps unimportant forStat binding to a single consensus binding site [119],these domains appear critical for binding to imperfect sites [120]. Combinatorial binding might be one mechanism ofachieving more specificity in signaling.
Coiled-coil domain and association with other transcription factors
Consisting of four α -helices, the coiled-coil domain fromamino acids 136-317 provides a structure suitable for many protein-proteininteractions. The coactivator proteins p48 and p300/CBP have been shown tointeract with Stats through this region [121,122,123,124,125], as well as another protein,Nmi [126]. Other transcription factors have been shownto associate with Stats such as the glucocorticoid receptor (with Stat5a andStat5b), Sp-1, c-Jun, and nuclear factor-κ B, but the exact domaininteractions have yet to be mapped [127,128,129,130,131,132].
DNA binding domain
The DNA binding region of Stats resides within the central 171amino acids, but relatively few direct contacts exist. Rather, the clamp-likestructure is imparted by phosphotyrosine-SH2 interactions [115,116]. Stats bind two types of DNAmotif: IFN-stimulated response elements (consensus: AGTTTNCNTTTCC) and γ-activated sequence elements (consensus: TTCNNNGAA). Stat1, Stat2, and p48 bindto IFN-stimulated response elements, whereas Stat1, Stat3, Stat4, Stat5a, andStat5b bind to γ -activated sequence element sites. Stat6 binds a similarbut distinct site: TTCNNNNGAA.
The src homology 2 domain and tyrosine phosphorylation site
The SH2 domain (amino acids 600-700) serves two criticalfunctions: to allow Stats to bind phosphorylated receptor subunits and bephosphorylated themselves by Jaks on a conserved tyrosine residue; and toenable Stat dimerization and DNA binding. The crystal structure of a Stat-DNAcomplex underscores the importance of the SH2 domain, as theSH2-phosphotyrosine interaction forms the hinge of the clamp that is largelyresponsible for DNA binding (Fig. 3).
Transcriptional activation domain
Stat1, Stat2, and Stat5 have been documented to havecarboxy-terminal transcriptional activation domains [119,133,134,135,136,137]. In addition to tyrosinephosphorylation, it has been shown that Stat1, Stat3, Stat4, and Stat5 are alsophosphorylated on serine residues in response to cytokine stimulation [133,138,139].For these proteins, a conserved site of serine phosphorylation, residing in aconsensus sequence for MAPK-mediated phosphorylation has been mapped within thecarboxy-terminal transcriptional activation domain [135,140] (Visconti et al,unpublished data). However, the functional significance of Stat serinephosphorylation and the identity of the kinase(s) responsible for this eventremain deeply controversial. Recently, a large number of reports have beenpublished that link STAT serine phosphorylation to the activation of variousMAPKs. Notably though, they provide significantly divergent results, perhapsdue to the differences in the Stat proteins investigated and in the systemsutilized [141,142,143,144,145,146].
Thus, it has been reported that p38, which is activated inresponse to IFNs, is indispensable for Stat1 serine 727 phosphorylation andtranscriptional activity [145]. Accordingly, we havefound that IL-12-induced Stat4 serine 721 phosphorylation and transcriptionalactivity requires p38 activity (Visconti et al, unpublished data).Other findings indicate that JNKs, but not p38, mediate Stat3 serine 727phosphorylation in response to various stress treatments and that this eventresults in the inhibition of Stat3 activity [146]. Incontrast, it has been shown that both JNKs and p38 are required for STAT3transcriptional activity induced by the Src oncoprotein [143]. Other MAPKs, including extracellular signal relatedkinase family members, can also phosphorylate serine residues in Stat proteinsand activate them [147,148].Others have found, however, that signaling through the extracellular signalrelated kinase pathway can also downregulate Stat activity [149]. The most plausible hypothesis at the moment is that theeffect of serine phosphorylation of Stat proteins depends on the cell type andon the class of serine kinases activated in response to different extracellularstimuli.
Nuclear translocation
Stats lack a classic nuclear localization signal, and in generaldimerization of the Stats is believed to be essential for nuclear localization.This appears not to be sufficient, however, and sequences in the amino-terminuscontribute to nuclear translocation (and perhaps deactivation, as well) [150]. For Stat1 at least, nuclear import has been shown to bedependent upon the activity of the small guanosine triphosphatase Ran and mayinvolve the importin receptor [151,152,153,154].One group has hypothesized that Stats may translocate to the nucleus via thenuclear localization signal of the ligands or receptors themselves, but theimportance of this possibility remains to be established [155,156]. Finally, regulation ofnuclear export of the Stats may also be an important means of nuclearlocalization. In any case, it is very clear that much needs to be learned aboutthe regulation of Stat intracellular trafficking.
Stat function
Stat1
Stat1 has been shown to be activated by the IFNs, cytokines suchas IL-2, IL-6, and IL-10, and noncytokine signals such as epidermal growthfactor (Table 1). Not surprisingly, Stat1 knockout micewere found to be highly susceptible to viral and some bacterial infections,reminiscent of defects observed in IFN-α receptor and IFN-γ receptorknockout mice and IFN-γ receptor deficient humans [157,158,159,160]. Interestingly, defects insignaling by cytokines other than IFNs have not been reported, but it does seemto be important for the fibroblast growth factor-mediated growth inhibition ofchondrocytes [161].
A role for IFN-γ signaling through Stat1 for tumorsurveillance has recently been underscored. Mice deficient in Stat1 or theIFN-γ receptor were much more susceptible to tumor development whenchallenged with a chemical carcinogen, and when bred with mice deficient in thetumor suppressor p53 gene, these mice developed a broader spectrum oftumors compared with mice lacking p53 alone [162]. A recent study [163] has shownthat Stat1 deficient mice are unable to clear immunogenic tumors that theirwild-type littermates easily controlled, and that they are unable to rejectpoorly immunogenic tumors when immunized with an IL-12 based vaccine. Severedefects in lytic activity in both T and natural killer cells were alsonoted.
Stat1 has additional functions in regulating apoptosis. That is,tumor necrosis factor-α dependent apoptosis is impaired in Stat1 deficientmice because of reduced expression of the caspases Ice, CPP32, and Ich-1 [164]. Unlike its role in mediating cytokine dependent geneexpression, however, Stat1 dimerization does not appear to be necessary for theregulation of these genes, because an SH2 mutant Stat1 supports expression. Themechanism by which this occurs is obscure, particularly in view of thestructural information present. The importance of Stat1 in apoptosis mediatedby type I cytokines was further underscored when it was found thatStat1-/- mice were resistant to virus-induced apoptosis [165].
Stat2
Like Stat1, Stat2 is also activated by interferons; indeed, onlyIFN-α/β has been reported to activate Stat2 (Table 1). Unlike other Stats, it requires Stat1 and p48 forinteraction with DNA [166]. As of yet, Stat2 knockoutmice have not been reported, but evidently they are severely deficient inIFN-α/β signaling, as might be expected (Schindler C, personalcommunication).
