Abstract
Introduction
Faecal blood loss has been measured using autologous erythrocytes labelled with radioactive chromium for several decades, using generally similar methods. We conducted a systematic review of studies employing this technology to determine the degree of blood loss associated with use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclo-oxygenase-2 selective inhibitors (coxibs).
Methods
A systematic search of PubMed and the Cochrane Library (to December 2006) was conducted to identify randomized trials in which treatment with aspirin, NSAIDs, or coxibs was continued for at least 7 days, and with at least 7 days of washout for crossover trials. Rates of faecal blood loss associated with these agents were determined in the randomized trials identified. Comparators were placebo, active, or no treatment. Outcomes of interest were mean daily faecal blood loss, and the number or proportion of individuals recording faecal blood above 5 ml/day and above 10 ml/day.
Results
Forty-five reports of 47 trials were included, including 1,162 individuals, mostly healthy volunteers and predominantly young men. Only 136 patients (as opposed to healthy volunteers; 12%) were included, and these were mostly older people with an arthritic condition. Most NSAIDs and low-dose (325 mg) aspirin resulted in a small average increase in faecal blood loss of 1 to 2 ml/day from about 0.5 ml/day at baseline. Aspirin at full anti-inflammatory doses resulted in much higher average levels of blood loss of about 5 ml/day. Some individuals lost much more blood than average, at least for some of the time, with 5% of those taking NSAIDs having daily blood loss of 5 ml or more and 1% having daily blood loss of 10 ml or more; rates of daily blood loss of 5 ml/day or 10 ml/day were 31% and 10%, respectively, for aspirin at daily doses of 1,800 mg or greater.
Conclusion
At baseline, or with placebo, faecal blood loss is measured at 1 ml/day or below. With low-dose aspirin and some NSAIDs, average values may be two to four times this, and anti-inflammatory doses of aspirin result in much higher average losses. A small proportion of individuals respond to aspirin or NSAIDs with much higher faecal blood loss of above 5 ml/day or 10 ml/day. There are significant limitations regarding the quality and validity of reporting of these studies, such as limited size and inclusion of inappropriate participants. The potential for blood loss and consequent anaemia requires more study.
Similar content being viewed by others
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective analgesics and anti-inflammatory drug therapy is an important pharmacological approach to treating various forms of pain, chronic musculoskeletal pain in particular. NSAIDs have a number of known adverse effects. NSAIDs (and aspirin) are associated with upper gastrointestinal injury [1], acute renal failure [2, 3] and congestive heart failure [4, 5]. Less well documented adverse events include associations with increased fracture rates [6] and lower gastrointestinal injury [7–9]. The latter includes bleeding [10–16] and permeability changes [17–19]. Cyclo-oxygenase-2 selective inhibitors (coxibs) are differentiated from traditional NSAIDs by lower rates of upper and lower gastrointestinal harm, and possibly by lack of effect on bone.
The gastrointestinal outcomes most often reported in modern, large, randomized trials and observational studies are upper gastrointestinal bleeding [20–22] or hospital admission for upper gastrointestinal bleeding [23–26]. Both outcomes represent a serious and significant clinical event that is probably at one extreme of a spectrum of blood loss. Much less is known about lower gastrointestinal bleeding and low-level chronic blood loss. Measurements of blood loss to the entire bowel demonstrate large differences between individuals, with some individuals losing significant amounts of blood on a daily basis, up to 50 ml or more [27, 28].
The clinical significance of low-level blood loss is unclear. Morris and colleagues [29] found small bowel lesions in 10 out of 15 patients with both rheumatoid arthritis and anaemia. In randomized trials anaemia was less common when patients were treated with celecoxib rather than NSAIDs [30], and there was lower rate of bowel injury with coxibs [14].
Various methods have been used to measure blood loss from the whole bowel [18, 31–33]. The use of radioactively labelled autologous erythrocytes with concomitant measurement of radioactivity in blood and faeces has been longest used. The method involves stool collection for a number of days after injection of 51Cr-erythrocytes. Methodological problems, notably those involving patients with long transit times [34], collection of all stool samples, avoidance of interfering behaviours and suitable methods for measuring radioactivity in blood and stool, were identified early on. Many randomized trials have been conducted over a number of decades using essentially similar methods. Typically, they compared the effects of aspirin, NSAID, or coxib on mean daily faecal blood loss, with comparators of placebo or aspirin. We chose to examine these trials systematically, both for effects on mean daily blood loss across groups and to identify individuals with greater levels of blood loss that might be connected with anaemia.
