Abstract
In this article, Ru(4,4′-dicarboxy-2,2′-bipyridine)2(NCS)2 dye (N3) and some derivatives were investigated using Density Functional Theory (DFT) calculations in solution to elucidate the influence of the environment and substituted groups on electronic properties. Full geometry optimization and investigation of electronic properties of N3 dye and some derivatives were performed using DFT and HF calculations. The singlet ground state geometries were fully optimized at the B3LYP/3-21G** level of theory through the Gaussian 98 program. Based on the computed results, the optoelectronic properties are sensitive to chemical solvent environments. Moreover, the properties of anatase cluster (TiO2) models have been investigated, and N3 dyes have been adsorbed on TiO2 nano-particle with diprotonated states. The modified N3 dyes highly affected the electronic structure. This leads to significant changes in the adsorption spectra as compared to the N3 dyes. Through hybrid methods, the properties of interfacial electronic coupling of the combined system were estimated. The results of some combined systems showed that the electronic coupling, lowest lowest unoccupied molecular orbitals, and the TiO2 conduction band resided in the visible region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
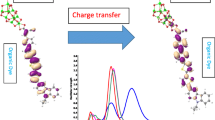
Photo-induced phenomenon, based on photon absorption with enough energy, causes the excitation of electrons and then the current generation. Electron transfer (ET) at organic/inorganic interfaces plays a key role in a variety of applications including photocatalysis, photoelectrolysis, and solar cell and nano-scale electronics [1–5]. The dye-sensitized nano-crystalline solar cell, or Grätzel cell, is a popular alternative in comparison to the costly traditional solar cell [3, 6]. The attached chromophore dye with tuned desired wavelength in a well-known Grätzel cell should absorb photons and generate electrons within dye-sensitized titanium dioxide nano-particles [7–10]. Quantum chemical calculations can provide information about geometrical and electronic structure in dye-sensitized solar cells (DSSCs) [11]. The N3 dye, as shown in Figure 1, is one conventional dye which can show optical adsorption spectra [12] related to the size and conjugation of linkers to the surface at semiconductor TiO2. Surface electron transfer accompanying the initial light absorption and excitation of ruthenium dyes often leads to efficient photo-induced charge separation across the dye and TiO2 interfaces.
TiO2 can be formed in several phases such as anatase, rutile and brookite. There are some properties of TiO2 nano-crystals such as electro-opticality, low cost, chemical stability, non-toxicity, abundance, availability, and lack of erosion and corrosion against light which are common in the literature [13]. The anatase crystal phase of TiO2 has an octahedral structure with wide gap energy, photo-induced activity, large surface area-to-bulk ratio, and can absorb UV light more than other phases. Due to the lack of anatase stability in its structure and a high degree of dangling bonds, calculation of structural and electronic properties of the large number of under coordinated atom is complicated. However, the size and complexity of TiO2 nano-particles impose challenges for choosing the methods. Several titanium oxide nano-crystals have been investigated in some studies [14–19]. The calculation for anatase (TiO2)5 and (TiO2)16 clusters has been performed by DFT, but because of computational demand for large systems, the semi-empirical methods have been used. The (TiO2)54 cluster has large surface and has more tendency toward sphericity, and it is selected as the basis for computing absorption dyes and its derivatives.
The Ru(4,4′-dicarboxy-2,2′-bipyridine)2(NCS)2 or ‘N3’ dye and its derivatives are probably the most well known sensitized dyes which transfer electron by adsorbing sunlight followed by electron injection to the conduction band of TiO2, so the gap energy will be increased [20, 21]. The electron transfer depends on different parameters, for example, the type of attachment such as bridge, chelating, mono-dentate which are shown in Figure 2[22, 23]. The dye is adsorbed in a prototype binding of the two carboxylic acid anchor groups of one of the bipyridine ligands in a bridging bi-dentate adsorption mode. Figure 2 shows the types of attachment anchor groups on the adsorbent layer [4].
The types of attaching anchor groups on the adsorbent layer [4].
The purpose of this paper is to investigate the role of acceptor and donor groups on N3 dyes and to understand the electron transfer phenomena, variation of gap energy, and the geometrical and electronic coupling among the parts in the combined system, (TiO2)54-N3 derivatives.
Methods
Through Density Function Theory (DFT), the full geometry optimization and investigation of the electronic properties of N3 dye and some derivatives were performed [11]. The results were obtained by DFT and supported by Hartree-Fock (HF) calculations. The singlet ground state geometries were fully optimized at the B3LYP/3-21G** level of theory. All calculations were performed with Gaussian 98 program (Gaussian, Inc., Wallingford, CT, USA) [24].
