Abstract
Access to safe drinking water is important as a health and development issue at national, regional, and local levels. About one billion people do not have healthy drinking water. More than six million people (about two million children) die because of diarrhea which is caused by polluted water. Developing countries pay a high cost to import chemicals including polyaluminium chloride and alum. This is the reason why these countries need low-cost methods requiring low maintenance and skill. The use of synthetic coagulants is not regarded as suitable due to health and economic considerations. The present study was aimed to investigate the effects of alum as coagulant in conjunction with bean, sago, and chitin as coagulants on the removal of color, turbidity, hardness, and Escherichia coli from water. A conventional jar test apparatus was employed for the tests. The study was taken up in three stages, initially with synthetic waters, followed by testing of the efficiency of coagulants individually on surface waters and, lastly, testing of blended coagulants. The experiment was conducted at three different pH conditions of 6, 7, and 8. The dosages chosen were 0.5, 1, 1.5, and 2 mg/l. The results showed that turbidity decrease provided also a primary E. coli reduction. Hardness removal efficiency was observed to be 93% at pH 7 with 1-mg/l concentration by alum, whereas chitin was stable at all the pH ranges showing the highest removal at 1 and 1.5mg/l with pH 7. In conclusion, using natural coagulants results in considerable savings in chemicals and sludge handling cost may be achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The explosive growth of the world's human population and subsequent water and energy demands have led to an expansion of standing surface water[1]. Nowadays, the concern about contamination of aquatic environments has increased, especially when water is used for human consumption. About one billion people do not have healthy drinking water. More than six million people (about two million children) die because of diarrhea which is caused by polluted water[2, 3].
In most of the cases, surface water turbidity is caused by the clay particles, and the color is due to the decayed natural organic matter. Generally, the particles that determine the turbidity are not separated by settling or through traditional filtration. Colloidal suspension stability in surface water is also due to the electric charge of particle surface. Thus, there is great importance in either the development of more sophisticated treatments or the improvement of the current ones[4].
The production of potable water from most raw water sources usually entails the use of a coagulation flocculation stage to remove turbidity in the form of suspended and colloidal material. This process plays a major role in surface water treatment by reducing turbidity, bacteria, algae, color, organic compounds, and clay particles. The presence of suspended particles would clog filters or impair disinfection process, thereby dramatically minimizing the risk of waterborne diseases[5, 6].
Many coagulants are widely used in conventional water treatment processes, based on their chemical characteristics. These coagulants are classified into inorganic, synthetic organic polymers, and natural coagulants[4]. Alum has been the most widely used coagulant because of its proven performance, cost effectiveness, relatively easy handling, and availability. Recently, much attention has been drawn on the extensive use of alum. Aluminum is regarded as an important poisoning factor in dialysis encephalopathy. Aluminum is one of the factors which might contribute to Alzheimer's disease[7–9]. Alum reaction with water alkalinity reduces water pH and its efficiency in cold water[10, 11]. However, some synthetic organic polymers such as acrylamide have neurotoxicity and strong carcinogenic effect[8, 12].
In addition, the use of alum salts is inappropriate in some developing countries because of the high costs of imported chemicals and low availability of chemical coagulants[3]. This is the reason why these countries need low-cost methods requiring low maintenance and skill.
For these reasons, and also due to other advantages of natural coagulants/flocculants over chemicals, some countries such as Japan, China, India, and the United States have adopted the use of natural polymers in the treatment of surface water for the production of drinking water[13]. A number of studies have pointed out that the introduction of natural coagulants as a substitute for metal salts may ease the problems associated with chemical coagulants.
Natural macromolecular coagulants are promising and have attracted the attention of many researchers because of their abundant source, low price, multi-purposeness, and biodegradation[11, 14, 15]. Okra, rice, and chitosan are natural compounds which have been used in turbidity removal[16–18]. The extract of the seeds has been mentioned for drastically reducing the amount of sludge and bacteria in sewage[19].
In view of the above discussion, the present work has been taken up to evaluate the efficiency of various natural coagulants on the physico-chemical contaminant removal of water. To date, most of the research has been concentrated on the coagulant efficiencies in synthetic water, but in this study, we move ahead making an attempt to test the efficiency of the natural coagulants on surface water. The efficiencies of the coagulants as stated by[20] might alter depending on many factors: nature of organic matter, structure, dimension, functional groups, chemical species, and others.
