Abstract
Background
The aim of this study was to explore if positive-pressure ventilation delivered by a conventional ICU ventilator at a moderately high frequency (HFPPV) allows a safe reduction of tidal volume (VT) below 6 mL/kg in a porcine model of severe acute respiratory distress syndrome (ARDS) and at a lower mean airway pressure than high-frequency oscillatory ventilation (HFOV).
Methods
This is a prospective study. In eight pigs (median weight 34 [29,36] kg), ARDS was induced by pulmonary lavage and injurious ventilation. The animals were ventilated with a randomized sequence of respiratory rates: 30, 60, 90, 120, 150, followed by HFOV at 5 Hz. At each step, VT was adjusted to allow partial pressure of arterial carbon dioxide (PaCO2) to stabilize between 57 and 63 mmHg. Data are shown as median [P25th,P75th].
Results
After lung injury, the PaO2/FiO2 (P/F) ratio was 92 [63,118] mmHg, pulmonary shunt 26 [17,31]%, and static compliance 11 [8,14] mL/cmH2O. Positive end-expiratory pressure (PEEP) was 14 [10,17] cmH2O. At 30 breaths/min, VT was higher than 6 (7.5 [6.8,10.2]) mL/kg, but at all higher frequencies, VT could be reduced and PaCO2 maintained, leading to reductions in plateau pressures and driving pressures. For frequencies of 60 to 150/min, VT progressively fell from 5.2 [5.1,5.9] to 3.8 [3.7,4.2] mL/kg (p < 0.001). There were no detrimental effects in terms of lung mechanics, auto-PEEP generation, hemodynamics, or gas exchange. Mean airway pressure was maintained constant and was increased only during HFOV.
Conclusions
During protective mechanical ventilation, HFPPV delivered by a conventional ventilator in a severe ARDS swine model safely allows further tidal volume reductions. This strategy also allowed decreasing airway pressures while maintaining stable PaCO2 levels.
Similar content being viewed by others
Background
Acute respiratory distress syndrome (ARDS) is a common cause of mortality and morbidity in critically ill patients [1]. Although indispensable in the support of ARDS patients, artificial ventilation involves the application of mechanical forces to the lung parenchyma that can further induce injury [2], adding morbidity and mortality [3].
Reducing tidal volumes (VTs) below 6 mL/kg of ideal body weight could potentially decrease the cyclic stretch imposed on the lung [4, 5]. Conversely, excessively low VTs have the potential to lead to clinically significant hypercapnia-related acidosis [6] with harmful side effects [7, 8].
In this scenario, high-frequency oscillatory ventilation (HFOV) has been tested, because of its ability to provide adequate gas exchange even at very low tidal volumes [9–13]. This technique, however, may be cumbersome because it requires a dedicated ventilator and special training. Additionally, the VT delivered can be susceptible to variations in airway resistance such as that which occurs with lung secretions [14]. Last but not least, it requires the use of high airway pressures, which may have deleterious effects, especially on the right ventricle [15]. Recently, two clinical studies in ARDS patients showed neutral [16] or disappointing [17] results in terms of mortality when HFOV was compared to a conventional mechanical ventilation strategy.
An alternative approach could be to apply moderately high frequency positive-pressure ventilation (HFPPV) using conventional mechanical ventilators. A similar strategy was explored in the 1980s [18, 19], but with special ventilators and before the well-established recognition of the importance of lung-protective strategies. HFPPV consists of applying respiratory rates intermediate between those used conventionally (≤ 35 breaths/min) and those used during HFOV (180 to 800 breaths/min). Potential advantages over HFOV would be the possibility to control the VT delivered, the use of conventional mechanical ventilators obviating the need for specialized training, and maintenance of a low mean airway pressure. In this feasibility study, we tested in a swine model of ARDS whether such a strategy could result in VT below 6 mL/kg while avoiding further increases in the partial pressure of arterial carbon dioxide (PaCO2) and maintaining a reasonable mean airway pressure (Pmean).
Methods
This study was approved by the Institutional Animal Research Ethics Committees of Hospital Sírio Libanês and of Faculdade de Medicina da Universidade de São Paulo, both in São Paulo, Brazil, and was performed according to the National Institutes of Health (USA) guidelines for the use of experimental animals. The experiments were done in eight previously healthy Agroceres pigs.