The variation of gap energies was investigated by inserting electron donor groups such as CH3, C2H5, NH2, N(CH3)2, or electron accepter groups such as F and Cl on the α position related to carboxylate on N3 dye as shown in Figure 1. To compare of the results, all calculations were done first in gas phase and then in solvent phase. In this study, PCM model of ethanol (dielectric constant, ϵ = 25) and acetonitrile (ϵ = 37) was used.
Since the DFT calculation of the large clusters is very costly, the (TiO2)54 nano-crystals have been optimized by MSINDO semi-empirical method [3]. Further, we used the integrate B3LYP:ZINDO1 approach for the combined dyes and (TiO2)54 cluster systems and some of the dye derivates.
To evaluate the accuracy of the proposed hybrid method results, the combined system of N3 and (TiO2)54 has been optimized via the DFT method by a 16-core supercomputer. The duration of the each calculation was about 2 months.
Results and discussion
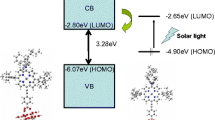
The computational results for the N3 dyes and some derivatives which were performed by B3LYP method and 3-21G** basis set in both gas and solvent phases are presented in Table 1. A visual comparison of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of N3 and some of its derivatives in the gas, acetonitrile, and ethanol phases shows an increasing and obvious shifting in absolute energy and reveal a clear difference in the energetic ordering of the frontier orbitals in different environments.
The results show that the substituted groups do not have an important role in transferring the absorption wavelength from UV to visible region in the gas phase. Moreover, the groups have a few influences on the decrease of N3 gap energy.
The energy silently decreases in solvent environment, so the difference between gas and solvent environment is about 0.5 eV for all groups. The solvent effect on the gap energy variation occurs through the interaction of the dye and ethanol or acetonitrile molecules. It has a significant influence on the optical properties of these complexes by the environment, so the wavelength of N3 complexes is shifted from 830 to 580 nm by inclusion of the solvent. The range of variation of optoelectronic properties is taken into account under a model for complexes with conjugated properties whether or not the theoretical modeling is capable of providing a physically realistic description of the complexes with extended conjugation.
Using the integrate B3LYP:ZINDO1 approach, the results of the combined systems of N3 dyes and some of the derivatives with (TiO2)54 cluster are summarized in Table 2. The table shows that some of the absorption wavelengths of a combined system are located in the visible area. Furthermore, the computed results by the mentioned methods for heterogeneous super molecule are given in Table 3. However, the gap energy almost differs between ONIOM and DFT; ONIOM results are in agreement with our expectation.
Conclusion
To significantly develop DSSCs and related photo-electrochemical devices, the quantum chemical calculations were performed to provide a better theoretical understanding of the basic physical and chemical processes of dye-sensitized semiconductors. As shown before, the modified N3 dyes have highly affected the electronic structure. This leads to significant changes in the absorption spectra as compared to the N3 dyes. The optical absorption spectra are related to the environment, but not probably related to the type of X-substituted groups. The results of the combination system showed that the electronic coupling of the lowest dye LUMOs and the TiO2 conduction band is negligible. Moreover, the lowest LUMO dye and TiO2 band conduction, using hybrid method and the electronic structure, agree with the experiment evidence. Finally, the absorption wavelength of some substituted dyes of combined system is located in the visible area.
References
Ferrere S, Gregg BA: Large increases in photocurrents and solar conversion efficiencies by UV illumination of dye sensitized solar cells. J. Phys. Chem. B 2001, 105: 7602–7605. 10.1021/jp011612o
Derosa PA, Seminario JM: Electron transport through single molecules: scattering treatment using density functional and Green function theories. J. Phys. Chem. B 2001, 105: 471–481.
O'Regan B, Grätzel M: A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO 2 films. Nature 1991, 353: 737–740. 10.1038/353737a0
Pan J, Benko G, Xu YH, Pascher T, Sun LC, Sundstrom V, Polivka T: Photoinduced electron transfer between a carotenoid and TiO 2 nanoparticle. J. Am. Chem. Soc 2002, 124: 13949–13957. 10.1021/ja0279186
Biju V, Micic M, Hu DH, Lu HP: Intermittent single-molecule interfacial electron transfer dynamics. J. Am. Chem. Soc 2004, 126: 9374–9381. 10.1021/ja040057b
McConnell RD: Assessment of the dye-sensitized solar cell. Renew. Sustain. Energy Rev 2002, 6: 273–295.
