Abstract
Agriculture, the world’s most dominant land use type, burdens freshwater biodiversity with a multitude of stressors such as diffuse pollution and hydromorphological alteration. However, it is difficult to directly link agricultural land use with biota response as agricultural stressors can also originate from other causes. Also, there is evidence for positive and negative effects of agriculture on organisms, agricultural impact differs strongly with the biological metric and study region considered and agricultural impact differs among practice and type, which in turn affects different organism groups with varying severity. Against this background, our study aimed at assessing, if agricultural land use has a consistent effect on river biota. We conducted a systematic review of the literature, which yielded 43 studies and 76 relationships between agriculture and aquatic organism groups. The relationships were subjected to a meta-analysis using Hedge’s g to calculate the standardized mean difference of effects. Overall, we detected a medium to strong effect g = − 0.74 of agricultural land use on freshwater biota, only marginally influenced by study design, river type and region. Strong differences in biota response could be observed depending on the biological metric assessed, with ecological quality indices of agricultural impairment performing best. Sensitive taxa declined with agricultural impact, while tolerant taxa tended to benefit. In addition, the biota response differed among agricultural types and practices and organism group, with macroinvertebrates showing the strongest effect. Our results quantify the effects of agriculture on riverine biota and suggest biological metric types for assessing agricultural impact. Further research is needed to discriminate between agricultural types and account for intensity.
Similar content being viewed by others
Introduction
Agriculture is the world’s dominant land use type covering approximately 51 million km2, which accounts for nearly 50% of the world’s habitable land [64]. More than 75% of this area is used for meat and dairy production, which accounts for less than 20% of the human calorie supply [1]. While large parts of the agricultural areas in developed regions like Europe are already characterized by farm management of high intensity [76], further expansion and intensification of agriculture is to be expected, given the increasing demand for food and the raising share of meat diet [33, 71]. The need for industrial crops arising from the growing world population further adds to agricultural intensification [83]. This steadily increasing intensity of agricultural land use causes biodiversity loss in many organism groups, such as birds [22], mammals [40] and insects [9].
Similarly, the biodiversity of rivers draining agricultural land is impaired. Agriculture affects river biota through a variety of stressors, particularly the influx of nutrients [73], agrochemicals [45] and fine sediment [43], as well as alteration of river morphology [78] and intensive river maintenance [7]. The detrimental effect of these stressors has been shown in a multitude of studies: Nutrient influx impacts macrophytes [58], insecticides cause impairment of macroinvertebrates [13], fish are affected by fine sediment influx [43], hydromorphological alteration impairs macroinvertebrates [35], while river management impairs various organism groups [10]. Often, agriculture is identified as the most relevant driver for the deterioration of river biota [72, 78]. However, several uncertainties obstruct general conclusions about the effects of agriculture on river biota.
First, stressors such as diffuse pollution and hydromorphological impairment can also be caused by other drivers like urbanization, flood protection, hydropower or navigation [23]. It therefore appears impossible to separate the effects of agriculture in case of multi-driver situations [17, 82]. Agricultural impacts clearly differ with distance to river shores and riparian vegetation [19]. Additionally, there is also evidence for the positive effects of agricultural land use on biota, such as riverine macrophyte diversity [6]. The effect of agriculture potentially differs from the biological metric used for describing the assemblage of an organism group. While the macrophyte species investigated by Baattrup-Pedersen et al. [6] benefit from nutrient influx, other species suffer from associated light deprivation and are consequently suppressed [39, 58]. Similar differences can be observed for macroinvertebrates: sensitive species of the order Ephemeroptera, Plecoptera or Trichoptera are suppressed by more tolerant filter feeders and grazers [46] and fluctuations in oxygen availability [42]. Taxa tolerant to anthropogenic disturbances are expected to be less impaired by agricultural land use [79] and therefore, metrics focusing on the quantity of organisms, such as the number of taxa, are likely to be less sensitive to agricultural stressors compared to metrics focusing on sensitive species [74]. Also, fish species tolerant to sedimentation have been found to benefit from agricultural land use [36], while benthic and substrates spawning species are impaired [14].
Second, the biotic response depends on the share of agricultural land use in river catchments [20], but also on agricultural types and practices. Cornfield farming, especially when close to riverbanks, can cause massive fine sediment influx combined with strong phosphorous enrichment [68]. Intensive livestock farming in the river catchment can result in nitrate influx into rivers after strong rainfall events [54], while the pasture is less problematic for riverine biodiversity [12]. However, the effect of individual stressors caused by agriculture clearly differs between organism groups. Insecticides, for instance, are more detrimental to invertebrates [4] compared to the aquatic flora, which tends to recover rapidly from pesticide exposure [11, 44] and to fish, for which acute toxic effects are rare and rather chronic effects are observed [57, 67]. The nutrient influx also mainly affects macroinvertebrates followed by fish [81], while aquatic flora may potentially even benefit [18, 52]. Fine sediment influx altering the habitat conditions is more detrimental for invertebrates, fish and diatoms compared to macrophytes [41, 43]. River management affects macrophytes most directly, while macroinvertebrate and fish are often indirectly affected by habitat alteration [51]. In conclusion, there is evidence that all the above-mentioned pathways can affect macroinvertebrates, but only some of them are relevant for fish, macrophytes and diatoms. Finally, the effects of agriculture on river biota may differ between spatial scales [2], study regions [53] and river types [29], soil type [21] or riparian vegetation [61].
Against this background, the main objective of this meta-analysis is to assess if agricultural land use has a consistent effect on river biota across ecoregions in different climate zones, independent of the individual study design. We analyse if there are consistent differences between organism groups, to which degree the effect is context specific and if there are generalizable stressor pathways. More specifically, we hypothesized that:
-
Agricultural land use affects river biota negatively and is widely from study design, region and organism group. Agricultural impacts on river biota can be better assessed with metrics of species composition (ecological quality indices) compared to metrics of species richness.
-
The biota response differs with agricultural types and practices present in the river catchments and in areas close to the river, with macroinvertebrates affected more strongly compared to fish, macrophytes and diatoms.