Methods
Natural coagulants and their preparation
Sago is a product prepared from the milk of tapioca root. Its botanical name is ‘Manihot esculenta Crantz syn. M. utilissima’. Hyacinth bean with botanical name Dolichos lablab is chosen as another coagulant. Both the coagulants were used in the form of powders (starches). Starch consists mainly of a homopolymer of α-d-glucopyranosyl units that comes in two molecular forms, linear and branched. The former is referred to as amylose and the latter as amylopectin[21]. These have the general structure as per[22] (Figure 1) .
The third coagulant was chitin ([C8H13O5N] n ), which is a non-toxic, biodegradable polymer of high molecular weight. Like cellulose, chitin is a fiber, and in addition, it presents exceptional chemical and biological qualities that can be used in many industrial and medical applications. The two plant originated coagulants were taken in the form of powder or starch. Chitin was commercially procured.
Stage I
The first stage included testing the efficiency of the four coagulants on the synthetic waters. Synthetic waters with turbidity of 70 and 100 nephelometric turbidity units (NTU) were prepared with fuller's earth in the laboratory and were used in this part of the study. The experiment was carried out using a jar test apparatus. The experiments were conducted in duplicates to eliminate any kind of error. Efficiency was evaluated by determination of reduction in turbidity of both the synthetic samples.
Stage II
In the second stage of the experiment, the individual coagulants were evaluated for their efficiency on the surface waters. The water samples for this stage and the preceding stage were collected from the surface reservoir, Mudasarlova, located at a distance of 5 km from the Environmental Monitoring Laboratory, GITAM University, where the experiments were carried out. This is the reservoir which serves as a source of domestic water for the nearby residents.
Care was taken while collecting the samples so that a representative sample is obtained. All samples were collected in sterile plastic containers. The samples were transported to the laboratory, and all the experiments were conducted within a duration of 24 h. The physical parameters like temperature and color were noted at the point of sample collection. The water samples were analyzed for the following parameters pre- and post-treatment with the coagulants (Table 1).
The coagulants were tested at various concentrations like 0.5, 1, 1.5, and 2 mg/l at three pH ranges of 6, 7, and 8.
Stage III
The results obtained from the second stage of the study have encouraged us to further extend the study in terms of blended coagulants. The blending of coagulants was taken up from the fact that alum was the most widely used coagulant, and hence, it was taken as one part. The remaining combinations were 2, 3, 4, and 5 parts of the natural coagulants, i.e., 1:2, 1:3, 1:4, and 1:5.
Testing of the following parameters was adopted for evaluating the efficiency of the blended coagulants (pre- and post-coagulation) (Table 2). All the analysis has been performed as per the standard methods given by APHA, 2005[23].
E. coli presence
The E. coli bacterial presence and absence were determined in the pre- and post-coagulated water using H2S strip bottle. The water sample was filled into the bottle and allowed to stand for 24 h at room temperature. After 24 h, the water sample was observed for color change; black color change indicates the presence of E. coli.
Results
Coagulant actions onto colloidal particles take place through charge neutralization of negatively charged particles. If charge neutralization is the predominant mechanism, a stochiometric relation can be established between the particles' concentration and coagulant optimal dose.
In the initial stage of the experiment, the coagulants were tested against synthetic turbid samples with 70 and 100 NTU. According to Figure 2a,b, the optimum dosage of alum was observed to be 1mg/l for both the turbid samples, and the optimum pH is observed to be 7.
It is understood from Figure 3a,b that the optimum dosage for chitin as coagulant is 1.5 mg/l (turbidity to 40 NTU) for 100 NTU, whereas not much difference was observed between pH 7 and 8 for both the turbid samples. The optimum pH is observed to be 7 for both 70 and 100 NTU samples.
Figure 4a,b exemplifies the trends of sago on the turbidity removal of the synthetic solutions. It is observed that sago was effective at both 1 and 1.5 mg/l (turbidity reduced to 50 and 45 NTU, respectively) for 100 NTU solution, and the efficiency was stable at pH 7 and 8.
Figure 5a,b illustrates the effect of bean on the synthetic turbid samples and turbidity removal. It is observed that bean was effective at 1mg/l (turbidity reduced to 55 NTU) for 100 NTU solution, and the efficiency was stable at pH 7 and 8.