Anderson NA, Lian TQ: Ultrafast electron transfer at the molecule-semiconductor nanoparticle interface. Ann. Rev. Phys. Chem 2005, 56: 491–519. 10.1146/annurev.physchem.55.091602.094347
Rego LGC, Batista VS: Quantum dynamics simulations of interfacial electron transfer in sensitized TiO 2 semiconductors. J. Am. Chem. Soc 2003, 125: 7989–7997. 10.1021/ja0346330
Stier W, Duncan WR, Prezhdo OV: Ab initio molecular dynamics of ultrafast electron injection from molecular donors to the TiO 2 acceptor. SPIE Proc 2003, 5223: 132–146. 10.1117/12.503646
Duncan WR, Stier WM, Prezhdo OV: Ab initio nonadiabatic molecular dynamics of the ultrafast electron injection across the alizarin-TiO2 interface. J. Am. Chem. Soc 2005, 127: 7941–7951. 10.1021/ja042156v
Lundqvist MJ: Quantum chemical modeling of dye-sensitized titanium dioxide. Uppsala University, Sweden; 2006. PhD thesis PhD thesis
Aghtar A: The making of the dye-sensitized solar cell with TiO2 nanoparticles for use in the photocatalytic oxidation–reduction reactions. Payame Noor University (PNU), Shiraz, Iran; 2009. MSc dissertation MSc dissertation
Diebold U: Structure and properties of TiO 2 surfaces: a brief review. Appl. Phys. A 2003, 76: 681–687. 10.1007/s00339-002-2004-5
Nilsing M, Lunell S, Persson P, Ojama L: Phosphonic acid adsorption at the TiO 2 anatase (101) surface investigated by periodic hybrid HF-DFT computations. Surf. Sci 2005, 582: 49–60. 10.1016/j.susc.2005.02.044
Homann T, Bredow T, Jug K: Adsorption of small molecules on the anatase (100) surface. Surf. Sci 2004, 555: 135–144. 10.1016/j.susc.2003.12.039
Vittadini A, Selloni A, Rotzinger FP, Gratzel M: Formic acid adsorption on dry and hydrated TiO 2 anatase (101) surfaces by DFT calculations. J. Phys. Chem. B 2000, 104: 1300–1306. 10.1021/jp993583b
Nilsing M, Persson P, Ojamae L: Anchor group influence on molecule-metal oxide interfaces: periodic hybrid DFT study of pyridine bound to TiO 2 via carboxylic and phosphonic acid. Chem. Phys. Lett 2005, 415: 375–380. 10.1016/j.cplett.2005.08.154
Rappoport D, Crawford NRM, Furche F, Burke K: Approximate density functionals: which should I choose? In Computational Inorganic and Bioinorganic Chemistry. Edited by: Solomon EI, King RB, Scott RA. Wiley, Chichester; 2009:159–172.
Nazeeruddin MK, De Angelis F, Fantacci S, Selloni A, Viscardi G, Liska P, Ito S, Takeru B, Gratzel M: Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc 2005, 127: 16835–16847. 10.1021/ja052467l
Wahab HS, Bredow T, Aliwi SM: Computational modeling of the adsorption and photodegradation of 4-chlorophenol on anatase TiO 2 particles. J. Mol. Struct. Theochem 2008, 863: 84–90. 10.1016/j.theochem.2008.05.019
Lundqvist MJ, Nilsing M, Persson P, Lunell S: DFT Modeling of bare and dye-sensitized TiO 2 nanocrystals. Int. J. Quantum Chem 2006, 106: 3214–3234. 10.1002/qua.21088
Lundqvist MJ, Galoppini E, Meyer GJ, Persson P: Calculated optoelectronic properties of ruthenium tris-bipyridine dyes containing oligophenyleneethynylene rigid rod linkers in different chemical environments. J. Phys. Chem. A 2007, 111: 1487–1497. 10.1021/jp064219x
Iozzi MF: Theoretical models of the interaction between organic molecules and semiconductor surfaces. Napoli University, Italy; 2006. PhD thesis PhD thesis
Frisch MJ, Trucks GW, Schlegel HB, Gill PMW, Johnson BG, Robb MA, Cheeseman JR, Keith T, Petersson GA, Montgomery JA, Raghavachari K, Al-Laham MA, Zakrzewski VG, Ortiz JV, Foresman JB, Cioslowski J, Stefanov BB, Nanayakkara A, Challacombe M, Peng CY, Ayala PY, Chen W, Wong MW, Andres JL, Replogle ES, Gomperts R, Martin RL, Fox DJ, Binkley JS, Defrees DJ: Gaussian 98: revision D.2. Gaussian Inc, Pittsburgh; 1999.
Acknowledgment
The authors greatly appreciate Dr. HS Wahab from the Baghdad University of Technology for his instructions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MO participated in the design and the sequence alignment of the study. LT carried out the jobs by Gaussian and participated in the evaluation of the data and in drafting the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oftadeh, M., Tavakolizadeh, L. Investigation of optoelectronic properties of N3 dye-sensitized TiO2 nano-crystals by hybrid methods: ONIOM (QM/MM) calculations. Int Nano Lett 3, 26 (2013). https://doi.org/10.1186/2228-5326-3-26
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-26