Instrumentation
The animals were fasted overnight before the experiment with free access to water. They received an intramuscular injection of midazolam (0.3 mg/kg; Dormonid®, Roche, São Paulo, Brazil) and acepromazine (0.5 mg/kg; Acepran®, Andrômaco, São Paulo, Brazil). Through an auricular vein, anesthesia was induced with thionembutal (12 mg/kg; Tiopental®, Abbott, São Paulo, Brazil) and muscular relaxation with pancuronium bromide (0.1 mg/kg; Pavulon®, AKZO Nobel, São Paulo, Brazil). They were then submitted to tracheal intubation (cuffed 7.5-French cannula) and connected to the Servo-300 mechanical ventilator (Maquet, Rastatt, Germany) with the following parameters in a volume-controlled mode: tidal volume of 8 to 10 mL/kg, positive end-expiratory pressure (PEEP) of 5 cmH2O, inspiratory fraction of oxygen (FiO2) adjusted to keep arterial saturation between 94% and 96%, and respiratory rate (RR) necessary to keep PaCO2 between 35 and 45 mmHg. Anesthesia was maintained during the study period with midazolam (0.3 mg/kg/h) and fentanyl citrate (5 μg/kg/h; Fentanyl®, Janssen-Cilag, São Paulo, Brazil) and muscular relaxation with pancuronium bromide (0.2 mg/kg/h). The adequate depth of anesthesia during the surgical period was evaluated with maintenance of physiological variables (heart rate and arterial pressure) and absence of reflexes (corneal and hind limb flexion response), as well as unresponsiveness to stimuli during manipulation. Supplementary boluses of 3 to 5 μg/kg of fentanyl and 0.1 to 0.5 mg/kg of midazolam were administered as necessary. A continuous drip of 1,000 mL/h of Lactated Ringer’s solution was infused until the end of the induction of pulmonary injury, and then a continuous infusion of 5 mL/kg/h of Lactated Ringer was maintained until the end of the study.
Monitoring with continuous electrocardiography, oxymetry, and blood pressures was done with a multiparametric monitor (Dixtal-Philips DX 2020, São Paulo, Brazil). The left femoral artery was cannulated for blood pressure monitoring and blood sampling. The right internal jugular vein was cannulated with a 9-French introducer sheath (Arrow, Reading, PA, USA) through which a pulmonary artery catheter (Edwards Lifesciences, Irvine, CA, USA) was introduced for monitoring of the mean pulmonary artery pressure (PAPm), cardiac output, central venous pressure (CVP), and mixed venous blood gases (SvO2). A central venous catheter was introduced in the left internal jugular vein. A surgical cystostomy was done to quantify the urine output. The animal was connected to the NICO2 device (Novametrix Medical Systems, Wallingford, CT, USA) for airway end-tidal pressure of carbon dioxide (EtCO2), tidal volume, airway pressures, and airway flow monitoring.
The regional ventilation was monitored with electrical impedance tomography (EIT; Dixtal-Philips, São Paulo, Brazil) [20, 21]. The lungs were split in sternal and vertebral regions of the same height. The amount of ventilation to the regions studied was reported according to the ventilator settings used. Arterial blood gas analyses were done with the ABL 800 device (Radiometer, Copenhagen, Denmark). After the surgical period, the animals were allowed to rest for 60 min prior to the baseline data acquisition.
Measurements
In all the steps of the study, the following data were collected:
-
1.
Hemodynamic: heart rate, cardiac output, CVP, mean systemic arterial blood pressure (ABPm), PAPm, pulmonary artery occlusion pressure (PAOP), SvO2, and norepinephrine use and dosage
-
2.
Respiratory: arterial partial pressure of oxygen (PaO2), PaCO2, EtCO2, V T, airway peak pressure (P peak), airway plateau pressure (P plateau) through expiratory valve occlusion after 2 s of inspiratory pause, intrinsic positive end-expiratory pressure (PEEPi) through expiratory valve occlusion after 4 s of expiratory pause, extrinsic positive end-expiratory pressure (PEEPe), mean airway pressure (P mean), inspiratory flow, inspiratory time (T insp), and ventilatory distribution EIT data
-
3.