Stat3
Stat3 was first identified as a factor activated by cytokinessignaling through gp130 (IL-6, leukemia inhibitory factor, and ciliaryneurotropic factor). Stat3 deficiency is embryonically lethal, perhaps due tothe absence of leukocyte inhibiting factor function, as well as its role inmaintaining stem cell pluripotency [167,168]. In contrast, gene targeting of Stat3 only in myeloidcells produced an exaggerated inflammatory response, resulting in prematuredeath largely due to impaired IL-10 function [169].These animals became highly susceptible to endotoxic shock with increasedproduction of inflammatory cytokines such as tumor necrosis factor-α,IL-1, IFN-γ, and IL-6. The suppressive effects of IL-10 on the productionof inflammatory cytokines by macrophages and neutrophils was completelyabolished, and these mice developed chronic enterocolitis with age.Additionally, these mice manifested an exaggerated Th1 response, which may alsohelp to explain the inflammatory bowel disease seen. These results mightsuggest a role for abnormal Stat3 signaling in other autoimmune processes, butno studies have been published to date. Although it is clear that Stat3 isessential for appropriate IL-10 signaling, its function for other cytokinesremains unclear because of the embryonic lethality seen in Stat3 knockoutembryos.
Stat4
Stat4 is activated by a limited number of cytokines; IL-12 is thepredominant cytokine that activates Stat4 in mice, whereas in humans bothIFN-α/β and IL-12 activate it. More recently it has been shown thatIL-2 is capable of activating Stat4 in natural killer cells [170]. Of note, though, Stat4 deficient mice only demonstratedefects of impaired IL-12 responses (ie defective Th1 development and impairedcell-mediated immune responses), a phenotype similar to that seen in IL-12 andIL-12 receptor knockout mice and IL-12 receptor deficient humans [171,172,173,174]. Although predominantlyexpressed in lymphoid cells, Stat4 has recently been found to be induciblyexpressed in activated macrophages, most notably those found in synovium fromrheumatoid arthritis patients (Frucht et al, submitted). The targetgenes of Stat4 in macrophages are currently unknown, but it is interesting tospeculate that macrophages may provide some functions of cellular immunitypreviously assigned only to lymphoid cells.
Stat5
Encoded by two genes, Stat5a and Stat5b share 93% identity at theprotein level [109,175], andare both activated by a plethora of cytokines, including prolactin, growthhormone, erythropoietin, thrombopoeitin, and IL-2. The development of knockoutmice, however, underscores the very different biologic functions they eachserve; Stat5a knockout mice have impaired mammary gland development [176], whereas Stat5b deficient mice are defective in bothsexually dimorphic growth as well as in growth hormone dependent regulation ofliver gene expression [177].
To assess potential redundancy in function, Stat5a/Stat5b doubleknockouts were created [178]; one-third of these micedied within 48 h of birth, with the surviving mice developing a smaller thannormal body size, which was apparently due to aberrant growth hormonesignaling. Despite the fact that lymphoid development is normal, T cells arehyporesponsive to IL-2, and these animals develop lymphoproliferative disease,similar to that in IL-2, IL-2 receptor α chain and IL-2 receptor βchain deficient mice [179]. These results underscore anessential role for Stat5 for IL-2 signaling; whether Stat5a or Stat5bindividually are critical is somewhat controversial at present, because severalstudies have clearly shown that IL-2 responsiveness is impaired in eitherStat5a or Stat5b deficient mice [180,181]. Clinically, adult females are infertile, but unlikeJak2 knockout mice, which have no blood, these animals are only moderatelyanemic; the severity of anemia, however, is also a point of contention [182] (Ihle J, personal communication).
Of additional interest is the current controversy regarding therole of Stat5 in T-cell receptor-mediated signaling. Initially found not to beinvolved with T-cell receptor signaling [183], a morerecent study has shown that Stat5 phosphorylation induced by T-cell receptorcrosslinking is abolished in lck deficient mice [184].Other studies [185] havesuggested a role for Stat3 but not Stat5 in T-cell receptor-mediated signaling;the different results may reflect the different model systems used.Nonetheless, the potential significance of antigen-mediated signaling throughthe Stats remains intriguing.
Stat6
Stat6 was originally identified as an IL-4 inducible transcriptionfactor [106]. It was therefore not surprising to findthat Stat6 deficient mice failed to develop Th2 immunity in response to IL-4 orIL-13, were unable to upregulate cell surface expression of majorhistocompatibility complex class II, CD23, or IL-4 receptor α chain inresponse to IL-4, and failed to produce immunoglobulin E in response tocross-linking of surface immunoglobulin D [186,187,188,189].Accordingly, lack of Stat6 dramatically attenuates allergic and asthmaticdiseases in several animal models [190,191,192,193].Stat6 deficient animals are also unable to clear parasites [194]. Remarkably, a recent study of Stat6 knockout mice in amurine acquired immune deficiency syndrome model revealed normal serumimmunoglobulin E levels and lymphoproliferation, indicating that B cells frommice with murine acquired immune deficiency syndrome activate uniqueIL-4-independent and STAT6-independent signaling pathways for B-cell activationand differentiation [195].
The generation of Stat4/Stat6 double knockout mice has provided aninteresting model system for the study of Th1/Th2 differentiation, a complexand exciting topic in immunology today. Interestingly, these mice develop Th1responses, suggesting that Stat4 may be dispensable for Th1 differentiation inthe absence of Th2 responses [196].
Activation of signal transducers and activators of transcription by noncytokine receptors
As with the Jaks, ligation of many receptors has been reported toactivate various Stats. As discussed above, T-cell receptor crosslinking, forinstance, has been reported to activate Stats, but this remains controversial[179,184, 197]. Less controversial are the findings that epidermalgrowth factor, angiotensin II, and other ligands activate Stats [198,199,200,201,202,203]. Through the use of knockoutmice or deficient cells, however, the essential function of a specific Stat fora noncytokine stimulus has yet to be established; this is clearly the majorchallenge in this area. It should be noted, though, that Stats areevolutionarily very old. Several Dictyostelium Stats have beenidentifed, but this organism has not been reported to have Jaks [204, 205]. If this is the case, itwould add credence to the notion that Stats have broader functions beyond typeI and II cytokine signaling mediated by Jaks. Interestingly, v-src andother oncogenes have been reported to activate Stats and, importantly, adominant-negative Stat3 construct has been shown to block transformation [206]. Conversely, a gain-of-function Stat3 construct istransforming [207].The importance of Stats in malignanttransformation will need to be established using knockout animals, but this isclearly an exciting area.