Materials and methods
Quality of Reporting of Meta-analyses guidelines were followed where appropriate [35]. PubMed and the Cochrane Library were searched to identify randomized trials using the autologous radioactive chromium method to measure faecal blood loss with aspirin, NSAIDs, or coxibs. The date of the last search was December 2006. A series of free text terms was used, using combinations of words in title and abstract, including faecal (or fecal) blood loss, occult blood loss, chromium, erythrocyte*, aspirin, N-acetylsalicylic acid, NSAID, nonsteroidal anti-inflammatory drug, cyclooxygenase-2 inhibitor, as well as the individual names of common drugs, including ibuprofen, diclofenac, naproxen, indomethacin, ketoprofen, rofecoxib, celecoxib, etoricoxib, valdecoxib and lumiracoxib. Electronic searches were supplemented by exploration of bibliographies of all papers obtained, and reviews of gastrointestinal damage caused by aspirin or NSAIDs. Trials identified as possibly relevant from title or abstract were obtained in full paper version.
Trials were included if they were randomized; if they used chromium-labelled autologous erythrocytes to measure faecal blood loss with collections in controlled conditions; if they had at least one placebo arm in the trial; if they involved administration of any dose of aspirin, NSAID, or coxib for at least 7 days; and if they were parallel group or had a crossover design with at least 7 days of washout between therapies.
Information from each trial was abstracted into a table. Relevant information concerned randomization, blinding, and withdrawal and dropouts was collected to assess reporting quality using a commonly used 5-point scoring system [36]. Methodological items noted were as follows: whether a history was taken to exclude possible pre-existing causes of intestinal bleeding; whether participants had a regular bowel habit; dependence of inclusion on either a negative faecal occult blood test or a measured low faecal blood loss on baseline screening; whether the study was conducted on an inpatient basis in controlled conditions; and how the method of calculating faecal blood loss was reported.
Other information noted included information on study design, participants (age, sex, volunteer or patient), treatments (including dose), duration, baseline faecal blood loss and daily faecal blood loss at the longest time period. As well as noting mean or median blood loss, we sought information about the number of participants with faecal blood loss of greater than 5 ml/day or greater than 10 ml/day.
It was recognized that trials would be heterogeneous in relation to active therapies used, including drug dose. The intention was to calculate weighted mean faecal blood loss using results from individual treatment arms reporting mean or median results, with weighting by number of participants in the study group. It was expected that dispersion information (standard deviation or standard error of the mean) would be irregularly reported. In addition, the proportion of participants with faecal blood loss above 5 ml/day or above 10 ml/day was calculated.
It was expected that for aspirin the dose range might be large, with studies using full-dose aspirin (arbitrarily set at ≥1,800 mg/day) or low-dose aspirin (arbitrarily set at ≤325 mg/day). Results within these two dose ranges would be calculated separately. For NSAIDs and coxibs, no extreme variation in dose was envisaged, but the analysis plan allowed for exclusion of very high or very low doses if these were outside the usual daily dose range (for example, ibuprofen 800 to 2,400 mg/day, diclofenac 50 to 150 mg/day and naproxen 500 to 1,000 mg/day). The analysis plan also allowed for comparison of results from healthy volunteers and patients. No other sensitivity analyses were planned, and no statistical testing was done.
Results
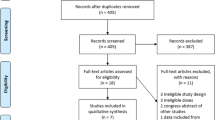
Figure 1 is a flow chart showing the choice of studies for inclusion. Of papers examined in detail, 43 did not involve chromium-labelled erythrocytes, were not randomized trials, or were reviews, and six appeared to be possible randomized trials but they did not include a drug under investigation. Ten randomized trials did involve both chromium-labelled autologous erythrocytes and a drug under review, but their duration was less than 7 days (seven trials), had no information for the first phase only of a crossover trial (two), had no washout between treatments in a crossover study (one), or included only eight participants in different trials, with data available on four for any treatment (one).