Implications from the stage 1 experiment articulate that the coagulants are quite stable at the pH ranges tested; hence, in the proceeding experiments, all the three pH ranges were considered. In the second stage of experiment, the environmental samples from the surface water source were collected and tested for the removal of turbidity and other chemical parameters. The dosages were the same as the previous stage. The results are graphically represented as shown in Figures 6,7,8,9.
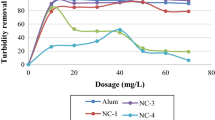
The turbidity removal efficiencies of the individual coagulants are depicted in Figure 6 wherein there was a broad variation among the pH ranges. The maximum reduction was observed with 1 mg/l (87%) of bean at pH 6 followed by 1 mg/l (82%) sago at the same pH. At pH 7, the maximum efficiency was shown by bean with 1.5 mg/l dosage (85.37%) followed by bean and sago with 1 (82.49%) and 1.5 mg/l (82.49%), respectively. Removal efficiencies of 41.46% and 36.59% were reported by 1 mg/l of bean and sago, respectively, at pH 8. The minimum reductions are not reported as there was a negative competence of the coagulants at different doses and pH variations. It can be observed from the graph that there was an increase in the turbidity of the water at these dosages like with 2 g of chitin the turbidity removal was −19.51. In the entire study, the best results were obtained with total hardness removal wherein no negative competence was reported as shown in Figure 7. The utmost removal was observed with 0.5-mg/l (97.67%) sago at pH 7. At pH 6, it was (90.70%) with 1.5 mg/l of bean. At pH 8, the reduction was (93.02%) with 0.5 mg/l of alum. Apart from these, the general observation was that all the coagulants were effective in an average removal of 65% total hardness at all pH variations and doses. The tracking for the least efficiency has showed chitin at pH 6 with 2-mg/l dose (34.88%).
The calcium hardness removal efficiencies are directly proportional with the total hardness removal; the highest removal was recorded by chitin (93.33%) at pH 7 with 1.5-mg/l dose as shown in Figure 8. Removal of 90% is at pH 8 and 7 with 0.5-mg/l alum and 1-mg/l chitin, respectively. Minimum effectiveness was observed by chitin (6.67%) at pH 6 with 2-mg/l dose. On an average, the removal competence was more than 60% with all coagulants at doses at all the pH conditions.
Figure 8 illustrates the chloride removal efficiency of the coagulants tested. The average competence was observed to be 40%. The maximum competence was noted at pH 7 by chitin (83.64%) at 1.5 mg/l followed by sago (81.82%) at 1 mg/l. Indeed at pH 7, the removal was observed to be superior as a whole. Similarly, pH has shown inferior effectiveness in the amputation of chloride. The remarkable point that was noted is that at pH 8, where the removal was superior, the increase in doses of sago and bean (1.5 and 2 mg/l) has shown a depressing outcome.
With the results obtained from the second stage experimentation, the study was carried forward for the evaluation of blended coagulants. From the literature, it was understood that blended coagulants show improved competence than that of the individual ones.
The regular test of turbidity was substituted with conductivity to establish a relation and test the difference with these parameters. The conductivity diminution was observed to be preeminent at the ratio of 1:2 of all the blended coagulants 26.12%, 26.00%, and 21.35% with alum/bean, alum/chitin, and alum/sago, respectively. The highest reduction was observed with alum/sago at pH 8 with 1:2 ratio (32.28%) (Figure 10).
The total hardness reduction trend of the blended coagulants was recorded as follows: at pH 7, all combinations of alum/bean have resulted in negative competence. Amputation of 100% was observed with alum/chitin and alum/sago at 1:2 and 1:4 and 1:5 doses, respectively (Figure 11). The overall competence of the alum/chitin and alum/sago were registered to be more than 80%. The calcium hardness efficiencies of the blended coagulants were similar to that of the total hardness. The highest removal efficiency was shown by alum/chitin with 1:5 ratio at pH 7 (Figure 12).
As said earlier, the turbidity was replaced by color determination taking into account the fact that turbidity is directly related to the color. pH 7 has been remarkably effective in the highest removal of color from the water. The blended coagulant alum/sago was found to be very effective with 98% to 100% reduction in color at all the ratios of dosage (Figure 13). The blended coagulants alum/chitin and alum/sago were relatively successful at an average rate of 80% decline in the color at almost all ratios of dosage at pH 7 and 8.