Model for cytokine signal transduction. Cytokines associate withcytokine receptor subunits and activate janus kinases (Jaks). The Jaks in turnphosphorylate tyrosine-based docking sites on the receptor. signal transducersand activators of transcription (Stats) then bind via their src homology (SH)2 domains. The STATs are then phosphorylated by the JAKs, formhomo-hetero-dimers and then translocate into the nucleus, where they bindtarget sequences like γ activated sequence (GAS) motif. Transcriptionalactivation of genes typically requires the coordinated function of multipleaccessory transcription factors. Additionally, serine phosphorylation of someStats may be important for maximal transcription of target genes.
Signal transducer and activator of transcription (Stat) binding toDNA. In this two-dimensional model, the heterodimerized or homodimerized Statmolecule binds to the appropriate DNA sequence via a 'C clamp'structure. Note that in addition to the specificity controlled by the DNAbinding domain, other regions of the Stat molecule may also be important in DNAinteractions. Tetramerization of the Stats may allow for an even greater degreeof stability in DNA binding to adjacent imperfect Stat binding sites; this ismediated by the Stat amino-terminus (not shown). Also not shown in thisillustration is the Stat transcriptional activation domain.
Attenuation of cytokine signaling
Equally as important as the ability to initiate cytokine signaling is the ability to terminate it. Indeed, one might speculate that this will be more important in terms of the pathogenesis of human autoimmune diseases. The ability to regulate cytokine signaling occurs by multiple proposed mechanisms. These include the following: phosphatases, cytokine-inducible inhibitor molecules, transcriptional repressors, and Stat degradation (Fig. 4).
Phosphatases
It is well recognized that cytokine-induced phosphorylation of various substrates, including the Jaks, cytokine receptors, and the Stats, is transient. The tyrosine phosphatase SHP-1 has been hypothesized as one regulator that can interact with cytokine receptors and downregulate their function [208,209,210]. Interestingly, motheaten and viable motheaten mice have a mutation in the gene that encodes SHP-1 and exhibit many characteristics of systemic autoimmunity [211,212,213]. Motheaten mice are characterized by increased levels of plasma cells in secondary lymphoid organs with abnormal levels of immunoglobulins and elevated serum anti-double-stranded DNA antibodies, a peripheral neutrophilia and monocytosis, decreased erythropoiesis, neutrophilic skin lesions, and a severe pneumonitis that is induced by activated macrophages. These mice rarely live beyond 8 weeks [214]. This striking presentation of autoimmune phenomena suggests that some manifestations of human disease might also be a result of aberrant downregulation of cytokine signaling. The regulation of cytokine signaling via SHP-1 is also important in bone remodeling, because motheaten mice have recently been shown to develop osteopenia due to a lack of SHP-1 mediated control of bone resorption [215].
Whether SHP-1 or another nuclear tyrosine phosphatase is responsible for Stat dephosphorylation is not clear. Indeed, one might speculate that a nuclear phosphatase would be required. Because some Stats are also serine phosphorylated, it is also reasonable to expect that a serine phosphatase might also regulate Stat function. Alternatively, it has been suggested that degradation of Stats via ubiquitination is also a means of terminating Stat induced signaling [216].
Suppressor of cytokine signaling/Jak binding/Stat-induced Statinhibitor/cytokine inducible SH2 protein family of inhibitors
A recently described family of SH2-containing molecules [alternatively named Jak binding, SOCS, Stat-induced Stat inhibitor and cytokine inducible SH2 protein (CIS)] comprises several molecules that are induced rapidly upon cytokine stimulation and serve as classic feedback inhibitors of signaling [11,217,218,219,220]. There are at least eight members of this family characterized by a central SH2 domain and a carboxy-terminal region of homology, termed the 'SOCS box' (Fig. 1); for the purpose of this paper, this family will be referred to as the 'SOCS' family. The exact function of this region remains unclear at present, but a recent study [221] has shown that the SOCS box mediates interactions with elongins B and C. In this manner, they may couple SOCS proteins and their activated substrates to the proteasomal protein degradation pathway. The proteins SOCS-1, SOCS-3, and CIS-1 have been the most carefully studied in terms of regulating cytokine signaling, and this is discussed further.
The first member of this family, CIS, was discovered in 1995 and was shown to associate with the IL-3 and ery-thropoietin receptors [217]. Subsequently, three different groups identified the second family member (named SOCS-1, Jak binding, or Stat-induced Stat inhibitor-1) based on its ability to interact with Jaks and/or inhibit Jak mediated Stat activation [218,219]. Via their SH2 domains, some SOCS members bind the phosphorylated activation loop tyrosine residue in Jaks, thereby inhibiting Jak activity [219,222].
The importance of this downregulation is highlighted in SOCS-1 knockout mice, which have marked growth retardation, display increased lymphocyte apoptosis, and perish within 3 weeks of birth [223,224]. Two very recent studies of these animals have shown that this lethality is due almost entirely to systemic hyper-responsiveness to IFN-γ and that aberrant T lymphocytes may be the source of the IFN-γ [225,226]. Although these mice have enhanced IFN-γ dependent ability to kill Leishmania major parasites, they exhibit exaggerated and lethal responses to viral infections. Deficiency of SOCS-1 also results in impaired lymphopoiesis, as thymi from these animals undergo a loss of cellularity and a switch from predominantly CD4+CD8+ to single positive cells. Additionally, peripheral T cells express activation markers and respond to IL-2 in the absence of T-cell receptor cross-linking. The relative specificity of SOCS-1 for IFN-γ signaling in this model is underscored by experiments that demonstrate that all pathology can be prevented by administering anti-IFN-γ antibodies or by crossing the mice with IFN-γ knockout mice. Clearly, the main role of SOCS-1, therefore, is to prevent uncontrolled and lethal IFN-γ signaling. It is interesting to speculate that SOCS-1 mutations or polymorphisms could potentially underlie immunologic diseases in humans.
SOCS-3 is another family member that has been shown in vitro to interact with the Jaks to regulate Stat activation [222,227,228]. However, a recent study in SOCS-3 deficient mice reveals the critical role for this protein in down-regulating fetal, but not adult, hematopoiesis [229]. This suggests a more specific role for SOCS-3 in Jak2 regulation, because Jak2 knockout mice have hematopoietic disorders as well [66]. CIS-1, another member of this family, may downregulate cytokine signaling by binding directly to receptors, rather than Jaks. In this regard, CIS-1 has been shown to interact with the IL-2 receptor β chain and inhibit IL-2 dependent signaling [230]. Recently, CIS-1 transgenic mice have been created, and their phenotype is remarkably similar to those of Stat5a and Stat5b knockout mice, indicating the critical role of CIS-1 as a negative regulator of Stat5 function [231]. It is becoming more evident with time that in vivo models are necessary to dissect the specificity of SOCS interactions. Also, we are learning that SOCS members may play a role in noncytokine signaling, including the leptin, growth hormone, and prolactin signaling pathways [232,233,234], but findings in SOCS knockout mice do not necessarily support a critical function.