There remained 45 reports of 47 randomized trials (Additional file 1 includes details of the trials, including trial design; nature of participants, and their sex, age and any illness; faecal blood loss results; and references). Two reports [37, 38] included extractable information on two randomized trials, and one [28] had additional information and analysis of two randomized trials [39, 40]. The trials had been published over 40 years (Table 1); 26 were described as double blind, and 25 had quality scores of 3 or more out of 5 (Table 1). Thirty-four trials reported therapy periods of 1 week (22 trials) or 2 weeks (12 trials), published mainly before the mid-1980s; 13 trials reported therapy periods of 3 weeks (two trials) or 4 weeks or longer (10 trials), published mainly since the mid-1980s.
A number of methodological criteria might reflect on the validity of a trial. For example, only 36 out of 47 trials indicated that patients had been screened to exclude a pre-existing history that might contribute to increased faecal blood loss, such as a prior history of gastrointestinal disease or surgery; oral, nasal, or rectal bleeding (including bleeding gums on brushing them); or haemorrhoids. Almost all trials excluded current or recent use of drugs that are likely to interfere with measurements; typically, these were analgesics or low-dose aspirin, and recent prior use of aspirin, NSAIDs, antacids, histamine antagonists, or proton pump inhibitors. Behavioural issues leading to exclusion were excessive alcohol use, excessive use of caffeinated beverages, or peculiarities of diet. Only 12 trials used a negative faecal occult blood test as an entry criterion, and only five used a baseline low daily faecal blood loss to exclude participants. Regular bowel habit was an entry criterion in 11 trials, whereas only five trials used controlled conditions (inpatient or dormitory accommodation). Detailed reporting of the method of measuring faecal and blood radioactivity was uncommon, and although most reported at least a reference four trials had neither details nor reference. Only two trials [39, 40] reported adequately on all of these points.
Almost all studies involved a baseline, pretreatment faecal blood loss measurement over a period of days, and elevated baseline faecal blood loss was also a reason for exclusion (and occasionally investigation). The total number of individuals investigated was 1,162. Most were healthy volunteers, predominantly young men; only 136 (12%) were patients, usually older with an arthritic condition.
Table 2 shows the weighted mean daily faecal blood loss for baseline measurements before treatment, on placebo and for each treatment. For aspirin, data are divided according to dose, with trials examining low-dose aspirin (all 325 mg/day) or full-dose aspirin (≥1,800 mg/day). Daily mean faecal blood loss for individual study arms is shown in Figure 2 for baseline, placebo, coxibs and NSAIDs with at least two trials and 25 patients.
Mean daily faecal blood loss in individual treatment arms. Daily faecal blood loss is shown on a logarithmic scale for aspirin, cyclo-oxygenase-2 selective inhibitors (coxibs) and nonsteroidal anti-inflammatory drugs (NSAIDs) with more than 20 participants. The size of the symbol is proportional to the number of individuals (inset scale).
Most information on mean daily faecal blood loss was available for baseline, the initial period before randomization and the start of the trial proper. This was available in 38 trial arms and 950 participants. Baseline mean daily faecal blood loss was below 1 ml/day in all but one study [41], in which it was 1.0 ml/day; the weighted mean was 0.46 ml/day. A somewhat higher mean daily faecal blood loss of 0.76 ml/day was measured with placebo in 172 participants (Table 2).
Mean daily faecal blood loss was available for low-dose and full-dose aspirin, 25 different NSAIDs, and rofecoxib and etoricoxib. There was information on 361 participants for aspirin at doses greater than 1,800 mg/day, but for other aspirin doses, NSAIDs and coxibs there was information on fewer than 100 participants, and mostly fewer than 50 participants. For 16 NSAIDs information available was on 20 individuals or fewer (Table 2).
For aspirin there appeared to be a dose-response relation (Figure 3), with maximum weighted mean values of up to 4 ml/day at 2,000 mg/day, 6 ml/day at 3,000 mg/day, and 10 ml/day at 4,000 mg/day. The most commonly used NSAIDs had mean daily faecal blood loss values of between 1 and 2 ml/day (ibuprofen and naproxen 2.0 mL/day), apart from diclofenac, meloxicam and etodolac, with values of about 0.5 to 0.9 ml/day (Table 2 and Figure 2). Rofecoxib and etoricoxib also had low mean daily faecal blood loss of 0.8 and 0.9 ml/day.