Alum/sago blend has a noteworthy effect on the removal of chloride from the water samples in which no negative result was noted. The highest reduction was observed with alum/chitin with dose of 1:5 (85.71%) at pH 7. Indeed, pH 7 can be optimized as perfect pH for this blend as all the ratios of dosages were quite efficient in the removal of chloride (Figure 14).
Discussion
Although many studies have used synthetic water in the experiments, this work chose to use raw water collected directly from the surface source. Therefore, it is important to consider that the natural compounds may cause variations in their composition, which interfere in the treatment process. All those factors are taken into account when evaluating the obtained results.
The characteristics of the superficial water used in this study are observed as that the water used has apparent color, turbidity, solids, and amount of compounds with a relatively high absorption in UV (254 nm). It is noticeable that the water has high turbidity and color.
The effectiveness of alum, commonly used as a coagulant, is severely affected by low or high pH. In optimum conditions, the white flocs were large and rigid and settled well in less than 10 min. This finding is in agreement with other studies at optimum pH[24, 25]. The optimum pH was 7 and was similar to the obtained results by Divakaran[26]. At high turbidity, a significant improvement in residual water turbidity was observed. The supernatant was clear after about 20-min settling. Flocs were larger and settling time was lower. The results showed that above optimum dosage, the suspensions showed a tendency to restabilize.
The effectiveness of the chitin in the present study in the removal of various contaminants with varied pH individually and also in blended form can be traced to the explanation from the literature that chitin has been studied as biosorbent to a lesser extent than chitosan; however, the natural greater resistance of the former compared to the last, due to its greater crystallinity, could mean a great advantage. Besides, the possibility to control the degree of acetylation of chitin permits to enhance its adsorption potential by increasing its primary amine group density. Recent studies regarding the production of chitin-based biocomposites and its application as fluoride biosorbents have demonstrated the potential of these materials to be used in continuous adsorption processes. Moreover, these biocomposites could remove many different contaminants, including cations, organic compounds, and anions[27].
Chitosan has high affinity with the residual oil and excellent properties such as biodegradability, hydrophilicity, biocompability, adsorption property, flocculating ability, polyelectrolisity, antibacterial property, and its capacity of regeneration in many applications[28]. It has been used as non-toxic floccules in the treatment of organically polluted wastewater[29].
The effects of coagulation process on hardness are observed for varying levels of hardness, which resulted in significant decrease of hardness removal. The study correlates with the results obtained by[27], wherein they had a maximum hardness removal of 84.3% by chitosan in low turbid water with initial hardness of about 204 mg/l as CaCO3.
Several experiments were carried out to determine the comparative performance of chitosan on E. coli in different turbidities. E. coli negative is present in the chitin-treated waters in all of the turbidities. The conclusive evidence was found for the negative influence of chitosan on E. coli. The regrowth of E. coli was not observed in the experiments after 24 h, which was similar to the observations by[27].
As far as sago is considered, the starch was effective both individually and as blended coagulant. Unlike polyaluminium chloride, the efficiency of the natural coagulants is not affected by pH. The pH increased their efficiency, which is one of the advantages of natural coagulants. The principle behind the efficiency of the sago from the literature can be stated as follows: Sago starch is a natural polymer that is categorized as polyelectrolyte and can act as coagulant aid. Coagulant aid can be classified according to the ionization traits, which are the anions, cations, and amphoteric (with dual charges). Bratskaya et al.[30] mentioned that among the three groups, cation polymer is normally used to remove adsorbed negatively charged particles by attracting the adsorbed particles through electrostatic force. They discovered that anion polymer and those non-ionized cannot be used to coagulate negatively charged particles.
The chemical oxygen demand (COD) reduction is influenced by the concentration of sago used; the lower the concentration the better the removal of the COD. Using less than 1.50 g L-1, better COD reduction is observed. At this low concentration, settling time did not influence the COD reduction. Similarly, concentration of sago used at lower than 1.50 g L-1 reduced the turbidity in less than 15 min of settling time. Sago concentration higher than 1.50 g L-1 increased the turbidity; however, settling time has an influence on the turbidity reduction at higher sago concentrations. This pattern is congruent with the COD removal[31].
The sago starch-graft-polyacrylamide (SS-g-PAm) coagulants were found to achieve water turbidity removal up to 96.6%. The results of this study suggest that SS-g-PAm copolymer is a potential coagulant for reducing turbidity during water treatment[32].