Protein inhibitors of activated Stats
Recently, a family of proteins that interact with Stats, termed protein inhibitors of activated Stats (PIAS), have been identified [235,236]. PIAS1 and PIAS3 bind to Stat1 and Stat3, respectively. They inhibit transcriptional activity of the Stats, but do not affect phosphorylation. Just how specific they are in terms of regulating cytokine signaling has not been determined; no knockouts have yet been reported. In addition, these molecules were cloned by yeast two-hybrid screens using baits other than Stat molecules, and therefore may affect proteins other than Stats. Thus, it will be important to characterize the physiologic function of this family of molecules.
Bcl-6
Bcl-6, a zinc-finger protein expressed in B cells and CD4+ T cells and frequently associated with non-Hodgkin's lymphoma, has also been recently shown to regulate Stat function negatively. Bcl-6 deficient mice develop a severe systemic inflammatory disease typical of a Th2-mediated hyperimmune response, which is characterized by infiltrates of immunoglobulin E-bearing B cells and eosinophils [237]. Because the Bcl-6 DNA recognition motif resembles sites bound by Stat6, it was surprising to find that when Bcl-6 mice were bred to either Stat6 or IL-4 deficient mice, the animals still manifested the same hyperinflammatory process. In vitro, however, Stat6 was required for the differentiation of Bcl-6 deficient T cells into Th2 cells. These findings indicate that this transcriptional repressor can regulate Th2 responses by pathways both dependent on and independent of IL-4 and Stat6 [238].
Attenuation of cytokine signaling. Suppressor of cytokinesignaling (SOCS) proteins are induced in response to cytokines and suppresssignal transduction in two ways. Some SOCS proteins bind directly to januskinases (Jaks) and inhibit their catalytic activity, whereas others likecytokine inducible src homology-2 protein (CIS) can bind to activatedreceptors and prevent docking by signaling intermediates such as the Stats.SHP-1 can either dephosphorylate Jaks or activated receptor subunits, dependingupon the pathway activated. Protein inhibitors of activated Stats (PIAS) familymembers inactivate Stat dimers by an as yet unknown mechanism. Stat dimers arealso probably downregulated by degradation and dephosphorylated by unknownmechanisms. The accumulation of STATs in the nucleus could be regulated at thelevel of nuclear import, nuclear export, or a combination of the two; themechanisms that control these processes are not well characterized. Finally,molecules like Bcl-6 can bind to consensus Stat binding sites and function asrepressors.
Conclusion
The Jaks are a small family of tyrosine kinases with very specific functions; these are best illustrated by humans with mutations and gene-targeted mice. The present data indicate that they have critical functions in transmitting cytokine-dependent signals. The Stats, too, appear to be a small, but conserved family of transcription factors that serve to further transmit signals initiated by receptor-Jak interactions, also with highly specific functions. Indeed, at least four of the six Stats have major functions in regulating host defense and immune responses. Although we have learned a great deal about the cytokine signaling pathways, relatively few cytokine-inducible genes have been identified. This, of course, will rapidly change with the advent of microarray and gene chip technologies. The challenge will remain to dissect how Stats interact with the growing list of other transcription factors to regulate the expression of these genes and how signals emanating from cytokine receptors affect transcriptional activation. Another important issue that needs to be resolved is to what extent Jaks and Stats function as essential intermediates for noncytokine receptors. Equally exciting as the discovery of the Jaks and Stats is the discovery of families of molecules that serve to attenuate cytokine signalling; it is exciting to consider that these molecules might be mutated or polymorphic in human autoimmune diseases.
A knowledge of these signaling pathways is of particular importance to rheumatologists, because cytokines clearly regulate the inflammatory and immune responses. One significant lesson gleaned from these investigations is that cytokines may act as a double-edged sword: That is, although cytokines play important physiologic roles in promoting immune development and fighting off infections, maladapted cytokine responses can lead to autoimmunity. Perhaps a clearer understanding of how members of the Jak and Stat families, as well as the more recently discovered SOCS family members, interact and function may allow us to target specific pathways, such as those involving proinflammatory cytokines including IFN-γ or IL-6, for therapeutic intervention. Knowledge in cytokine signaling pathways has grown exponentially over the past decade and, conceivably, manipulation of these pathways through pharmaceutical intervention may provide the rheumatologist with a unique way to treat autoimmune diseases.
References
Darnell JEJ, Kerr IM, Stark GR: Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994, 264: 1415-1421.
Pellegrini S, Dusanter-Fourt I: The structure, regulation and function of the Janus kinases (JAKs) and the signal transducers and activators of transcription (STATs). Eur J Biochem. 1997, 248: 615-633.
Ihle JN, Thierfelder W, Teglund S: Signaling by the cytokine receptor superfamily. Ann N Y Acad Sci. 1998, 865: 1-9.
O'Shea JJ: Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet?. Immunity. 1997, 7: 1-11.
Bach EA, Aguet M, Schreiber RD: The IFN-gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997, 15: 563-593.
Leonard WJ, O'Shea JJ: Jaks and STATs: biological implications. Annu Rev Immunol. 1998, 16: 293-322.
Carter-Su C, Smit LS: Signaling via JAK tyrosine kinases: growth hormone receptor as a model system. Recent Prog Horm Res. 1998, 53: 61-82; discussion 82-83.
Aringer M, Cheng A, Nelson JW: Janus kinases and their role in growth and disease. Life Sci. 1999, 64: 2173-2186.
Hoey T, Grusby MJ: STATs as mediators of cytokine-induced responses. Adv Immunol. 1999, 71: 145-162.
Starr R, Hilton DJ: Negative regulation of the JAK/STAT pathway. Bioessays. 1999, 21: 47-52. 10.1002/(SICI)1521-1878(199901)21:1<47::AID-BIES6>3.3.CO;2-E.
Hilton DJ: Negative regulators of cytokine signal transduction. Cell Mol Life Sci. 1999, 55: 1568-1577.
Baird AM, Gerstein RM, Berg LJ: The role of cytokine receptor signaling in lymphocyte development. Curr Opin Immunol. 1999, 11: 157-166.
Krolewski JJ, Lee R, Eddy R, Shows TB, Dalla-Favera R: Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene. 1990, 5: 277-282.
Wilks AF, Harpur AG, Kurban RR: Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991, 11: 2057-2065.
Harpur AG, Andres AC, Ziemiecki A, Aston RR, Wilks AF: JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992, 7: 1347-1353.
Kawamura M, McVicar DW, Johnston JA: Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci USA. 1994, 91: 6374-6378.
Sofer L, Kampa D, Burnside J: Molecular cloning of a chicken JAK homolog from activated T cells. Gene. 1998, 215: 29-36. 10.1016/S0378-1119(98)00284-4.
Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N: Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995, 14: 2857-2865.
Binari R, Perrimon N: Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994, 8: 300-312.
Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O: Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995, 13: 369-398.
Velazquez L, Fellous M, Stark GR, Pellegrini S: A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992, 70: 313-322.
Watling D, Guschin D, Muller M: Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993, 366: 166-170.