Methods
Literature search
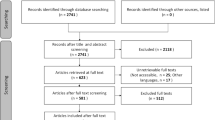
We conducted a systematic literature search with a search string including the attributes country, freshwater ecosystem type and agricultural stressor. We only considered studies published between January 1990 and May 2022, as we expected both agricultural practice and biodiversity data to have changed over time and reported agricultural effects on freshwater biota to be less standardized in the years before, or untypical for today’s situation (e.g. addressing pesticides that are meanwhile prohibited). To further restrict the variability of impacts and responses, we limited our search to references from subpolar, temperate and subtropical regions in North America, Europe and Oceania.
The following search string was used for an all-database literature search in the Web of science:
(europe* OR albania* OR austria* OR belarus OR belgium* OR bosnia* OR bulgaria* OR croatia* OR cyprus OR czech* OR denmark* OR England* OR UK OR estonia* OR finland* OR france OR germany* OR greece OR hungary* OR ireland* OR italy* OR itali* OR latvia* OR lithuania* OR luxemb* OR macedonia* OR malta* OR moldova* OR montenegro OR netherlands* OR norway* OR poland* OR portugal OR romania* OR slovakia* OR slovenia* OR serbia* OR sweden* OR switzerland OR spain OR ukraine OR “new Zealand” OR australi* OR oceanic OR USA* OR “united states” OR “north america” OR U.S* OR canada).
(Topic) and (stream* OR river* OR watershed* OR catchment* OR floodplain*).
(Topic) and (diatom* OR macrophy* OR fish OR phytobenth* OR phytoplan* OR macrobenth* OR macrozoobenth* OR "aquatic plant" OR "aquatic larvae" OR "water plant" OR invertebrate*) (Topic) and (agri* OR farm* OR agronom* OR cultiv* OR cropl* OR pasture OR livestock OR ranching).
(Topic) not (marin* OR sea* OR ocean*).
From the initial population of 6484 papers, we screened the titles and abstracts and excluded studies that:
-
did not focus on agricultural impacts,
-
did not report effects on river biota,
-
did not include data from Europe, North America or Oceania,
-
did not provide empirical data,
-
provided a review, lacking the original data required for a meta-analysis.
This initial screening resulted in 387 studies that met all criteria and that were subjected to a full-text scan, to identify studies qualified for a meta-analysis, that is studies that provided the data required to calculate the effect size (see below). We excluded studies, for which no comparable effect sizes could be calculated because they either were lacking a control group or a gradient in agricultural land use, or because of missing data that could not be retrieved. Eventually, the meta-analysis was performed with 43 studies, from many of which several different effect sizes were derived.
Data extraction
From the selected articles, we extracted data from narrative descriptions, tables and figures (using webplot digitizer: https://apps.automeris.io/wpd/index.de_DE.html, last accessed on June 23rd, 2022) to calculate effect sizes. More specifically, we compiled data on sample size, mean, variance and correlation coefficients, or used the raw data provided to calculate these values. If important test statistics were not directly retrievable, we tried to estimate mean values following Wan et al. [80] and standard deviations (SD) following Higgins and Green [38]:
where m = median, q = quartile
where n is the sampling size and CI is the confidence interval.
Furthermore, we extracted information on the organismic group (macroinvertebrates, fish, macrophytes and diatoms), agricultural types and practices and the type of response metric. Abundance metrics entailed counts and biomass of specimens, while richness metrics included species richness and number of taxa. Quality metrics refer to ecologically relevant assessment indices (e.g. ecological quality ratios, indices of biological integrity or taxa scores). The analysed studies discriminated between livestock farming, grassland, arable land and a mixture (or not further defined), which we refer to as “Livestock” including livestock farming and grassland, “Arable land” and “Mixture” including mixture and not further defined agriculture. Further, we distinguished metrics of “tolerant” and “sensitive” taxa according to their tolerance towards pollution [49, 63]. Disregarding in-group variability, Oligochaeta, Chironomidae and Diptera were classified as tolerant taxa, while sensitive taxa include Ephemeroptera, Plecoptera and Trichoptera (EPT). Finally, we extracted information on geographical region, time of fieldwork, river type, river size and the prevailing agricultural stressors. We gathered information on study design, agricultural type and practices (including the spatial scale addressed: riparian zone or entire catchment) and whether agriculturally used areas were compared to forests, best attainable or other control areas or if an agricultural gradient was reported.
Statistical analysis
We used Hedge’s g, a measure of standardized mean difference, to quantify biota response to agricultural land use [8]. Hedge’s g is a modified version of Cohen´s d, corrected for small or unequal sample sizes. Hedge’s g compares the means of the treatment (agricultural land use) and control groups (XT and XC, respectively), which are standardized by dividing the pooled standard deviation (SDp) and multiplied by a correction factor J, to avoid sample size bias.
The pooled standard deviation was calculated using the sample sizes of control and treatment groups (nT and nC) and the squared standard deviations of the respective groups (SD2T and SD2C):
The correction factor J was calculated with the sample sizes:
If studies reported correlation coefficients (r) rather than means and standard deviations, they were transformed to Hedge’s g following Borenstein et al. [8]:
An effect size =|1| indicates a deviance of one standard deviation between the treatment and the control group. Positive and negative effect sizes indicate higher and lower metric values, respectively, in the treatment group, while an effect size = 0 indicates no effect. Following Cohen [16], an effect size of ≤|0.2| is considered as small, a value of |0.5| as medium and a value of ≥|0.8| as a large effect.
To test Hypothesis 1 (general effects of agricultural land use on biota and quality metrics assessing agricultural impact best), we ran a meta-analysis with the metafor package in R [77]. We also ran a multi-level meta-analysis with the function rmv.ma to account for potential high heterogeneity which is typical for meta-analyses in ecology [70] using the Study ID as a random effect. Subsequently, we used meta-regression and subgroup analysis to investigate dominant co-variables (e.g. metric category, geographical region, ecoregion or river type and size), referred to as moderators, and hence, further explain heterogeneity. To test Hypothesis 2 (effects of agricultural types and practices on different organism types), we used measures of agricultural land use types, agricultural stressors reported, as well as the spatial basis (riparian or catchment land use) and the organism groups as moderators.