At its optimum concentration, D. lablab seed powder does not affect the pH of the water. Total and calcium hardness remained almost constant and were within acceptable levels according to World Health Organization standards for drinking water. Moreover, coagulation of medium to high turbidity water with D. lablab seed powder with the finest grain size reduced turbidity further. The best performance of the finest seed powder could be due to its large total surface area, whereby most of the water-soluble proteins are at the solid–liquid interface during the extraction process as stated by Gassenschmidtet al.[33]. This might have increased the concentration of active coagulation polymer in the extract, which improved the coagulation process. The coagulant extract from seeds has shown antimicrobial activity in the comparative culture test, which was also observed in the study of Tandonet al.[34].
D. lablab demonstrated the best performance with turbid water, in which a turbidity removal efficiency of 87% was observed. The restabilization of destabilized colloidal particles, which was associated with higher residual turbidities, occurred at dosages above the optimum. It is commonly observed that particles are destabilized by small amounts of hydrolyzing metal salts and that optimum destabilization corresponds with neutralization of the particles' charge. Larger amounts of coagulants cause charge reversal so that the particles become positively charged and, thus, restabilization occurs, which results in elevated turbidity levels[35]. It has also been observed that the reduction in turbidity is associated with significant improvements in bacteriological quality. The effect of natural coagulants on turbidity removal and the antimicrobial properties against microorganisms may render them applicable for simultaneous coagulation and disinfection of water for rural and peri-urban people in developing countries[36].
It is observed that blended coagulants gave utmost efficiency as compared to the traditional alum coagulants. Here in this blending process, we reduce the alum dose up to 80%; thus, we reduce the drawbacks of the alum. Also, we can reduce the cost of the treatment using the natural coagulants instead of the traditional coagulant.
E. coli is the best coliform indicator of fecal contamination from human and animal wastes. E. coli presence is more representative of fecal pollution because it is present in higher numbers in fecal material and generally not elsewhere in the environment[37]. Results showed the absence of E. coli increases with increasing time. A greater percentage of E. coli was eliminated in higher turbidities. The aggregation and, thus, removal of E. coli was directly proportional to the concentration of particles in the suspension. Chitosan and other natural coagulants showed antibacterial effects of 2 to 4 log reductions.
Antimicrobial effects of water-insoluble chitin and coagulants were attributed to both its flocculation and bactericidal activities. A bridging mechanism has been reported for bacterial coagulation by chitosan[38]. Especially with reference to chitosan, molecules can stack on the microbial cell surface, thereby forming an impervious layer around the cell that blocks the channels, which are crucial for living cells[39]. On the other hand, cell reduction in microorganisms, such as E. coli, occurred without noticeable cell aggregation by chitosan.
This indicates that flocculation was not the only mechanism by which microbial reduction occurred. It was found that when samples were stored during 24 h, regrowth of E. coli was not observed for all turbidities. It should be noted that the test water contained no nutrient to support regrowth of E. coli, and chitosan is not a nutrient source for it. Another experiment was designed to check the effect of alum alone. Regrowth of E. coli was not observed for unaided alum after 24 h. The number of E. coli after resuspension of sediment reached to the initial numbers after 24 h and showed that it cannot be inactivated by alum. Such findings have been previously reported by Bina[40].
Conclusion
Access to clean and safe drinking water is difficult in rural areas of India. Water is generally available during the rainy season, but it is muddy and full of sediments. Because of a lack of purifying agents, communities drink water that is no doubt contaminated by sediment and human feces. Thus, the use of natural coagulants that are locally available in combination with solar radiation, which is abundant and inexhaustible, provides a solution to the need for clean and safe drinking water in the rural communities of India. Use of this technology can reduce poverty, decrease excess morbidity and mortality from waterborne diseases, and improve overall quality of life in rural areas.
The application of coagulation treatment using natural coagulants on surface water was examined in this study. The surface water was characterized by a high concentration of suspended particles with a high turbidity. At a varied range of pH, the suspended particles easily dissolved and settled along with the coagulants added. Research has been undertaken to evaluate the performance of natural starches of sago flour, bean powder, and chitin to act as coagulants individually and in blended form. In all three cases, the main variable was the dosage of the coagulant. The study shows that natural characteristics of starch and other coagulants can be an efficient coagulant for surface water but would need further study in modifying it to be efficient to the maximum. Thus, it can be concluded that the blended coagulants are the best which give maximum removal efficiency in minimum time.