Silvennoinen O, Ihle JN, Schlessinger J, Levy DE: Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature. 1993, 366: 583-585.
Witthuhn BA, Quelle FW, Silvennoinen O: JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993, 74: 227-236.
Argetsinger LS, Campbell GS, Yang X: Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993, 74: 237-244.
Witthuhn BA, Silvennoinen O, Miura O: Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994, 370: 153-157.
Johnston JA, Kawamura M, Kirken RA: Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994, 370: 151-153.
Ihle JN: The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol. 1995, 60: 1-35.
Gurniak CB, Berg LJ: Murine JAK3 is preferentially expressed in hematopoietic tissues and lymphocyte precursor cells. Blood. 1996, 87: 3151-3160.
Sharfe N, Dadi HK, Shahar M, Roifman CM: Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997, 94: 3168-3171.
Tortolani PJ, Lal BK, Riva A: Regulation of JAK3 expression and activation in human B cells and B cell malignancies. J Immunol. 1995, 155: 5220-5226.
Musso T, Johnston JA, Linnekin D: Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J Exp Med. 1995, 181: 1425-1431.
Russell SM, Johnston JA, Noguchi M: Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994, 266: 1042-1045.
Miyazaki T, Kawahara A, Fujii H: Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994, 266: 1045-1047.
Boussiotis VA, Barber DL, Nakarai T: Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994, 266: 1039-1042.
Oakes SA, Candotti F, Johnston JA: Signaling via IL-2 and IL-4 in JAK3-deficient severe combined immunodeficiency lymphocytes: JAK3-dependent and independent pathways. Immunity. 1996, 5: 605-615.
Candotti F, Oakes SA, Johnston JA: In vitro correction of JAK3-deficient severe combined immunodeficiency by retroviral-mediated gene transduction. J Exp Med. 1996, 183: 2687-2692.
Macchi P, Villa A, Gillani S: Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995, 377: 65-68.
Russell SM, Tayebi N, Nakajima H: Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995, 270: 797-800.
Noguchi M, Yi H, Rosenblatt HM: Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993, 73: 147-157.
Nosaka T, van Deursen JM, Tripp RA: Defective lymphoid development in mice lacking Jak3. Science. 1995, 270: 800-802.
Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ: Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995, 270: 794-797.
Park SY, Saijo K, Takahashi T: Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity . 1995, 3: 771-782.
Thomis DC, Berg LJ: The role of Jak3 in lymphoid development, activation, and signaling. Curr Opin Immunol. 1997, 9: 541-547.
Maeurer MJ, Lotze MT: Interleukin-7 (IL-7) knockout mice. Implications for lymphopoiesis and organ-specific immunity. Int Rev Immunol. 1998, 16: 309-322.
Maraskovsky E, Teepe M, Morrissey PJ: Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J Immunol. 1996, 157: 5315-5323.
von Freeden-Jeffry U, Vieira P, Lucian LA: Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995, 181: 1519-1526.
Puel A, Ziegler SF, Buckley RH, Leonard WJ: Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nature Genet. 1998, 20: 394-397.
Lodolce JP, Boone DL, Chai S: IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998, 9: 669-676.
Candotti F, Oakes SA, Johnston JA: Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood. 1997, 90: 3996-4003.
Suzuki H, Kundig TM, Furlonger C: Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995, 268: 1472-1476.
Willerford DM, Chen J, Ferry JA: Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995, 3: 521-530.
Fujii H, Ogasawara K, Otsuka H: Functional dissection of the cytoplasmic subregions of the IL-2 receptor betac chain in primary lymphocyte populations. EMBO J. 1998, 17: 6551-6557.
Saijo K, Park SY, Ishida Y, Arase H, Saito T: Crucial role of Jak3 in negative selection of self-reactive T cells. J Exp Med . 1997, 185: 351-356.
Brugnoni D, Notarangelo LD, Sottini A: Development of autologous, oligoclonal, poorly functioning T lymphocytes in a patient with autosomal recessive severe combined immunodeficiency caused by defects of the Jak3 tyrosine kinase. Blood. 1998, 91: 949-955.
Villa A, Sironi M, Macchi P: Monocyte function in a severe combined immunodeficient patient with a donor splice site mutation in the Jak3 gene. Blood. 1996, 88: 817-823.
Colamonici O, Yan H, Domanski P: Direct binding to and tyrosine phosphorylation of the alpha subunit of the type I interferon receptor by p135tyk2 tyrosine kinase. Mol Cell Biol . 1994, 14: 8133-8142.
Novick D, Cohen B, Rubinstein M: The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994, 77: 391-400.
Abramovich C, Shulman LM, Ratovitski E: Differential tyrosine phosphorylation of the IFNAR chain of the type I interferon receptor and of an associated surface protein in response to IFN-alpha and IFN-beta. EMBO J. 1994, 13: 5871-5877.
Muller M, Briscoe J, Laxton C: The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993, 366: 129-135.
Quelle FW, Sato N, Witthuhn BA: JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994, 14: 4335-4341.
Stahl N, Boulton TG, Farruggella T: Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994, 263: 92-95.
Zou J, Presky DH, Wu CY, Gubler U: Differential associations between the cytoplasmic regions of the interleukin-12 receptor subunits beta1 and beta2 and JAK kinases. J Biol Chem. 1997, 272: 6073-6077.
Rodig SJ, Meraz MA, White JM: Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998, 93: 373-383.
Neubauer H, Cumano A, Muller M: Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998, 93: 397-409.
Parganas E, Wang D, Stravopodis D: Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998, 93: 385-395.
Lacronique V, Boureux A, Valle VD: A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997, 278: 1309-1312.
Peeters P, Raynaud SD, Cools J: Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997, 90: 2535-2540.
Hanissian SH, Geha RS: Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997, 6: 379-387.
Jabara HH, Buckley RH, Roberts JL: Role of JAK3 in CD40-mediated signaling. Blood. 1998, 92: 2435-2440.
Rane SG, Reddy EP: JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene. 1994, 9: 2415-2423.
Lai KS, Jin Y, Graham DK: A kinase-deficient splice variant of the human JAK3 is expressed in hematopoietic and epithelial cancer cells. J Biol Chem. 1995, 270: 25028-25036.
Hubbard SR, Wei L, Ellis L, Hendrickson WA: Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994, 372: 746-754.
Gauzzi MC, Velazquez L, McKendry R: Interferon-alpha-dependent activation of Tyk2 requires phosphorylation of positive regulatory tyrosines by another kinase. J Biol Chem. 1996, 271: 20494-20500.
Feng J, Witthuhn BA, Matsuda T: Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997, 17: 2497-2501.
Zhou YJ, Hanson EP, Chen YQ: Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc Natl Acad Sci USA. 1997, 94: 13850-13855.
Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C: Identification of SH2-Bbeta as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol. 1997, 17: 6633-6644.
Rui L, Carter-Su C: Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA. 1999, 96: 7172-7177.
Frank SJ, Gilliland G, Kraft AS, Arnold CS: Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology. 1994, 135: 2228-2239.