At baseline there was no obvious difference between healthy young volunteers (0.44 ml/day, 835 individuals) and patients (0.56 ml/day, 103 individuals). With aspirin at doses above 1,800 mg/day, the mean daily faecal blood loss was about twice as high with volunteers (5.8 ml/day, 249 individuals) as with patients (3.2 ml/day, 112 individuals).
A number of the studies either gave information on individual patients or provided suitable information to identify whether any participants individually had daily faecal blood loss above 5 or 10 ml/day. For instance, a range of individual mean values was often provided. Such information permitted determination that all patients had faecal blood loss of under than 5 and 10 ml/day if the upper value of the range was below 5 ml/day, but if the top of the range was above 10 mL/day then this identified only one such individual; others who may have had higher values could not be identified by this method, which therefore provides a minimum estimate.
The estimated number and percentage of patients who individually had higher blood loss is shown in Table 3. Blood loss greater than 5 ml/day did not occur with placebo in any participant. For aspirin at 1,800 mg/day or more, 31% of participants had blood loss of 5 ml/day or greater, and 10% had loss of 10 ml/day or greater. Lower rates of 5% and 1%, respectively, were found for all NSAIDs combined.
Discussion
Systematic reviews have several utilities. They can shed new light on a topic or resolve a difference of opinion, but sometimes they can indicate only what is in the literature, without extensive analysis. For any analysis to make sense, it must be based on information that is of sufficient quality to avoid bias, it must be valid (at least within a reasonable definition for a topic), and the volume of data analyzed must be of sufficient size to prevent conclusions from being wafted about on the winds of chance.
Systematic reviews can only attempt to make sense of the information presented. In the case of the present review, the number of trials of sufficient quality limited, increasing the likelihood of bias. We have defined validity, inter alia, by duration of therapy at a minimum of 1 week and washouts of 1 week in crossover studies. Analysis is limited by clinical factors, design and reporting heterogeneity. There were only 1,162 participants in 47 trials (average 25/trial, split between several groups in the parallel group trials). There were different levels of reporting quality, both crossover and parallel designs, duration periods of 1 to 4 weeks, and 30 different treatments (including placebo, and some at different doses); the bulk of studies were conducted in young healthy men, but some were conducted in young healthy women, and a minority (12%) evaluated older patients. Other variables include whether studies maintained participants in a controlled environment to collect stool samples reliably, and the mechanics of measuring radioactivity in stool samples. In the face of so many variables, our judgement was that no statistical approach could possibly be justified. Although we would like to know how any of these variables might have affected the results, we cannot see how such an analysis may be achieved and so we resorted to a descriptive analysis.
Given the clear limitations of quality and validity in these individually small trials, even a descriptive analysis can only be undertaken with circumspection. Comment is, however, still required on the results that are available.
In these randomized trials, conducted over five decades, some (but not all) of the drugs investigated resulted in a small average increase in faecal blood loss of 1 to 2 ml/day. Aspirin appeared to be associated with increased faecal blood loss in a dose-dependent manner; full anti-inflammatory doses (>1,800 mg/day) resulted in much higher average levels of blood loss of about 5 ml/day, although even 325 mg/day resulted in daily faecal blood loss equivalent to that with standard dose NSAIDs. For aspirin and NSAIDs, some individuals lost much more blood on average, with 5% of those taking NSAIDs having daily blood loss of 5 ml or more and 1% having daily blood loss of 10 ml or more. Individual daily blood loss above 50 ml was reported in some healthy young men with ibuprofen [28], and with aspirin over as few as 5 days [32], and individual rather high blood loss was reported sporadically.
These headline results must be qualified in several ways. Most importantly, almost 90% of individuals investigated were healthy, most were young (age <40 years) and most were men. It is not known whether these results can be extrapolated to older populations, often with comorbid conditions, using aspirin or NSAIDs. We found no conclusive evidence that baseline faecal blood loss, or faecal blood loss with aspirin at doses above 1,800 mg/day, was higher in patients than in healthy volunteers.