It is chitin and chitosan which can readily be derivatized by utilizing the reactivity of the primary amino group and the primary and secondary hydroxyl groups to find applications in diversified areas. In this work, an attempt has been made to increase the understanding of the importance and effects of chitin at various doses and pH conditions, upon the chemical and biological properties of water. In view of this, this study will attract the attention of academicians and environmentalists.
Authors' information
SV is an assistant professor at the Department of Environmental Studies, GITAM Institute of Science, GITAM University, India.
References
Rosenberg DM, McCully P, Pringle CM: Global-scale environmental effects of hydrological alterations: introduction. BioScience 2000, 50: 746–751. 10.1641/0006-3568(2000)050[0746:GSEEOH]2.0.CO;2
Choi KJS, Kim G, Kim CW, Park JK: Removal efficiencies of endocrine disrupting chemicals by coagulation/flocculation, ozonation, powdered/granular activated carbon adsorption, and chlorination. Korean J. Chem. Eng 2006, 23: 399–408. 10.1007/BF02706741
Rook JJ: The formation of haloforms during chlorination of natural waters. Wat. Treatment & Exam 1974, 23: 234–243.
GrasieleScaramal M, GeovannaBordini S, AngélicaMarquetottiSalcedo V, Letícia N, Karina Cordeiro C, Rosângela B: Study of the effect of saline solution on the extraction of the Moringa oleifera seed's active component for water treatment. Water Air Soil Pollut 2010, 211: 409–415. 10.1007/s11270-009-0309-0
Mackenzie LD, Cornwell DA: Introduction to Environmental Engineering. 2nd edition. McGraw Hill, New York; 1991:157–163.
Fatoki O, Ogunfowokan A: Effect of coagulant treatment on the metal composition of raw water. Water SA 2002,28(3):293–298.
McLachlan DRC: Aluminum and the risk for Alzheimer's disease. Environmetrics 1995, 6: 233–275. 10.1002/env.3170060303
Ko YS, Lee YJ, Nam SH: Evaluation of a pilot scale dual media biological activated carbon process for drinking water. Korean J. Chem. Eng 2007, 24: 253–260. 10.1007/s11814-007-5038-8
Moon CH, Lee YJ, Ko YS, Nam SH: J. of KSSE. 2003, 25: 227.
Merlet N, Prevost M, Merlet Y, Coallier J: Enlevement de la matiere organique dans les filters CAB. Sciences de I'Eau 1991, 5: 143–164.
Rook JJ: Haloforms in drinking water. AWWA 1976, 68: 168–172.
Joret JC, Levi Y, Volk C: Biodegradable dissolved organic carbon (BDOC) content of drinking water and potential regrowth of bacteria. Water Sci Technol 1991, 24: 95–101.
Kawamura S: Effectiveness of natural polyelectrolytes in water treatment. Journal Awa, Japan 1991,79(6):88–91.
Singer PC, Chang SD: Correlations between trihalomethanes and total organic halides formed during water treatment. J. AWWA 1989, 81: 61–65.
Edzwald JK, Becker WC, Wattier KL: Surrogate parameters for Monitoring Organic Matter and THM precursors. J. AWWA 1985, 77: 122–132.
Reckhow DA, Singer PC, Malcolm RL: Chlorination of humic materials: byproduct formation and chemical interpretations. Environ Sci Technol 1990, 24: 1655–1664. 10.1021/es00081a005
Camp TR, Root DA: Effect of temperature on rate of floc formation. J. AWWA 1913, 1940: 32.
Kawamura SJ: AWWA. 1976, 70: 328.
Muyibi SA, Evison LM: Moringa oleifera seeds for softening hard water. Water Res 1995,29(4):1099–1105. 10.1016/0043-1354(94)00250-B
Driscoll CT, Letterman RD: Factors regulating residual aluminium concentrations in treated waters. Environmetrics 1995, 6: 287–309. 10.1002/env.3170060306
Taggart P: Starch as an ingredient: manufacture and applications. In Starch in Food: Structure, Function and Applications. Edited by: Eliasson AC. Woodhead Publishing Limited and CRC Press LLC, New York; 2004.