Luo H, Rose P, Barber D: Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997, 17: 1562-1571.
Fujitani Y, Hibi M, Fukada T: An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene. 1997, 14: 751-761. 10.1038/sj/onc/1200907.
Frank SJ, Yi W, Zhao Y: Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J Biol Chem. 1995, 270: 14776-14785.
Zhao Y, Wagner F, Frank SJ, Kraft AS: The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J Biol Chem. 1995, 270: 13814-13818.
Kohlhuber F, Rogers NC, Watling D: A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol Cell Biol. 1997, 17: 695-706.
Chen M, Cheng A, Chen YQ: The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc Natl Acad Sci USA. 1997, 94: 6910-6915.
Cacalano NA, Migone TS, Bazan F: Autosomal SCID caused by a point mutation in the N-terminus of Jak3: mapping of the Jak3-receptor interaction domain. EMBO J. 1999, 18: 1549-1558.
Yan H, Piazza F, Krishnan K, Pine R, Krolewski JJ: Definition of the interferon-alpha receptor-binding domain on the TYK2 kinase. JBiol Chem. 1998, 273: 4046-4051.
Richter MF, Dumenil G, Uze G, Fellous M, Pellegrini S: Specific contribution of tyk2 JH regions to the binding and the expression of the interferon alpha/beta receptor component IFNAR1. J Biol Chem. 1998, 273: 24723-24729.
Takeshita T, Arita T, Higuchi M: STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity. 1997, 6: 449-457.
Asao H, Sasaki Y, Arita T: Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J Biol Chem. 1997, 272: 32785-32791.
Tanaka N, Kaneko K, Asao H: Possible involvement of a novel STAM-associated molecule "AMSH" in intracellular signal transduction mediated by cytokines. J Biol Chem. 1999, 274: 19129-19135.
Livnah O, Stura EA, Middleton SA: Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999, 283: 987-990. 10.1126/science.283.5404.987.
Remy I, Wilson IA, Michnick SW: Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999, 283: 990-993. 10.1126/science.283.5404.990.
Tibbles LA, Woodgett JR: The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999, 55: 1230-1254.
Campbell KS: Signal transduction from the B cell antigen-receptor. Curr Opin Immunol. 1999, 11: 256-264.
Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE: The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999, 17: 701-738.
Kurosaki T: Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999, 17: 555-592.
Hardy K, Chaudhri G: Activation and signal transduction via mitogen-activated protein (MAP) kinases in T lymphocytes. Immunol Cell Biol. 1997, 75: 528-545.
Su B, Karin M: Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996, 8: 402-411.
Fu XY, Schindler C, Improta T, Aebersold R, Darnell JEJ: The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci USA. 1992, 89: 7840-7843.
Shuai K, Schindler C, Prezioso VR, Darnell JEJ: Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992, 258: 1808-1812.
Zhong Z, Wen Z, Darnell JEJ: Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci USA. 1004, 91: 4806-4810.
Akira S, Nishio Y, Inoue M: Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell . 1994, 77: 63-71.
Yamamoto K, Quelle FW, Thierfelder WE: Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol Cell Biol. 1994, 14: 4342-4349.
Quelle FW, Shimoda K, Thierfelder W: Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995, 15: 3336-3343.
Hou J, Schindler U, Henzel WJ: An interleukin-4-induced transcription factor: IL-4 Stat. Science . 1994, 265: 1701-1706.
Wakao H, Gouilleux F, Groner B: Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994, 13: 2182-2191.
Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL: Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995, 2: 321-329.
Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L: Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA. 1995, 92: 8831-8835.
Darnell JEJ: STATs and gene regulation. Science. 1997, 277: 1630-1635.
Schindler C, Fu XY, Improta T, Aebersold R, Darnell JEJ: Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci USA. 1992, 89: 7836-7839.
Shuai K, Stark GR, Kerr IM, Darnell JEJ: A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993, 261: 1744-1746.
Greenlund AC, Morales MO, Viviano BL: Stat recruitment by tyrosine-phosphorylated cytokine receptors: an ordered reversible affinity-driven process. Immunity. 1995, 2: 677-687.
Schindler U, Wu P, Rothe M, Brasseur M, McKnight SL: Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity. 1995, 2: 689-697.
Becker S, Groner B, Muller CW: Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998, 394: 145-151.
Chen X, Vinkemeier U, Zhao Y: Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998, 93: 827-839.
Vinkemeier U, Moarefi I, Darnell JEJ, Kuriyan J: Structure of the amino-terminal protein interaction domain of STAT-4. Science. 1998, 279: 1048-1052.
Xu X, Sun YL, Hoey T: Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science. 1996, 273: 794-797.
Mikita T, Campbell D, Wu P, Williamson K, Schindler U: Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996, 16: 5811-5820.
John S, Vinkemeier U, Soldaini E, Darnell JEJ, Leonard WJ: The significance of tetramerization in promoter recruitment by Stat5. Mol Cell Biol. 1999, 19: 1910-1918.
Bhattacharya S, Eckner R, Grossman S: Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature. 1996, 383: 344-347.
Zhang JJ, Vinkemeier U, Gu W: Two contact regions between Stat1 and CBP/p300 in interferon gamma signaling. Proc Natl Acad Sci USA. 1996, 93: 15092-15096.
Horvai AE, Xu L, Korzus E: Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc Natl Acad Sci USA. 1997, 94: 1074-1079.
Korzus E, Torchia J, Rose DW: Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998, 279: 703-707.
Pfitzner E, Jahne R, Wissler M, Stoecklin E, Groner B: p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol . 1998, 12: 1582-1593.
Zhu M, John S, Berg M, Leonard WJ: Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell. 1999, 96: 121-130.
Stocklin E, Wissler M, Gouilleux F, Groner B: Functional interactions between Stat5 and the glucocorticoid receptor. Nature . 1996, 383: 726-728.
Moriggl R, Berchtold S, Friedrich K: Comparison of the trans-activation domains of Stat5 and Stat6 in lymphoid cells and mammary epithelial cells. Mol Cell Biol. 1997, 17: 3663-3678.
Cella N, Groner B, Hynes NE: Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol Cell Biol. 1998, 18: 1783-1792.
Look DC, Pelletier MR, Tidwell RM, Roswit WT, Holtzman MJ: Stat1 depends on transcriptional synergy with Sp1. J Biol Chem. 1995, 270: 30264-30267.
Schaefer TS, Sanders LK, Nathans D: Cooperative transcriptional activity of Jun and Stat3 beta, a short form of Stat3. Proc Natl Acad Sci USA. 1995, 92: 9097-9101.
Shen CH, Stavnezer J: Interaction of stat6 and NF-kappaB: direct association and synergistic activation of interleukin-4-induced transcription. Mol Cell Biol. 1998, 18: 3395-3404.
Wen Z, Zhong Z, Darnell JEJ: Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995, 82: 241-250.
Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S: Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995, 267: 1990-1994.