Other than obvious differences between trials in terms of drugs and doses tested, the main difference between trials was in the duration of therapy. We set a minimum limit of 7 days of treatment, in part to limit under-estimation of faecal blood loss caused by slow intestinal transit time [34]. More recent studies have tended to include longer treatment duration and older studies tended to involve shorter treatment durations, but there was an insufficient number of common therapies to allow a sensible comparison to be conducted, especially because the number of patients in each treatment arm was small. There was the added complication of crossover trials, with disparate washout periods, although a minimum of 7 days was imposed as an inclusion criterion.
Additional limitations arose from the apparent differences between trials in participant inclusion. Although most trials screened participants for a history of potential for increased gastrointestinal damage, or drugs that might interfere or confound measurements, fewer than one-third excluded participants with higher blood loss by means of faecal occult blood screening or measurement. Moreover, only one trial in 10 collected stool samples in controlled conditions from inpatients, and full description of the methods used to measure stool or blood radioactivity was uncommon. All of these features indicated that a cautious descriptive analysis of these data was all that was possible.
Although frank bleeding from the upper and lower gastrointestinal tracts is associated with high levels of anaemia, including very low haemoglobin levels (<100 g/l) [42], there is no direct evidence linking anaemia with low-level blood loss into the bowel, although aspirin, NSAIDs and perhaps coxibs are known to be associated with varying frequencies of frank gastrointestinal bleeding. There is circumstantial evidence that NSAIDs are linked both to increased faecal blood loss and to increased anaemia [30]. For low-dose aspirin, which produced faecal blood loss similar to that of NSAIDs in these trials, a limited body of literature has examined only small numbers, with one study [43] suggesting an association between low-dose aspirin use and anaemia and another one [44] finding no association. A small comparative study of misoprostol and no treatment in 21 patients with small bowel enteropathy and iron deficiency anaemia showed a rise of 15 g/l in haemoglobin with misoprostol, as compared with no change without treatment [45].
It is interesting that individual participants could have bleeding rates that were much higher than the average, more frequently with full-dose aspirin than with NSAIDs. It is tempting to consider that individuals with daily faecal blood loss of more than 5 ml or 10 ml might be more likely to develop anaemia, especially because some individuals had daily blood loss of 50 ml or more (for example, in the study conducted by Bowne and coworkers [28]). Caution is needed, however, because in most cases the information we obtained indicates that high blood loss was identified over just a few days, and we have no good evidence about the longer term. The exception [28], a re-analysis of ibuprofen used in healthy male volunteers, indicated that very high blood loss may occur infrequently and intermittently. Longer studies and larger numbers would be needed to demonstrate the cause and effect between daily faecal blood loss and anaemia.
Anaemia is common in older people living in the community; 13% of a Canadian population of 17,000 had anaemia using the World Health Organization definition (<120 g/l for women and <130 g/l for men), and 4% had haemoglobin levels below 110 g/l [46]. Anaemia is also common (prevalence about 50%) in some rheumatological conditions [47]. Of 72,000 older patients admitted to hospital with myocardial infarction, 43% satisfied a World Health Organization definition of anaemia [48]. Anaemia in older people is associated with greater mortality in association with heart failure [49, 50], angina [51] and myocardial infarction, especially with compromised renal function [52]. Anaemic older patients in hospital are less likely to resume activities of daily living [53] and anaemia is associated with poor cognition [54].
Where a cause for anaemia can be found and treated, quality of life improves. This is the case with blood transfusion in older people, or when haemoglobin levels rise upon treatment with erythropoietin in cancer [55] or renal disease. There are no reliable studies demonstrating improvements in hard clinical outcomes or mortality.
Conclusion
At baseline, or with placebo, faecal blood loss is generally measured at 1 ml/day or below. With low-dose aspirin and some NSAIDs, average values in studies may be two to four times this, and with anti-inflammatory doses of aspirin there is consistent evidence for much higher average losses. A small proportion of individuals respond to aspirin or NSAIDs with much higher faecal blood loss of above 5 ml/day or above 10 ml/day. Blood loss of 5 ml/day would represent almost 2 l if continued over a year, a not insubstantial loss, which might be a consequence of NSAID use in up to one in 20 users. Similar concerns apply to low-dose aspirin. There are significant limitations regarding the quality and validity of reporting of these studies, such as limited size and inclusion of inappropriate participants. The potential for blood loss and consequent anaemia requires more study.