Metabolic: pH, lactate, temperature, and fluid balance
Calculated variables
To obtain the calculated variables, we used the following formulas:
● Cardiac index (CI) = Cardiac output/Weight
● Systemic vascular resistance index = (ABPm − CVP) × 80/CI
● Pulmonary vascular resistance index = (PAPm − PAOP) × 80/CI
● Blood oxygen content (C × O2) = P × O2 × 0.0031 + 1.36 × Hb × S × O2
● Minute ventilation = VT × RR
● PEEPtotal = PEEPi + PEEPe
● Alveolar oxygen partial pressure (PAO2) = 643 × FiO2/100 − (PaCO2/0.8)
● Alveolar-arterial oxygen [(A-a)O2] gradient = PAO2 − PaO2
● Pulmonary capillary oxygen content (CcO2) = PAO2 × 0.0031 + 1.36 × Hb
● Pulmonary shunt = (CcO2 − CaO2) × 100/(CcO2 − CvO2)
● Static compliance (Cstatic) = VT/(Pplateau − PEEPtotal)
● Dynamic compliance (Cdyn) = VT/(Ppeak − PEEPtotal)
● Resistance (Ppeak − Pplateau)/Inspiratory flow
ARDS induction
After the baseline data collection, ARDS was induced with repeated whole-lung lavage using 1 L of isotonic saline (37°C) until the PaO2 was below 100 mmHg for at least 10 min. Lung injurious ventilation was then started with the animal ventilated in pressure control mode with PEEP = 3 cmH2O, FiO2 = 1, inspiratory/expiratory time ratio (I/E) = 1:1, Ppeak = 42 cmH2O, and a RR of 20 to 30 breaths/min [22]. Arterial blood gases were obtained every 15 min, and the PEEP could be increased up to 19 cmH2O targeting a PaO2 level between 55 and 80 mmHg, whereas the inspiratory pressure was limited at 48 cmH2O. The injurious ventilation was maintained until one of the following parameters was reached:
● An interval of 240 min of injurious ventilation
● A PAPm > 50 mmHg
● A Cstatic < 10 mL/cmH2O (with a PEEP = 10 cmH2O and VT = 6 mL/kg)
● A PEEP persistently ≥ 15 cmH2O for at least two consecutive arterial blood sample analyses
● An ABPm < 70 mmHg in spite of the use of norepinephrine in a dosage higher than 0.5 μg/kg/min
After lung injury induction, the stabilization step started. The animal was ventilated according to the recommendations of the interventional group in the "ARMA" study [5] in a volume-controlled mode, with VT = 6 mL/kg, RR = 35 breaths/min (the maximal respiratory rate allowed by the protocol - because of hypercapnia and acidosis), initial PEEP = 10 cmH2O (mean value used in the ARMA study), and FiO2 = 1.
An arterial blood sample was obtained every 10 min. Subsequently, PEEP and FiO2 were titrated according to the ARMA study PEEP table (aiming at a PaO2 = 55 to 80 mmHg) [5]. VT and RR were kept constant during the stabilization step with no attempt to correct the PaCO2 level.
Experimental protocol
After reaching a PaCO2 equilibrium (variation < 5% in three consecutive arterial blood samples), we considered that the stabilization phase was finished. The same PEEP level titrated at this time was used in the following steps of the study.
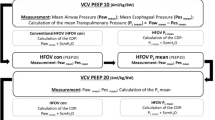
Four sequences of five different RRs were randomly tested, and these sequences were chosen to alternate higher and lower respiratory frequencies (Figure 1). The five RRs (ventilatory modes) randomized were as follows: RR = 30, 60, 90, 120, 150 breaths/min. At each sequence, VT was adjusted to reach a PaCO2 target of 57 to 63 mmHg.
Timeline of the study. PEEPT, total end-expiratory positive pressure (intrinsic end-expiratory positive pressure plus extrinsic end-expiratory positive pressure should be the same as the end-expiratory positive pressure titrated following the ARMA PEEP table); VT, tidal volume; FiO2, inspiratory fraction of oxygen; PaCO2, partial arterial carbon dioxide pressure.
The randomization was done using sealed envelopes containing the proportion of 1:1:1:1 of the following RR sequences:
● Sequence 1 (60, 150, 90, 120, 30)
● Sequence 2 (90, 30, 120, 60, 150)
● Sequence 3 (120, 150, 90, 30, 60)
● Sequence 4 (150, 90, 30, 120, 60)
During this part of the protocol, the animals were ventilated using volume control ventilation, with FiO2 = 1 and square inspiratory flow = 1 L/s. At each step, PEEPi was measured every 10 min, and PEEPe was corrected in order to keep the PEEPtotal equal to the PEEP obtained during the equilibrium step using the ARMA PEEP table.
After completion of these randomized sequences, the animals were submitted to HFOV (Figure 1) at 5 Hz (SensorMedics 3100B, Yorba Linda, CA, USA) with a Pmean set at 30 cmH2O, I/E = 1:2, bias flow = 30 L/min, and the initial pressure amplitude = 80 cmH2O [9]. Unlike the other five RRs, of which the sequence was randomized, HFOV was always performed last, because of its higher Pmean, which could induce lung recruitment.
An arterial blood sample was obtained each 10 min throughout the remainder of the study. After VT changes or after pressure amplitude changes during HFOV, we waited until there were three consecutive measurements with the PaCO2 levels stable between 57 and 63 mmHg. Data were then collected, and the next step of the sequence was started. Between consecutive steps, a 40-s disconnection from the ventilator was done in order to avoid the carryover of the time-dependent alveolar recruitment. At the end of the experiments, the anesthesia was deepened with propofol overdose, and the animals were euthanized with a bolus of 10 mL of potassium chloride 19.1%.