Wen Z, Darnell JEJ: Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res . 1997, 25: 2062-2067.
Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JEJ: Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci USA. 1996, 93: 7673-7678.
Moriggl R, Gouilleux-Gruart V, Jahne R: Deletion of the carboxyl-terminal transactivation domain of MGF-Stat5 results in sustained DNA binding and a dominant negative phenotype. Mol Cell Biol. 1996, 16: 5691-5700.
Cho SS, Bacon CM, Sudarshan C: Activation of STAT4 by IL-12 and IFN-alpha: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J Immunol. 1996, 157: 4781-4789.
Beadling C, Ng J, Babbage JW, Cantrell DA: Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway. EMBO J. 1996, 15: 1902-1913.
Yamashita H, Xu J, Erwin RA: Differential control of the phosphorylation state of proline-juxtaposed serine residues ser725 of stat5a and ser730 of stat5b in prolactin-sensitive cells. J Biol Chem. 1998, 273: 30218-30224.
Woetmann A, Nielsen M, Christensen ST: Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci USA. 1999, 96: 10620-10625.
Zauberman A, Zipori D, Krupsky M, Ben-Levy R: Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene. 1999, 18: 3886-3893. 10.1038/sj/onc/1202738.
Turkson J, Bowman T, Adnane J: Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in stat3 transcriptional activity induced by the src oncoprotein. Mol Cell Biol. 1999, 19: 7519-7528.
Bode JG, Gatsios P, Ludwig S: The mitogen-activated protein (MAP) kinase p38 and its upstream activator MAP kinase kinase 6 are involved in the activation of signal transducer and activator of transcription by hyperosmolarity. J Biol Chem. 1999, 274: 30222-30227.
Goh KC, Haque SJ, Williams BR: p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999, 18: 5601-5608.
Lim CP, Cao X: Serine phosphorylation and negative regulation of stat3 by JNK. J Biol Chem. 1999, 274: 31055-31061.
David M, Petricoin ER, Benjamin C: Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science . 1995, 269: 1721-1723.
Chung J, Uchida E, Grammer TC, Blenis J: STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997, 17: 6508-6516.
Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB: Rapid inhibition of interleukin-6 signaling and stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1998, 95: 11107-11112.
Strehlow I, Schindler C: Amino-terminal signal transducer and activator of transcription (STAT) domains regulate nuclear translocation and STAT deactivation. J Biol Chem. 1998, 273: 28049-28056.
Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y: Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem. 1996, 271: 31017-31020.
Sekimoto T, Yoneda Y: Nuclear import and export of proteins: the molecular basis for intracellular signaling. Cytokine Growth Factor Rev. 1998, 9: 205-211.
Ullman KS, Powers MA, Forbes DJ: Nuclear export receptors: from importin to exportin. Cell. 1997, 90: 967-970.
Gorlich D: Transport into and out of the cell nucleus. EMBO J. 1998, 17: 2721-2727.
Subramaniam PS, Mujtaba MG, Paddy MR, Johnson HM: The carboxyl terminus of interferon-gamma contains a functional polybasic nuclear localization sequence. J Biol Chem. 1999, 274: 403-407.
Johnson HM, Torres BA, Green MM: Hypothesis: ligand/receptor-assisted nuclear translocation of STATs. Proc Soc Exp Biol Med. 1998, 218: 149-155.
Car BD, Eng VM, Schnyder B: Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med . 1994, 179: 1437-1444.
Durbin JE, Hackenmiller R, Simon MC, Levy DE: Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996, 84: 443-450.
Meraz MA, White JM, Sheehan KC: Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996, 84: 431-442.
Jouanguy E, Lamhamedi-Cherradi S, Lammas D: A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nature Genet. 1999, 21: 370-378.
Sahni M, Ambrosetti DC, Mansukhani A: FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999, 13: 1361-1366.
Kaplan DH, Shankaran V, Dighe AS: Demonstration of an interferon gamma-dependent tumor surveillance system in immuno-competent mice. Proc Natl Acad Sci USA. 1998, 95: 7556-7561.
Fallarino F, Gajewski TF: Cutting edge: differentiation of antitumor CTL In vivo requires host expression of stat1. J Immunol . 1999, 163: 4109-4113.
Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR: Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997, 278: 1630-1632.
Tanaka N, Sato M, Lamphier MS: Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells. 1998, 3: 29-37. 10.1046/j.1365-2443.1998.00164.x.
Bluyssen HA, Levy DE: Stat2 is a transcriptional activator that requires sequence-specific contacts provided by stat1 and p48 for stable interaction with DNA. J Biol Chem. 1997, 272: 4600-4605.
Takeda K, Noguchi K, Shi W: Targeted disruption of the mouse Stat3 gene leads to early 'embryonic' lethality. Proc Natl Acad Sci USA. 1997, 94: 3801-3804.
Escary JL, Perreau J, Dumenil D, Ezine S, Brulet P: Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993, 363: 361-364.
Takeda K, Clausen BE, Kaisho T: Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999, 10: 39-49.
Wang KS, Ritz J, Frank DA: IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J Immunol. 1999, 162: 299-304.
Kaplan MH, Sun YL, Hoey T, Grusby MJ: Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996, 382: 174-177.
Magram J, Connaughton SE, Warrier RR: IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996, 4: 471-481.
Wu C, Ferrante J, Gately MK, Magram J: Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997, 159: 1658-1665.
de Jong R, Altare F, Haagen IA: Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998, 280: 1435-1438.
Lin JX, Mietz J, Modi WS, John S, Leonard WJ: Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5BDNA binding activity in COS-7 cells. J Biol Chem. 1996, 271: 10738-10744.
Liu X, Robinson GW, Wagner KU: Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11: 179-186.
Udy GB, Towers RP, Snell RG: Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997, 94: 7239-7244.
Teglund S, McKay C, Schuetz E: STAT5a and STAT5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998, 93: 841-850.
Moriggl R, Topham DJ, Teglund S: Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999, 10: 249-259.
Nakajima H, Liu XW, Wynshaw-Boris A: An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997, 7: 691-701.
Imada K, Bloom ET, Nakajima H: Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998, 188: 2067-2074.
Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF: Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999, 98: 181-191.
Beadling C, Guschin D, Witthuhn BA: Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994, 13: 5605-5615.
Welte T, Leitenberg D, Dittel BN: STAT5 interaction with the T cell receptor complex and stimulation of T cell proliferation. Science. 1999, 283: 222-225. 10.1006/abio.2000.4639.
Gerwien J, Nielsen M, Labuda T: Cutting edge: TCR stimulation by antibody and bacterial superantigen induces stat3 activation in human T cells. J Immunol. 1999, 163: 1742-1745.
Kaplan MH, Schindler U, Smiley ST, Grusby MJ: Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996, 4: 313-319.
Takeda K, Tanaka T, Shi W: Essential role of Stat6 in IL-4 signalling. Nature. 1996, 380: 627-630.
Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S: Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. JImmunol. 1996, 157: 3220-3222.