Abbreviations
- coxib:
-
= cyclo-oxygenase-2 selective inhibitor
- NSAID:
-
= nonsteroidal anti-inflammatory drug.
References
Hernández-Diaz S, García Rodriguez LA: Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding and perforation: An overview of epidemiological studies published in the 1990s. Arch Intern Med. 2000, 160: 2093-2099. 10.1001/archinte.160.14.2093.
Henry D, Page J, Whyte I, Nanra R, Hall C: Consumption of non-steroidal anti-inflammatory drugs and the development of functional renal impairment in elderly subjects. Results of a case-control study. Br J Clin Pharmacol. 1997, 44: 85-90. 10.1046/j.1365-2125.1997.00631.x.
Griffin MR, Yared A, Ray WA: Nonsteroidal antiinflamatory drugs and acute renal failure in elderly persons. Am J Epidemiol. 2000, 151: 488-496.
Page J, Henry D: Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000, 160: 777-784. 10.1001/archinte.160.6.777.
Garcia Rodriguez LA, Hernandez-Diaz S: Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology. 2003, 14: 240-246. 10.1097/00001648-200303000-00020.
Vestergaard P, Rejnmark L, Mosekilde L: Fracture risk associated with use of nonsteroidal anti-inflammatory drugs, acetylsalicylic acid, and acetaminophen and the effects of rheumatoid arthritis and osteoarthritis. Calcif Tissue Int. 2006, 79: 84-94. 10.1007/s00223-006-0020-8.
Fortun PJ, Hawkey CJ: Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol. 2007, 23: 134-141. 10.1097/MOG.0b013e328020045a.
Adebayo D, Bjarnason I: Is non-steroidal anti-inflammaory drug (NSAID) enteropathy clinically more important than NSAID gastropathy?. Postgrad Med J. 2006, 82: 186-191. 10.1136/pgmj.2005.039586.
Laine L, Smith R, Min K, Chen C, Dubois RW: Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2006, 24: 751-767. 10.1111/j.1365-2036.2006.03043.x.
Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI: Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992, 327: 749-754.
Holt S, Rigoglioso V, Sidhu M, Irshad M, Howden CW, Mainero M: Nonsteroidal antiinflammatory drugs and lower gastrointestinal bleeding. Dig Dis Sci. 1993, 38: 1619-1623. 10.1007/BF01303169.
Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sainz R: Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997, 112: 683-689. 10.1053/gast.1997.v112.pm9041228.
Laine L, Connors LG, Reicin A, Hawkey CJ, Burgos-Vargas R, Schnitzer TJ, Yu Q, Bombardier C: Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003, 124: 288-292. 10.1053/gast.2003.50054.
Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG: Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005, 3: 133-141. 10.1016/S1542-3565(04)00619-6.
Graham DY, Opekun AR, Willingham FF, Qureshi WA: Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005, 3: 55-59. 10.1016/S1542-3565(04)00603-2.
Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I: A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005, 128: 1172-1178. 10.1053/j.gastro.2005.03.020.
Sigthorsson G, Crane R, Simon T, Hoover M, Quan H, Bolognese J, Bjarnason I: COX-2 inhibition with rofecoxib does not increase intestinal permeability in healthy subjects: a double blind crossover study comparing rofecoxib with placebo and indomethacin. Gut. 2000, 47: 527-532. 10.1136/gut.47.4.527.
Smecuol E, Bai JC, Sugai E, Vazquez H, Niveloni S, Pedreira S, Maurino E, Meddings J: Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut. 2001, 49: 650-655. 10.1136/gut.49.5.650.
Smale S, Bjarnason I: Determining small bowel integrity following drug treatment. Br J Clin Pharmacol. 2003, 56: 284-291. 10.1046/j.1365-2125.2003.01942.x.
Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, VIGOR Study Group: Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000, 343: 1520-1528. 10.1056/NEJM200011233432103.
Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS: Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 2000, 284: 1247-1255. 10.1001/jama.284.10.1247.
Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Matchaba P, TARGET Study Group: Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004, 364: 665-674. 10.1016/S0140-6736(04)16893-1.
Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE: Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002, 54: 320-326. 10.1046/j.1365-2125.2002.01636.x.
Mamdani M, Rochon PA, Juurlink DN, Kopp A, Anderson GM, Naglie G, Austin PC, Laupacis A: Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ. 2002, 325: 624-10.1136/bmj.325.7365.624.
Norgard B, Pedersen L, Johnsen SP, Tarone RE, McLaughlin JK, Friis S, Sorensen HT: COX-2-selective inhibitors and the risk of upper gastrointestinal bleeding in high-risk patients with previous gastrointestinal diseases: a population-based case-control study. Aliment Pharmacol Ther. 2004, 19: 817-825. 10.1111/j.1365-2036.2004.01913.x.
Lanas A, Garcia-Rodriguez LA, Arroyo MT, Gomollon F, Feu F, Gonzalez-Perez A, Zapata E, Bastida G, Rodrigo L, Santolaria S, Guell M, de Argila CM, Quintero E, Borda F, Pique JM, Asociacion Espanola de Gastroenterologia: Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006, 55: 1731-1738. 10.1136/gut.2005.080754.
Dybdahl JH, Daae LN, Larsen S, Ekeli H, Frislid K, Wiik I, Aanstad L: Acetylsalicylic acid-induced gastrointestinal bleeding determined by a 51Cr method on a day-to-day basis. Scand J Gastroenterol. 1980, 15: 887-895.
Bowen B, Yuan Y, James C, Rashid F, Hunt RH: Time course and pattern of blood loss with ibuprofen treatment in healthy subjects. Clin Gastroenterol Hepatol. 2005, 3: 1075-1082. 10.1016/S1542-3565(05)00605-1.
Morris AJ, Howden CW, Robertson C, Duncan A, Torley H, Sturrock RD, Russell RI: Increased intestinal permeability in ankylosing spondylitis – primary lesion or drug effect?. Gut. 1991, 32: 1470-1472. 10.1136/gut.32.12.1470.
Moore RA, Derry S, Makinson GT, McQuay HJ: Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Res Ther. 2005, 7: R644-R665. 10.1186/ar1704.
Fall DJ, Kuiper DH, Pollard HM: Use of isotopes in determining occult blood. Cancer. 1971, 28: 135-136. 10.1002/1097-0142(197107)28:1<135::AID-CNCR2820280126>3.0.CO;2-3.
Dybdahl JH, Daae LN, Larsen S: Occult faecal blood loss determined by chemical tests and a 51Cr method. Scand J Gastroenterol. 1981, 16: 245-252.
Cohen A, Boeijinga JK, van Haard PM, Schoemaker RC, van Vliet-Verbeek A: Gastrointestinal blood loss after non-steroidal anti-inflammatory drugs. Measurement by selective determination of faecal porphyrins. Br J Clin Pharmacol. 1992, 33: 33-38.
Chafetz N, Taylor A, Schleif A, Verba J, Hooser CW: A potential error in the quantitation of fecal blood loss: concise communication. J Nucl Med. 1976, 17: 1053-1054.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of meta-analyses of randomised controlled: the QUOROM statement. Lancet. 1999, 354: 1896-1900. 10.1016/S0140-6736(99)04149-5.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996, 17: 1-12. 10.1016/0197-2456(95)00134-4.
Savon JJ, Allen ML, DiMarino AJ, Hermann GA, Krum RP: Gastrointestinal blood loss with low dose (325 mg) plain and enteric-coated aspirin administration. Am J Gastroenterol. 1995, 90: 581-585.
Johnson PC: The effect of zomepirac on red blood cell survival and immune system. J Clin Pharmacol. 1980, 20: 406-408.
Hunt RH, Bowen B, Mortensen ER, Simon TJ, James C, Cagliola A, Quan H, Bolognese JA: A randomized trial measuring fecal blood loss after treatment with rofecoxib, ibuprofen, or placebo in healthy subjects. Am J Med. 2000, 109: 201-206. 10.1016/S0002-9343(00)00470-8.
Hunt RH, Harper S, Callegari P, Yu C, Quan H, Evans J, James C, Bowen B, Rashid F: Complementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxib. Aliment Pharmacol Ther. 2003, 17: 201-210. 10.1046/j.1365-2036.2003.01407.x.