Publication bias
Studies with large and strongly significant results are more likely to be published as opposed to studies reporting weak and insignificant effects. This misbalance of published effects is called publication bias, which requires thoughtful consideration in meta-analyses. We used a funnel plot including data augmentation following Duval [24] to discover asymmetry of effect sizes and their variances and to estimate the number of potentially missing studies. In an unbiased dataset, the association should result in a funnel-shaped graph with most studies located within the funnel area. Additionally, we applied Egger´s regression test to analyse the funnel asymmetry [25]. Finally, we applied the fail-safe n test as a second measure to detect publication bias, calculating the number of effect sizes needed to reduce the significance level to non-significance. A study is considered robust and thus publication bias is negligible, when the fail-safe number is greater than 5 k + 10 (k = number of effect sizes). We also tested for time-lag bias, arising when initially published studies have larger effects than those of later studies often reported in ecological meta-analyses [75].
Results
Overview of studies and results
A total of 43 studies were distributed between Europe, North America and Oceania with 18, 16 and 9 studies, respectively. The effects on macroinvertebrates were reported by 43 studies, 11 studies reported effects on fish, 3 studies on macrophytes and 11 studies on diatoms (multiple assignments possible). While 75% of the 43 studies named nutrient influx as a major stressor, 50% listed fine sediment, followed by morphological alteration and pesticide influx with 40–30%, respectively (multiple assignments possible). Concerning study design, 28 studies compared control and treatment, while 15 studies reported effects as a correlation coefficient. In total, 76 extracted effect sizes were used for the overall meta-analysis, with 43 cases belonging to the metric category “Richness & Abundance metrics” and 33 to “Quality metrics”.
The overall effect size pooled from all 76 cases was g = − 0.74 [CI − 1.01; − 0.48] with Q = 660, a total variability of I2 = 93% and an estimated total heterogeneity of Tau2 = 1.1, showing strong heterogeneity following [37]. Because the 76 effect sizes were retrieved from 43 studies only, we applied a multilevel meta-analysis with the Study ID as a random effect yielding no change in overall effect size. Nearly, all heterogeneity is explained by the effect size level (Tau2Level 2 = 1.08) with the study size level explaining less than 1 percent (Tau2 Level 3 < 0.00).
Conducting meta-regression analysis to identify moderators explaining heterogeneity based on the Akaike Information criterion [3], we were able to explain R2 = 54.3% of the heterogeneity using four moderators: metric category, geographical region, agricultural types and organism types.
Effects of agriculture on river biota and metrics describing them best
Individually accounting for R2 = 40.4% of heterogeneity, “metric category” was the most important moderator (Fig. 1): Richness & Abundance metrics had a g = − 0.16 [CI − 0.50; 0.17], which is considered a non-significant low effect, while for Quality metrics a very large effect was observed: g = − 1.41 [CI − 1.66; − 1.17]. We also applied multilevel meta-analysis rendering a small change of the subgroup effect sizes to g = − 0.18 [CI − 0.92; 0.57] for Richness & Abundance metrics and g = − 1.47 [CI − 1.81; − 1.14] for Quality metrics with Tau2Level 2 = 0.50, Tau2Level 3 = 0.14. The attempt to investigate, which studies caused study level heterogeneity (Tau2Level 3) was not successful. In conclusion, the effect sizes did not change from the two-level meta-analysis for the overall meta-analysis and only marginally for the subgroups and most heterogeneity was found on the effect size level. Against this background, we limited our further analysis and the graphical representation to a two-level meta-analysis to reduce complexity.
Effect size of agricultural land use on different organism groups accounting for different metric types. Shown are individual effect sizes per study, pooled effect sizes for two subgroups divided by metric type and an overall pooled effect size with 95% confidence intervals. Effect sizes are significant if 95% CI does not overlap the vertical zero line. Richness = species-, taxa richness; abundance = density, mass, count; ASPT Average core er taxa; IBI Index of biotic integrity, EPT Ephemeroptera, Plecoptera, Trichoptera, EQR Ecological quality ratio, O/E Observed/Expected, SPEAR Species at risk, WQ sensitive Water quality sensitivity index
Further subgroup analysis suggests that agricultural effects are stronger in the northern hemisphere compared to the southern rendering strong negative effects in the northern hemisphere and no effects in the southern hemisphere (Fig. 2). Only small differences were observed between ecoregions with slightly stronger effects in the subtropical compared to temperate and subpolar regions (Fig. 3). No major differences were observed for study design, river types and size, suggesting similar effects for lowland and mountain rivers (Table 1).
Biota response to different agricultural types and practices
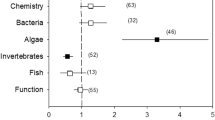
The comparison between different agricultural types suggests that arable land affects river biota more strongly than livestock farming. Geographically, most studies from North America and Europe report the effects of arable land use, while the studies from Oceania almost exclusively report livestock effects (Fig. 2). No major differences were observed between catchment- and buffer land use (Table 1) and meta-regression with the number of stressors reported did not explain any heterogeneity (not shown). Small differences in the response of different organism types could be observed with macroinvertebrates affected most. We observed a large effect for macroinvertebrates, a medium effect for fish and macrophytes and only a small effect for diatoms (Fig. 4).
Sensitive vs tolerant taxa
We conducted another smaller meta-analysis with the 16 studies reporting effects for either pollution-sensitive or pollution-tolerant macroinvertebrate taxa (Fig. 5). We observed strong differences in effect size between those (g = − 0.6 [CI − 1.22; − 0.70] vs g = 1.51 [CI 0.66; 2.34], with sensitive taxa being strongly impaired and tolerant taxa benefiting.
Effect size of agricultural land use on sensitive vs. tolerant taxa. Shown are individual effect sizes per study, pooled effect sizes for two subgroups divided by metric type and an overall pooled effect size with 95% confidence intervals. Effect sizes are significant if 95% CI does not overlap the vertical zero line. EPT Ephemeroptera, Plecoptera and Trichoptera; ET Ephemeroptera and Trichoptera
Publication bias
The Egger´s regression test suggests no significant publication bias (z = − 0.56, p = 0.58), and likewise the funnel plot only suggests a small lack of studies with positive effects (empty circles, Fig. 6). Also, no time-lag bias could be found and the fail-safe number of 7730 is greater than 5 k + 10 (> 390) and therefore highly significant. Consequently, the results of the meta-analysis can be considered robust.
Discussion
Effects of agriculture on river biota and metrics describing them best
We expected that agricultural land use is negatively related to riverine biota, irrespective of region and organism group considered (Hypothesis 1). This expectation was supported by a medium to a strong negative overall effect size of g =− 0.74 [− 1.21; − 0.62] [16], despite differences among geographical regions and ecoregions. The observed strong heterogeneity between the effect sizes most likely resulted from the combination of several studies of different geographical regions with varying agricultural land use, considered target organisms and effect endpoints.
The positive effects reported in the literature can be partly explained by the type of indicator considered. Fitzpatrick et al. [31] addressed the response of fish species richness, which is known to increase with river size [34], as agricultural land cover is also correlated to catchment size; this may explain the positive relationship between catchment agricultural area and fish richness. Likewise, the positive impact of agriculture on diatoms reported by Zheng et al. [85] was observed for overall species richness, while the authors reported a decline of pollution-sensitive taxa along a gradient of agricultural area. Similar relationships were found for extensive pasture in New Zealand, which showed positive effects for overall species richness but negative effects for more pollution-sensitive taxa (e.g. [56, 62]. These findings match our expectation that overall species richness is a poor indicator of agricultural stress because the loss of stress-sensitive species may be balanced by stress-tolerant ones. Consequently, agricultural impact on riverine biota is better reflected by metrics of species composition (quality metrics) as compared to metrics of species richness/abundance.
This was further supported by the subgroup analysis showing a very strong negative effect for quality metrics, while we only found a small negative effect for richness/abundance metrics (Fig. 1). Additional support was obtained by the analysis of sensitive vs. tolerant taxa, which showed a strong negative effect of agriculture for sensitive taxa, but a strong positive effect for tolerant taxa (Fig. 6). Together, these findings suggest a stronger land use effect on community compositional metrics. Compositional metrics used to assess the impact of agriculture should include sensitive taxa, because those are the first to respond with a decline in their abundance or richness. Stress-tolerant species, however, may increase due to subsidy effects by nutrient enrichment [60, 84] or due to the release from competition with sensitive taxa [46]. These opposing effects on sensitive and tolerant taxa also explain why metrics of whole-community richness performed much weaker. Likewise, [30] found that the loss of sensitive taxa due to hydromorphological degradation may be largely balanced by more tolerant ones. Thus, metrics of whole-community richness are likely to be insufficient for the analysis of agricultural stress.
Consequently, the observed positive effects in Oceania (Fig. 2) can be partly explained by the number of richness metrics reported compared to quality metrics. Only four effect sizes belong to the category of quality metrics with eleven belonging to richness metrics. The geographical differences can be further explained by different types and practices of agriculture (compare Hypothesis 2 below). The differences between ecoregions reflect climate conditions. Rivers in arid zones, for example in the Mediterranean, are frequently impaired by water abstraction pressures [53] and by particularly intense land use close to the rivers [55] leading to multiplying stressed biota [69]. Subpolar regions, on the other hand, are known for lower crop production and livestock populations [26, 27].
Biota response to agricultural types and practices
We expected that types and practices of agriculture in the river catchments would be reflected in the biota response (Hypothesis 2). This expectation was supported by differing effects between arable land and livestock farming (Fig. 4), with arable land impairing river biota more strongly. Those effects differed across continents, with agriculture in Northern America most affecting river biota most followed by Europe and surprisingly positive effects in Oceania (partly explained by the metric category assessed as described above). The positive effect in Oceania can be further explained as most studies assessed the effects of extensive pasture farming and no studies with arable land use were incorporated in this analysis (Fig. 2). Although most studies did not report more detailed information on agricultural types and practices, differences in biota response across continents are likely to be linked to different agricultural practices. In particular, agriculture in North America is generally known for large scale highly intensive machinery farming with higher nutrient input concentrations compared to Europe and Oceania [59].
Several studies report strongly differing effects of agricultural types and practices with livestock farming in river catchments resulting in nitrate influx [54], or corn production causing massive fine sediment influx [68]. The fact that we did not find an effect for livestock farming can be explained by a multitude of studies with extensive pasture from Oceania, which is considered less influential on river biota [12]. Additionally, the differences between arable land and livestock farming can be explained by pesticide usage. Unlike livestock farming arable land probably mirrors their exponentially increasing use [72] causing severe pressure on pesticide-sensitive species [50]. Although we could not investigate this further as in-depth information on the agricultural types was lacking, we expect for the effects of agricultural types to differ based on crop specific pesticide treatment [5]. However, 75% of the studies named nutrient influx as major stressor followed by fine sediment, morphological alteration, and pesticide influx with 50%, 40% and 30%, respectively. The presumed importance of pesticides can be explained by complex analysis to assess pesticide pressures (e.g. [15]. Additionally, several studies focusing on pesticides could not be incorporated into this analysis lacking comparable effect sizes or predictors (e.g. [15, 66].
We also expected stronger responses for macroinvertebrates compared to fish, macrophytes and diatoms. While macrophytes were only slightly stronger affected compared to fish and macrophytes, a strong difference could be found in comparison to diatoms as shown in the subgroup analysis (Fig. 5). This coincides with the reported predominant stressors and invertebrates suffering from all, nutrients [48], pesticides [28], fine sediment [32] and morphological alterations for instance resulting in water temperature rise [35].