Shimoda K, van Jong J, Sangster MY: Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996, 380: 630-633.
Akimoto T, Numata F, Tamura M: Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998, 187: 1537-1542.
Kuperman D, Schofield B, Wills-Karp M, Grusby MJ: Signal transducer and activator of transcription factor 6 (Stat6)- deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998, 187: 939-948.
Miller RL, Eppinger TM, McConnell D, Cunningham-Rundles C, Rothman P: Analysis of cytokine signaling in patients with extrinsic asthma and hyperimmunoglobulin E. J Allergy Clin Immunol. 1998, 102: 503-511.
Miyata S, Matsuyama T, Kodama T: STAT6 deficiency in a mouse model of allergen-induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin Exp Allergy. 1999, 29: 114-123. 10.1046/j.1365-2222.1999.00405.x.
Kaplan MH, Whitfield JR, Boros DL, Grusby MJ: Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998, 160: 1850-1856.
Morse HCR, McCarty T, Giese NA, Taddesse-Heath L, Grusby MJ: STAT6-deficient mice exhibit normal induction of murine AIDS and expression of immunoglobulin E following infection with LP-BM5 murine leukemia viruses. J Virol. 1999, 73: 7093-7095.
Kaplan MH, Wurster AL, Grusby MJ: A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J Exp Med. 1998, 188: 1191-1196.
Cantrell D: T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996, 14: 259-274.
David M, Wong L, Flavell R: STAT activation by epidermal growth factor (EGF) and amphiregulin. Requirement for the EGF receptor kinase but not for tyrosine phosphorylation sites or JAK1. J Biol Chem. 1996, 271: 9185-9188.
Zhong Z, Wen Z, Darnell JEJ: Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994, 264: 95-98.
Leaman DW, Pisharody S, Flickinger TW: Roles of JAKs in activation of STATs and stimulation of c-fos gene expression by epidermal growth factor. Mol Cell Biol. 1996, 16: 369-375.
Bhat GJ, Thekkumkara TJ, Thomas WG, Conrad KM, Baker KM: Activation of the STAT pathway by angiotensin II in T3CHO/AT1A cells. Cross-talk between angiotensin II and interleukin-6 nuclear signaling. J Biol Chem. 1995, 270: 19059-19065.
Marrero MB, Schieffer B, Paxton WG: Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995, 375: 247-250.
Mellado M, Rodriguez-Frade JM, Aragay A: The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998, 161: 805-813.
Kawata T, Shevchenko A, Fukuzawa M: SH2 signaling in a lower eukaryote: a STAT protein that regulates stalk cell differentiation in dictyostelium. Cell. 1997, 89: 909-916.
Mohanty S, Jermyn KA, Early A: Evidence that the Dictyostelium Dd-STATa protein is a repressor that regulates commitment to stalk cell differentiation and is also required for efficient chemotaxis. Development. 1999, 126: 3391-3405.
Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JEJ: Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998, 18: 2553-2558.
Bromberg JF, Wrzeszczynska MH, Devgan G: Stat3 as an oncogene. Cell. 1999, 98: 295-303.
Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF: Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995, 80: 729-738.
Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR: Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998, 273: 33893-33896.
Migone TS, Cacalano NA, Taylor N: Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci USA. 1998, 95: 3845-3850.
Tsui HW, Siminovitch KA, de Souza L, Tsui FW: Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nature Genet. 1993, 4: 124-129.
Bignon JS, Siminovitch KA: Identification of PTP1C mutation as the genetic defect in motheaten and viable motheaten mice: a step toward defining the roles of protein tyrosine phosphatases in the regulation of hemopoietic cell differentiation and function. Clin Immunol Immunopathol. 1994, 73: 168-179. 10.1006/clin.1994.1185.
Shultz LD, Schweitzer PA, Rajan TV: Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993, 73: 1445-1454.
Green MC, Shultz LD: Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975, 66: 250-258.
Aoki K, Didomenico E, Sims NA: The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in me(v)/me(v) mutant mice. Bone. 1999, 25: 261-267. 10.1016/S8756-3282(99)00174-X.
Kim TK, Maniatis T: Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1996, 273: 1717-1719.
Yoshimura A, Ohkubo T, Kiguchi T: A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995, 14: 2816-2826.
Starr R, Willson TA, Viney EM: A family of cytokine-inducible inhibitors of signalling. Nature. 1997, 387: 917-921.
Endo TA, Masuhara M, Yokouchi M: A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997, 387: 921-924.
Naka T, Narazaki M, Hirata M: Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997, 387: 924-929.
Zhang JG, Farley A, Nicholson SE: The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999, 96: 2071-2076.
Sasaki A, Yasukawa H, Suzuki A: Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999, 4: 339-351.
Naka T, Matsumoto T, Narazaki M: Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci USA. 1998, 95: 15577-15582.
Starr R, Metcalf D, Elefanty AG: Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998, 95: 14395-14399.
Alexander WS, Starr R, Fenner JE: SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999, 98: 597-608.
Marine JC, Topham DJ, McKay C: SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999, 98: 609-616.
Adams TE, Hansen JA, Starr R: Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998, 273: 1285-1287.
Cohney SJ, Sanden D, Cacalano NA: SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999, 19: 4980-4988.
Marine JC, McKay C, Wang D: SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999, 98: 617-627.
Aman MJ, Migone TS, Sasaki A: CIS associates with the interleukin-2 receptor beta chain and inhibits interleukin-2-dependent signaling. J Biol Chem. 1999, 274: 30266-30272.
Matsumoto A, Seki Y, Kubo M: Suppression of STAT5 functions in liver, mammary glands, and T cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol Cell Biol. 1999, 19: 6396-6407.
Pezet A, Favre H, Kelly PA, Edery M: Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem. 1999, 274: 24497-24502.
Karlsson H, Gustafsson JA, Mode A: Cis desensitizes GH induced Stat5 signaling in rat liver cells. Mol Cell Endocrinol. 1999, 154: 37-43.
Bjorbaek C, Elmquist JK, El-Haschimi K: Activation of SOCS-3 messenger ribonucleic acid in the hypothalamus by ciliary neurotrophic factor. Endocrinology. 1999, 140: 2035-2043.
Liu B, Liao J, Rao X: Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci USA . 1998, 95: 10626-10631.
Chung CD, Liao J, Liu B: Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997, 278: 1803-1805.
Ye BH, Cattoretti G, Shen Q: The BCL-6 protooncogene controls germinal-centre formation and Th2-type inflammation. Nature Genet. 1997, 16: 161-170.
Dent AL, Hu-Li J, Paul WE, Staudt LM: T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc Natl Acad Sci USA. 1998, 95: 13823-13828.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ortmann, R.A., Cheng, T., Visconti, R. et al. Janus kinases and signal transducers and activators of transcription: their roles in cytokine signaling, development and immunoregulation. Arthritis Res Ther 2, 16 (1999). https://doi.org/10.1186/ar66
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar66