Abstract
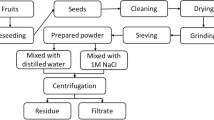
Several coagulants/flocculants have been studied in order to remove the color and turbidity of raw water, employing natural ones demonstrated advantages in relation to chemicals. Moringa oleifera Lam is a natural polymer that has been gaining prominence in water treatment. It acts as a clarifying agent, providing a cationic protein that destabilizes the particles contained in a liquid medium. The main objective of the present work is to study the efficiency in terms of removing color and turbidity of raw water in order to obtain drinking water. For this purpose, different coagulant solutions were obtained utilizing three solutions of KCl in different concentrations (0.01, 0.1, and 1 M) and pure water combined with M. oleifera Lam seed. Each coagulant solution obtained was studied with concentrations ranging from 50 to 600 ppm of Moringa in solution. The pH was varied (4.0, 6.0, and 8.0) with 25% and 50% sodium hydroxide solution (NaOH) and hydrochloric acid (HCl), respectively. The tests were conducted with the “Jar Test Device” and the efficiency of the process was evaluated regarding the reduction of color and turbidity. The best results were found employing the coagulant solutions extracted with 1 M salt solution, pH 8.0, and different concentrations of coagulant solution. It is important to explain that the best results were in various concentration ranges, as the concentration of protein in solution becomes higher, the greater is its power as a coagulant. The lowest content of protein was found in the solution extracted with water, which consequently had the lowest values of color and turbidity removal.






Similar content being viewed by others
References
Amagloh, F. K., & Benang, A. (2009). Effectiveness of Moringa oleifera seed as coagulant for water purification. African Journal of Agricultural Research, 4(1), 119–123.
Akhtar, M., Moosa Hasany, S., Bhanger, M. I., & Iqbal, S. (2007). Sorption potential of Moringa oleifera pods for the removal of organic pollutants from aqueous solutions. Journal of Hazardous Materials, 141(3), 546–556. doi:10.1016/j.jhazmat.2006.07.016.
APHA—American Public Health Association. (1995). Standard methods for the examination for water and wastewater, 19th ed. Washington, D.C.
Bhatia, S., Othman, Z., & Ahmad, A. L. (2007). Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. Journal of Hazardous Materials, 145(1–2), 120–126. doi:10.1016/j.jhazmat.2006.11.003.
Driscoll, C. T., & Letterman, R. D. (1995). Factors regulating residual aluminium concentrations in treated waters. Environmetrics, 6, 287–309.
Ghebremichael, K. A., Gunaratna, K. R., Henriksson, H., Brumer, H., & Dalhammar, G. (2005). A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Research, 39, 2338–2344. doi:10.1016/j.watres.2005.04.012.
Katayon, S., Noor, M. J. M. M., Asma, M., Ghani, L. A. A., Thamer, A. M., & Azni, I. (2006). Effects of storage conditions of Moringa oleifera seeds on its performance in coagulation. Bioresource Technology, 97(13), 1455–1460. doi:10.1016/j.biortech.2005.07.031.
Kawamura, S. (1991). Effectiveness of natural polyelectrolytes in water treatment. Journal Awa, Japan, 79(6), 88–91.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265.
McLachlan, D. R. C. (1995). Aluminum and the risk for Alzheimer's disease. Environmetrics, 6, 233–275.
Muyibi, S. A., & Evison, L. M. (1995). Moringa oleifera seeds for softening hardwater. Water Research, 29(4), 1099–1105.
Ndabigengesere, A., & Narasiah, K. S. (1998). Quality of water treated by coagulation using Moringa oleifera seeds. Water Research, 32(3), 781–791.
Nkurunziza, T., Nduwayezu, J. B., Banadda, E. N., & Nhapi, I. (2009). The effect of turbidity levels and Moringa oleifera concentration on the effectiveness of coagulation in water treatment. Water Science & Technology, 59, 1551–1558.
Okuda, T., Baes, A. U., Nishijima, W., & Okada, M. (1999). Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Research, 33(15), 3373–3378. doi:10.1016/S0043-1354/(99)00046-9.
Okuda, T., Baes, A. U., Nishijima, W., & Okada, M. (2001). Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Research, 35(3), 830–834. doi:10.1016/S0043-1354(00)00296-7.
Vieira, A. M. S., Vieira, M. F., Silva, G. F., Araújo, A. A., Fagundes-Klen, M. R. Veit, M. T., et al. (2009). Use of Moringa oleifera seed as a natural adsorbent for wastewater treatment. Water, Air, Soil Pollut, published on line. doi: 10.1007/s11270-009-0104-y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madrona, G.S., Serpelloni, G.B., Salcedo Vieira, A.M. et al. Study of the Effect of Saline Solution on the Extraction of the Moringa oleifera Seed’s Active Component for Water Treatment. Water Air Soil Pollut 211, 409–415 (2010). https://doi.org/10.1007/s11270-009-0309-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0309-0