Study implications
This study shows clearly that freshwater biota are impaired by agricultural land, with small differences based on geographical region, ecoregion or organism types, but results vary strongly based on the metric used. Quality metrics encompassing various ecologically relevant assessment indices reflect stronger effects than metrics focusing on richness or abundance. The obvious reason for these differences is the differential effects of stressors on individual taxa, that is not all taxa are equally impaired, with some tolerant species even benefiting from less competition or enhanced food availability. For future assessment of agricultural impacts, we suggest the use of metrics considering the tolerance of organisms, ideally metrics that are specific for individual stressor types caused by agriculture [32].
Agricultural stress is likely depending on the soil and climate conditions and agricultural types and practices, partly reflected in the ecoregions with stronger agricultural effects in the subtropical region compared to temperate and subpolar regions, potentially caused by water stress. Large parts of heterogeneity we could not explain are likely to be situated in the intensity and type of agriculture, in particular cropland densities [20], pesticide use [5], fertilizer use and biomass production [47]. Hence, in order to truly account for the impact of agricultural land use on river biota, further systematic investigations on the role of agricultural types, intensities and spatial arrangement are needed, not disregarding interactions with riparian vegetation as hinted by Palt et al. [61].
The present meta-analysis could only render hints suggesting the strongest agricultural impacts in North America, which is known for large-scale high intensity agriculture and in general stronger impacts for arable land compared to livestock farming. Still, the lower effects of livestock farming need to be taken with care, as they mostly receive concentrated feed imported from other regions [65]. Hence, parts of the environmental impact are spread, and animal production is less connected to the local river conditions.
Accordingly, after several years of research on agricultural impacts on freshwater biota, it is still not possible to quantitatively discriminate between agricultural practices, intensities, and types (such as individual crop types) and their interaction with riparian buffers. This is a significant knowledge gap that obstructs tailor-made solutions for minimizing the effects of agriculture on river biota. Obviously, these solutions are urgently required, given the overall strong impact of agriculture. Therefore, we call for further research to directly link agricultural types and intensities with biota response to derive management directives. Approaches could include but are not limited to accounting for crop-specific pesticide and nutrient application rates, different management practices including organic farming and mosaic farming or the occurrence of monocultures.
Availability of data and materials
The dataset used and/or analysed during the current study is available from the corresponding author upon reasonable request.
References
Alexander P, Brown C, Arneth A, Finnigan J, Rounsevell MD (2016) Human appropriation of land for food: the role of diet. Glob Environ Chang 41:88–98. https://doi.org/10.1016/j.gloenvcha.2016.09.005
Allan JD (2004) Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu Rev Ecol Evol Syst 35(1):257–284. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723. https://doi.org/10.1109/TAC.1974.1100705
Anderson JC, Dubetz C, Palace VP (2015) Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci Total Environ 505:409–422. https://doi.org/10.1016/j.scitotenv.2014.09.090
Andert S, Bürger J, Gerowitt B (2015) On-farm pesticide use in four Northern German regions as influenced by farm and production conditions. Crop Prot 75:1–10. https://doi.org/10.1016/j.cropro.2015.05.002
Baattrup-Pedersen A, Göthe E, Riis T, O’Hare MT (2016) Functional trait composition of aquatic plants can serve to disentangle multiple interacting stressors in lowland streams. Sci Total Environ 543:230–238. https://doi.org/10.1016/j.scitotenv.2015.11.027
Baattrup-Pedersen A, Ovesen NB, Larsen SE, Andersen DK, Riis T, Kronvang B, Rasmus-sen JJ (2018) Evaluating effects of weed cutting on water level and ecological status in Danish lowland streams. Freshw Biol 63(7):652–661. https://doi.org/10.1111/fwb.13101
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2021) Introduction to meta-analysis. John Wiley & Sons, New York
Börschig C, Klein AM, von Wehrden H, Krauss J (2013) Traits of butterfly communities change from specialist to generalist characteristics with increasing land-use intensity. Basic Appl Ecol 14(7):547–554. https://doi.org/10.1016/j.baae.2013.09.002
Bączyk A, Wagner M, Okruszko T, Grygoruk M (2018) Influence of technical maintenance measures on ecological status of agricultural lowland rivers–systematic review and implications for river management. Sci Total Environ 627:189–199. https://doi.org/10.1016/j.scitotenv.2018.01.235
Bighiu MA, Gottschalk S, Arrhenius Å, Goedkoop W (2020) Pesticide mixtures cause short-term, reversible effects on the function of autotrophic periphyton assemblages. Environ Toxicol Chem 39(7):1367–1374. https://doi.org/10.1002/etc.4722
Blake WH, Ficken KJ, Taylor P, Russell MA, Walling DE (2012) Tracing crop-specific sediment sources in agricultural catchments. Geomorphology 139:322–329. https://doi.org/10.1016/j.geomorph.2011.10.036
Brock TCM, Lahr J, Van den Brink, PJ. (2000). Ecological risks of pesticides in freshwater ecosystems; Part 1: herbicides. (No 88). Alterra. Wageningen, The Netherlands, 142
Casatti L, Teresa FB, Zeni JDO, Ribeiro MD, Brejao GL, Ceneviva-Bastos M (2015) More of the same: high functional redundancy in stream fish assemblages from tropical agroecosystems. Environ Manage 55(6):1300–1314. https://doi.org/10.1007/s00267-015-0461-9
Chiu MC, Hunt L, Resh VH (2016) Response of macroinvertebrate communities to temporal dynamics of pesticide mixtures: a case study from the Sacramento River watershed, California. Environ Pollut 219:89–98. https://doi.org/10.1016/j.envpol.2016.09.048
Cohen, J. (1988). Stafisfical power analysis for the behavioural sciences. 2. Hillside.
Dahm V, Hering D, Nemitz D, Graf W, Schmidt-Kloiber A, Leitner P, Melcher A, Feld CK (2013) Effects of physico-chemistry, land use and hydromorphology on three riverine organism groups: a comparative analysis with monitoring data from Germany and Austria. Hydrobiologia 704(1):389–415. https://doi.org/10.1007/s10750-012-1431-3
Dala-Corte RB, Giam X, Olden JD, Becker FG, Guimarães TDF, Melo AS (2016) Revealing the pathways by which agricultural land-use affects stream fish communities in South Brazilian grasslands. Freshw Biol 61(11):1921–1934. https://doi.org/10.1111/fwb.12825
Dala-Corte RB, Melo AS, Siqueira T, Bini LM, Martins RT, Cunico AM, Pes AM, Magalhães AL, Godoy BS, Leal CG, Monteiro-Júnior CS (2020) Thresholds of freshwater biodiversity in response to riparian vegetation loss in the Neotropical region. J Appl Ecol 57(7):1391–1402. https://doi.org/10.1111/1365-2664.13657
Davis NG, Hodson R, Matthaei CD (2022) Long-term variability in deposited fine sediment and macroinvertebrate communities across different land-use intensities in a regional set of New Zealand rivers. NZ J Mar Freshwat Res 56(2):191–212. https://doi.org/10.1080/00288330.2021.1884097
Dobbie KE, Smith KA (2003) Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables. Glob Change Biol 9(2):204–218. https://doi.org/10.1046/j.1365-2486.2003.00563.x
Donald PF, Sanderson FJ, Burfield IJ, Van Bommel FP (2006) Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agr Ecosyst Environ 116(3–4):189–196. https://doi.org/10.1016/j.agee.2006.02.007
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny ML, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81(2):163–182. https://doi.org/10.1017/S1464793105006950
Duval SJ (2005) The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M (eds) Publication bias in meta-analysis: Prevention, assessment, and adjustments. Wiley, Chichester, pp 127–144
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
EUROSTAT (2022a): Crop production in EU standard humidity by NUTS 2 regions [apro_cpshr]. https://ec.europa.eu/eurostat/web/products-datasets/-/apro_cpshr Accessed 23 June 2022
EUROSTAT (2022b): Animal populations by NUTS 2 regions [agr_r_animal]. https://ec.europa.eu/eurostat/web/products-datasets/-/agr_r_animal Accessed 23 June 2022
Englert D, Bundschuh M, Schulz R (2012) Thiacloprid affects trophic interaction between gam-marids and mayflies. Environ Pollut 167:41–46. https://doi.org/10.1016/j.envpol.2012.03.024
Feld CK (2013) Response of three lotic assemblages to riparian and catchment-scale land use: implications for designing catchment monitoring programmes. Freshw Biol 58(4):715–729. https://doi.org/10.1111/fwb.12077
Feld CK, de Bello F, Dolédec S (2014) Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshw Biol 59(2):233–248. https://doi.org/10.1111/fwb.12260
Fitzpatrick FA, Scudder BC, Lenz BN, Sullivan DJ (2001) Effects of multi-scale environmental characteristics on agricultural stream biota in eastern wisconsin 1. JAWRA J Am Water Resources Association 37(6):1489–1507. https://doi.org/10.1111/j.1752-1688.2001.tb03655.x
Gieswein A, Hering D, Lorenz AW (2019) Development and validation of a macroinvertebrate-based biomonitoring tool to assess fine sediment impact in small mountain streams. Sci Total Environ 652:1290–1301. https://doi.org/10.1016/j.scitotenv.2018.10.180
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967):812–818. https://doi.org/10.1126/science.1185383
Grenouillet G, Pont D, Hérissé C (2004) Within-basin fish assemblage structure: the relative influence of habitat versus stream spatial position on local species richness. Can J Fish Aquat Sci 61(1):93–102. https://doi.org/10.1139/f03-145
Haidekker A, Hering D (2008) Relationship between benthic insects (Ephemeroptera, Plecoptera, Coleoptera, Trichoptera) and temperature in small and medium-sized streams in Germany: a multivariate study. Aquat Ecol 42(3):463–481. https://doi.org/10.1007/s10452-007-9097-z
Harding JS, Benfield EF, Bolstad PV, Helfman GS, Jones Iii EBD (1998) Stream biodiversity: the ghost of land use past. Proc Natl Acad Sci 95(25):14843–14847. https://doi.org/10.1073/pnas.95.25.1484
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions, Chapter 7.7.3.2 Version 5.1.0. The Cochrane Collaboration, 2011. www.handbook.cochrane.org Accessed March 2011
Hilton J, O’Hare M, Bowes MJ, Jones JI (2006) How green is my river? A new paradigm of eutrophication in rivers. Sci Total Environ 365(1–3):66–83. https://doi.org/10.1016/j.scitotenv.2006.02.055
Janova E, Heroldova M (2016) Response of small mammals to variable agricultural landscapes in central Europe. Mamm Biol 81(5):488–493. https://doi.org/10.1016/j.mambio.2016.06.004
Jones JI, Douthwright TA, Arnold A, Duerdoth CP, Murphy JF, Edwards FK, Pretty JL (2017) Diatoms as indicators of fine sediment stress. Ecohydrology 10(5):e1832. https://doi.org/10.1002/eco.1832
Kaenel BR, Buehrer H, Uehlinger U (2000) Effects of aquatic plant management on stream metabolism and oxygen balance in streams. Freshw Biol 45(1):85–95. https://doi.org/10.1046/j.1365-2427.2000.00618.x
Kemp P, Sear D, Collins A, Naden P, Jones I (2011) The impacts of fine sediment on riverine fish. Hydrol Process 25(11):1800–1821. https://doi.org/10.1002/hyp.7940
King RS, Brain RA, Back JA, Becker C, Wright MV, Toteu Djomte V, Scott WC, Virgil SR, Brooks BW, Hosmer AJ, Chambliss CK (2016) Effects of pulsed atrazine exposures on autotrophic community structure, biomass, and production in field-based stream mesocosms. Environ Toxicol Chem 35(3):660–675. https://doi.org/10.1002/etc.3213
Lam S, Pham G, Nguyen-Viet H (2017) Emerging health risks from agricultural intensification in Southeast Asia: a systematic review. Int J Occup Environ Health 23(3):250–260. https://doi.org/10.1080/10773525.2018.1450923
Lange K, Townsend CR, Matthaei CD (2014) Can biological traits of stream invertebrates help disentangle the effects of multiple stressors in an agricultural catchment? Freshw Biol 59(12):2431–2446. https://doi.org/10.1111/fwb.12437
Levers C, Müller D, Erb K, Haberl H, Jepsen MR, Metzger MJ, Meyfroidt P, Plieninger T, Plutzar C, Stürck J, Verburg PH, Kuemmerle T (2018) Archetypical patterns and trajectories of land systems in Europe. Reg Environ Change 18(3):715–732. https://doi.org/10.1007/s10113-015-0907-x
Liess A, Le Gros A, Wagenhoff A, Townsend CR, Matthaei CD (2012) Landuse intensity in stream catchments affects the benthic food web: consequences for nutrient supply, periphyton C: nutrient ratios, and invertebrate richness and abundance. Freshwater Sci 31(3):813–824. https://doi.org/10.1899/11-019.1
Liess M, Schäfer RB, Schriever CA (2008) The footprint of pesticide stress in communities—species traits reveal community effects of toxicants. Sci Total Environ 406(3):484–490. https://doi.org/10.1016/j.scitotenv.2008.05.054
Liess M, Liebmann L, Vormeier P, Weisner O, Altenburger R, Borchardt D, Brack W, Chatzinotas A, Escher B, Foit K, Gunold R, Henz S, Hitzfeld KL, Schmitt-Jansen M, Kamjunke N, Kaske O, Knillmann S, Krauss M, Küster E, Link M, Lück M, Möder M, Müller A, Paschke A, Schäfer RB, Schneeweiss A, Schreiner AC, Schulze T, Schüürmann G, Tümpling Wv, Weitere M, Wogram J, Reemtsma T (2021) Pesticides are the dominant stressors for vulnerable insects in lowland streams. Water Res 201:117262. https://doi.org/10.1016/j.watres.2021.117262
Lusardi RA, Jeffres CA, Moyle PB (2018) Stream macrophytes increase invertebrate production and fish habitat utilization in a California stream. River Res Appl 34(8):1003–1012. https://doi.org/10.1002/rra.3331
Mebane CA, Simon NS, Maret TR (2014) Linking nutrient enrichment and streamflow to macrophytes in agricultural streams. Hydrobiologia 722(1):143–158. https://doi.org/10.1007/s10750-013-1693-4
Metzger MJ, Bunce RGH, Jongman RHG, Mücher CA, Watkins JW (2005) A climatic stratification of the environment of Europe. Glob Ecol Biogeo 14(6):549–563. https://doi.org/10.1111/j.1466-822X.2005.00190.x
Mouri G, Aisaki N (2015) Using land-use management policies to reduce the environmental impacts of livestock farming. Ecol Complex 22:169–177. https://doi.org/10.1016/j.ecocom.2015.03.003
Mücher CA, Klijn JA, Wascher DM, Schaminée JHJ (2010) A new European Landscape Classification (LANMAP): A transparent flexible and user-oriented methodology to distinguish landscapes. Ecol Indic 10(1):87–103. https://doi.org/10.1016/j.ecolind.2009.03.018
Niyogi DK, Koren M, Arbuckle CJ, Townsend CR (2007) Stream communities along a catchment land-use gradient: subsidy-stress responses to pastoral development. Environ Manage 39(2):213–225. https://doi.org/10.1007/s00267-005-0310-3
Nowell LH, Moran PW, Schmidt TS, Norman JE, Nakagaki N, Shoda ME, Mahler BJ, Van Metre PC, Stone WW, Sandstorm MW, Hladik ML (2018) Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern US streams. Sci Total Environ 613:1469–1488. https://doi.org/10.1016/j.scitotenv.2017.06.156
O’Hare MT, Baattrup-Pedersen A, Baumgarte I, Freeman A, Gunn ID, Lázár AN, Sinclair R, Wade AJ, Bowes MJ (2018) Responses of aquatic plants to eutrophication in rivers: a revised conceptual model. Front Plant Sci 9:451. https://doi.org/10.3389/fpls.2018.00451
Pellegrini P, Fernández RJ (2018) Crop intensification, land use, and on-farm energy-use efficiency during the worldwide spread of the green revolution. Proc Natl Acad Sci 115(10):2335–2340. https://doi.org/10.1073/pnas.1717072115
Piggott JJ, Lange K, Townsend CR, Matthaei CD (2012) Multiple stressors in agricultural streams: a mesocosm study of interactions among raised water temperature, sediment addition and nutrient enrichment. PLoS ONE 7(11):e49873. https://doi.org/10.1371/journal.pone.0049873
Palt M, Le Gall M, Piffady J, Hering D, Kail J (2022) A metric-based analysis on the effects of riparian and catchment landuse on macroinvertebrates. Sci Total Environ 816:151590. https://doi.org/10.1016/j.scitotenv.2021.151590
Quinn JM, Cooper AB, Davies-Colley RJ, Rutherford JC, Williamson RB (1997) Land use effects on habitat, water quality, periphyton, and benthic invertebrates in Waikato, New Zealand, hill-country streams. NZ J Mar Freshwat Res 31(5):579–597. https://doi.org/10.1080/00288330.1997.9516791
Raitif J, Plantegenest M, Roussel JM (2019) From stream to land: ecosystem services provided by stream insects to agriculture. Agr Ecosyst Environ 270:32–40. https://doi.org/10.1016/j.agee.2018.10.013
Ritchie, H., & Roser, M. (2019). Land use. Our world in data. https://ourworldindata.org/landuse?fbclid=IwAR2ZHUKQViHe1cB1YszWkbdwJ8HxfaCpbOyOvHTk0mb5Lv_kv7oxdiXH4nM Accessed 15 Feb 2022
Sauvant D, Ponter A (2004) Tables of composition and nutritional value of feed materials. Pigs, poultry, cattle, sheep, goats, rabbits, horses and fish, Wageningen Acad. Publ; INRA Ed. http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10686757