Rider JA: Comparison of fecal blood loss after use of aspirin and diflunisal. Pharmacotherapy. 1983, 3: 61S-64S.
Peura DA, Lanza FL, Gostout CJ, Foutch PG: The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997, 92: 924-928.
Black DA, Fraser CM: Iron deficiency anaemia and aspirin use in old age. Br J Gen Pract. 1999, 49: 729-730.
Hammerman-Rozenberg R, Jacobs JM, Azoulay D, Stessman J: Aspirin prophylaxis and the prevalence of anaemia. Age Ageing. 2006, 35: 514-517. 10.1093/ageing/afl066.
Morris AJ, Murray L, Sturrock RD, Madhok R, Capell HA, Mackenzie JF: Short report: the effect of misoprostol on the anaemia of NSAID enteropathy. Aliment Pharmacol Ther. 1994, 8: 343-346.
Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR: Impact of anemia on hospitalization and mortality in older adults. Blood. 2006, 107: 3841-3846. 10.1182/blood-2005-10-4308.
Wilson A, Yu HT, Goodnough LT, Nissenson AR: Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004, 50S-57S. 10.1016/j.amjmed.2003.12.012. Suppl 7A
Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM: Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001, 345: 1230-1236. 10.1056/NEJMoa010615.
McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R: Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol. 2002, 13: 1928-1936. 10.1097/01.ASN.0000018409.45834.FA.
Mozaffarian D, Nye R, Levy WC: Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol. 2003, 41: 1933-1939. 10.1016/S0735-1097(03)00425-X.
Muzzarelli S, Pfisterer M, TIME Investigators: Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J. 2006, 152: 991-996. 10.1016/j.ahj.2006.06.014.
Langston RD, Presley R, Flanders WD, McClellan WM: Renal insufficiency and anemia are independent risk factors for death among patients with acute myocardial infarction. Kidney Int. 2003, 64: 1398-1405. 10.1046/j.1523-1755.2003.00200.x.
Maraldi C, Ble A, Zuliani G, Guralnik JM, Mussi C, Fellin R, Volpato S: Association between anemia and physical disability in older patients: role of comorbidity. Aging Clin Exp Res. 2006, 18: 485-492.
Denny SD, Kuchibhatla MN, Cohen HJ: Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006, 119: 327-334. 10.1016/j.amjmed.2005.08.027.
Cortesi E, Gascon P, Henry D, Littlewood T, Milroy R, Pronzato P, Reinhardt U, Shasha D, Thatcher N, Wilkinson P: Standard of care for cancer-related anemia: improving hemoglobin levels and quality of life. Oncology. 2005, 22-32. 10.1159/000083130. Suppl 1
Acknowledgements
Pain Research is supported in part by the Oxford Pain Research Trust, and this work was also supported by an unrestricted educational grant from Pfizer Ltd. Neither organization had any role in design, planning or execution of the study, or in writing the manuscript. The terms of the financial support from Pfizer included freedom for the authors to reach their own conclusions, and an absolute right to publish the results of their research, irrespective of any conclusions reached. Pfizer did have the right to view the final manuscript before publication, and did so. We wish to thank Richard Hunt and Barry Bowen for helpful comments on an earlier version of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
RAM and HJM have received consulting and/or lecture fees from pharmaceutical companies and other organizations. The authors have received research support from charities and government sources at various times. No author has any direct stock holding in any pharmaceutical company.
Authors' contributions
RAM, HJM and SD were involved with the original concept and planning of the study. RAM performed searches, and led on data extraction, analysis and preparing the manuscript. SD helped with data extraction, analysis and writing. HJM helped with writing and advice. All authors read and approved the final manuscript.
Electronic supplementary material
13075_2007_2205_MOESM1_ESM.pdf
Additional file 1: A PDF file which contains information on each included study, with reference, quality score, design, treatments, main results, and comments. (PDF 69 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Moore, R.A., Derry, S. & McQuay, H.J. Faecal blood loss with aspirin, nonsteroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors: systematic review of randomized trials using autologous chromium-labelled erythrocytes. Arthritis Res Ther 10, R7 (2008). https://doi.org/10.1186/ar2355
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar2355