Dzulkefly K, See Yaw K, Anuar K, Atan S, AbdHalim A: Chemical modification of sago starch by solventless esterification with fatty acid chlorides. The Malaysian Journal of Analytical Sciences 2007,11(2):395–399.
American Public Health Association: Standard Methods for the Examination of Water and Wastewater. APHA, Washington, DC; 2005.
Lin SD, Evans RL, Beuscher DB: Algal Removal by Alum Coagulation. Illinois State Water Survey Division: Urbana. Report of Investigation 68. Illinois State Water Survey, Illinois; 1971:1–19.
Ebeling JM, Sibrell P, Ogden S, Summerfelt S: Evaluation of chemical coagulation–flocculation aids for the removal of suspended solids and phosphorus from intensive recirculating aquaculture effluent discharge. AquacEng 2003, 29: 23–42.
Divakaran R, Pillai VN: Flocculation of river silt using chitosan. Water Res 2002, 36: 2414–2418. 10.1016/S0043-1354(01)00436-5
Jose Rene R-M, Vladimir Alonso Escobar B, Jose Luis D-R: Chitin based biocomposites for removal of contaminants from water: a case study of fluoride adsorption. In Biopolymers. Edited by: Elnashar M. Sciyo, Croatia; 2010:163–180.
Ravi Kumar MNV: A review of chitin and chitosan applications. React Funct Polym 2000,46(1):1–27. 10.1016/S1381-5148(00)00038-9
An HK, Park BY, Kim DS: Crab shell for the removal of heavy metals from aqueous solution. Water Res 2001,35(15):3551–3556. 10.1016/S0043-1354(01)00099-9
Bratskaya S, Schwarz S, Liebert T, Heinze T: Starch derivatives of high degree of functionalization 10. Flocculation of kaolin dispersions. Colloids and Surfaces. A. Physicochemical and Engineering Aspects 2005, 254: 75–80. 10.1016/j.colsurfa.2004.11.030
FatehahMohd O, NikNorulainiNik Abdul R, Anees A: COD reduction in semiconductor wastewater by natural and commercialized coagulants using response surface methodology. Water Air Soil Pollut 2008, 195: 345–352. 10.1007/s11270-008-9751-7
Qudsieh IY, Fakhru'l-Razi A, Kabbashi NA, Mirghani MES, Fandi KG, Alam MZ, Muyibi SA, Nasef MM: Preparation and characterization of a new coagulant based on the sago starch biopolymer and its application in water turbidity removal. J Appl Polym Sci 2008,109(5):3140–3147. 10.1002/app.28399
Gassenschmidt U, Jany KD, Tauscher B, Niebergall H: Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochem Biophys Acta 1995,1243(3):477–481. 10.1016/0304-4165(94)00176-X
Tandon P, Chhibber S, Reed RH: Inactivation of Escherichia coli and coliform bacteria in traditional brass and earthenware water storage vessels. Ant van Leeuw 2005, 88: 35–48. 10.1007/s10482-004-7366-6
Ghebremichael KA, Gunaratna KR, Henriksson H, Brumer H, Dalhammar G: A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res 2005,39(11):2338–2344. 10.1016/j.watres.2005.04.012
Özacar M, Sengil A: Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles. Colloids and Surfaces A: Physicochem Eng Aspects 2003,229(1–3):85–96.
Tyagi VK, Chopra AK, Kazmi AA, Kumar A: Alternative microbial indicators of faecalpollution:current perspective. Iran. J. Environ. Health. Sci. Eng 2006,3(3):205–216.
Roussy J, Van Vooren M, Dempsey B, Guibal E: Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res 2005, 39: 3247–3258. 10.1016/j.watres.2005.05.039
Qin C, Li H, Xiao Q, Liu Y, Zhu J, Du Y: Water-solubility of chitosan and its antimicrobial activity. Carbohydr Polym 2006, 63: 367–374. 10.1016/j.carbpol.2005.09.023
Bina B: The use of Moringa oleifera seed as natural plant coagulant in removal of clay particles and E.coli. Water & Sewage 1995, 14: 4–12.
Acknowledgments
The author thanks GITAM University for providing necessary facilities for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that she has no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vara, S. Screening and evaluation of innate coagulants for water treatment: a sustainable approach. Int J Energy Environ Eng 3, 29 (2012). https://doi.org/10.1186/2251-6832-3-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-3-29