Schäfer RB, Pettigrove V, Rose G, Allinson G, Wightwick A, Von Der Ohe PC, Kefford BJ (2011) Effects of pesticides monitored with three sampling methods in 24 sites on macroinvertebrates and microorganisms. Environ Sci Technol 45(4):1665–1672. https://doi.org/10.1021/es103227q
Schäfer RB, van den Brink PJ, Liess M (2011) Impacts of pesticides on freshwater ecosystems. Ecol Impacts Toxic Chem 2011:111–137. https://doi.org/10.2174/978160805121211101010111
Secchi S, Gassman PW, Jha M, Kurkalova L, Kling CL (2011) Potential water quality changes due to corn expansion in the Upper Mississippi River Basin. Ecol Appl 21(4):1068–1084. https://doi.org/10.1890/09-0619.1
Segurado P, Almeida C, Neves R, Ferreira MT, Branco P (2018) Understanding multiple stressors in a Mediterranean basin: Combined effects of land use water scarcity and nutrient enrichment. Sci Total Environ 624(1):1221–1233. https://doi.org/10.1016/j.scitotenv.2017.12.201
Senior AM, Grueber CE, Kamiya T, Lagisz M, Santos ES, Nakagawa S (2016) Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97(12):3293–3299. https://doi.org/10.1002/ecy.1591
Shoyama K, Braimoh AK, Avtar R, Saito O (2018) Land transition and intensity analysis of cropland expansion in Northern Ghana. Environ Manage 62(5):892–905. https://doi.org/10.1007/s00267-018-1085-7
Stehle S, Schulz R (2015) Agricultural insecticides threaten surface waters at the global scale. Proc Natl Acad Sci 112(18):5750–5755. https://doi.org/10.1073/pnas.1500232112
Strokal M, Ma L, Bai Z, Luan S, Kroeze C, Oenema O, Velthof G, Zhang F (2016) Alarming nutrient pollution of Chinese rivers as a result of agricultural transitions. Environ Res Lett 11(2):024014. https://doi.org/10.1088/1748-9326/11/2/024014
Suriano MT, Fonseca-Gessner AA, Roque FO, Froehlich CG (2011) Choice of macroinvertebrate metrics to evaluate stream conditions in Atlantic Forest Brazil. Environ Monit Assess 175(1):87–101. https://doi.org/10.1007/s10661-010-1495-3
Trikalinos TA, Ioannidis JP (2005) Assessing the evolution of effect sizes over time. In: Rothstein HR, Sutton AJ, Borenstein M (eds) Publication bias in meta-analysis: prevention, assessment, and adjustments. Wiley, Chichester, pp 241–259
Václavík T, Lautenbach S, Kuemmerle T, Seppelt R (2013) Mapping global land system archetypes. Glob Environ Chang 23(6):1637–1647. https://doi.org/10.1016/j.gloenvcha.2013.09.004
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Soft 36(3):1–48. https://doi.org/10.1863/jss.v036.i03
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467(7315):555–561. https://doi.org/10.1038/nature09440
Wahl CM, Neils A, Hooper D (2013) Impacts of land use at the catchment scale constrain the habitat benefits of stream riparian buffers. Freshw Biol 58(11):2310–2324. https://doi.org/10.1111/fwb.12211
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):1–13. https://doi.org/10.1186/1471-2288-14-135
Wang HH, Tan TK, Schotzko RT (2007) Interaction of potato production systems and the environment: a case of waste water irrigation in central Washington. Waste Manage Res 25(1):14–23. https://doi.org/10.1177/0734242X07066337
Weijters MJ, Janse JH, Alkemade R, Verhoeven JT (2009) Quantifying the effect of catchment land use and water nutrient concentrations on freshwater river and stream biodiversity. Aquat Conserv Mar Freshwat Ecosyst 19(1):104–112. https://doi.org/10.1002/aqc.989
Wirsenius S, Azar C, Berndes G (2010) How much land is needed for global food production under scenarios of dietary changes and livestock productivity increases in 2030? Agric Syst 103(9):621–638. https://doi.org/10.1016/j.agsy.2010.07.005
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A, Malmqvist B, Mickie BG, Tiegs SD, Cariss H, Dobson M, Elosegi A, Ferreira V, Graca MAS, Fleituch T, Lacoursiére JO, Nistorescu M, Pozo J, Risnoveanu G, Schindler M, Vadineanu A, Vought LB-M, Chauvet E (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336(6087):1438–1440. https://doi.org/10.1126/science.1219534
Zheng L, Gerritsen J, Beckman J, Ludwig J, Wilkes S (2008) Land use, geology, enrichment, and stream Biota in the Eastern Ridge and Valley Ecoregion: implications for nutrient criteria development 1. JAWRA Journal of the American Water Resources Association 44(6):1521–1536. https://doi.org/10.1111/j.1752-1688.2008.00257.x
Acknowledgements
This work was financially supported by a scholarship funding from the German Federal Environmental Foundation (DBU), which is gratefully acknowledged. We are grateful to the authors of the papers considered, who provided their original data. References of studies only included in the meta-analysis are found in the Additional file 1.
Funding
Open Access funding enabled and organized by Projekt DEAL. The scientific work of Christian Schürings was sponsored by The German Federal Environmental Foundation (DBU).
Author information
Authors and Affiliations
Contributions
CS: Conceptualization, investigation, literature review and analysis, drafting and writing of manuscript. CKF: Conceptualization and review, editing. JK: Conceptualization and review, editing. DH: Conceptualization and review, editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1
. References of studies incorporated in meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schürings, C., Feld, C.K., Kail, J. et al. Effects of agricultural land use on river biota: a meta-analysis. Environ Sci Eur 34, 124 (2022). https://doi.org/10.1186/s12302-022-00706-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-022-00706-z