Statistical analysis
The Shapiro-Wilk goodness-of-fit model showed a non-parametrical distribution for most variables; therefore, data are reported as median [P25th,P75th]. Wilcoxon’s signed rank test was used to test variables before and after lung injury induction and to compare the upper and lower regional ventilation with the EIT. In order to avoid type I error, a modified Bonferroni’s correction was used to account for the multiple comparisons between upper and lower regions of ventilation. Therefore, the p value considered significant was 0.007 when comparing upper and lower regions during the various frequencies studied and 0.012 when comparing the effects of inspiratory pauses and the alveolar recruitment with a RR of 60 breaths/min. The analysis of variance for repeated measures on ranks (Friedman’s test) was used for analyses during the ventilatory modes tested. The post hoc analyses were done using Student-Newman-Keuls’ test. A p < 0.05 was considered significant. The analyses and graphs were done with the SigmaPlot 12.0 statistical package software (Systat Software, Inc. San Jose, CA, USA).
Results
Eight pigs weighing 34 [29,39] kg were used. ARDS was induced using 10 [7,16] L of normal saline followed by injurious mechanical ventilation for 210 [40,225] min. The respiratory variables at baseline and after the induction of lung injury are shown in Table 1.
The FIO2 during the stabilization step was 0.7 [0.5,0.9]. The time to PaCO2 equilibrium was similar in the different phases of the experiment and equal to 50 [40,75] min. The most important respiratory data with different RRs are shown in Figure 2 and Table 2. During the stabilization step, PaCO2 was 81 [78,92] mmHg. In all other experimental phases, the PaCO2 was kept in the planned range of 57 to 63 mmHg (Figure 2A). VT could be progressively reduced with increasing RRs (Figure 2B), as did regional ventilation (Figure 3). The ventilation to the dependent parts of the lung reduced to a greater extent leading to an increase in the sternal/vertebral ratio of regional ventilation (Figure 4). Additionally, low values of plateau and driving pressures were maintained at all RRs (Figure 2C,D, respectively). The HFOV led to the highest oxygenation, the lowest VT, and the most homogeneous distribution of ventilation (Table 2, Figures 2B and 4, respectively).
Respiratory variables during the ventilatory modes tested. (A) PaCO2 (mmHg; Friedman’s test, p = 0.011). (B) Tidal volume (mL/kg; Friedman’s test, p < 0.001). (C) Plateau pressure (cmH2O; Friedman’s test, p < 0.001). (D) Driving pressure (cmH2O; Friedman’s test, p < 0.001). VT, RR, HFPPV, and HFOV denote tidal volume, respiratory rate, high-frequency positive-pressure ventilation, and high-frequency oscillatory ventilation, respectively. The whiskers denote the P10th and P90th. *Student-Newman-Keuls’ post hoc analysis, p < 0.05 vs stabilization step (VT = 6 mL/kg and RR = 35 breaths/min); #Student-Newman-Keuls’ post hoc analysis, p < 0.05 vs HFPPV = 150.
Regional ventilation (mL/kg) in the sternal and vertebral portions of the thorax measured through EIT. ©Student-Newman-Keuls’ post hoc analysis, p < 0.05 vs stabilization step (VT = 6 mL/kg and RR = 35 breaths/min) (Friedman’s test, p < 0.001); #Student-Newman-Keuls’ post hoc analysis, p < 0.05 vs HFPPV = 150 (Friedman’s test, p < 0.001); §Wilcoxon’s test, p < 0.007 (Bonferroni’s correction for multiple comparisons) vs the vertebral region (gravitational-dependent region).
Distribution of regional ventilation (%) in the sternal and vertebral portions of the thorax measured through EIT. ©Student-Newman-Keuls’ post hoc analysis, p < 0.05 vs stabilization step (VT = 6 mL/kg and RR = 35 breaths/min) (Friedman’s test, p < 0.001); #Student-Newman-Keuls’ post hoc analysis, p < 0.05 vs HFPPV = 150 (Friedman’s test, p < 0.001); §Wilcoxon’s test, p < 0.007 (Bonferroni’s correction for multiple comparisons) vs the vertebral region (gravitational-dependent region).
Only one animal needed norepinephrine during HFPPV, and the dose varied between 2.4 μg/kg/min (HFPPV = 60) and 3.2 μg/kg/min (HFOV). The hemodynamic and metabolic data with different RRs are shown in Table 3. Of note, the stabilization step with VT = 6 mL/kg and RR = 35 breaths/min was associated with higher pulmonary artery pressures and lower pH.
Discussion
Our main finding was that, during protective mechanical ventilation of a severe ARDS swine model, the use of HFPPV with a conventional ventilator allows further reductions in VT and PaCO2, leading to reductions in driving pressures and plateau pressures without increasing mean airway pressure. We did not identify any significant detrimental effect of the high RRs applied, even after careful assessment of hemodynamics, respiratory system mechanics, and gas exchange.
The possibility of further reducing the ventilator-associated lung injury is of utmost importance, with possible implications in terms of reducing death and multiple organ failure in ARDS patients [23]. Ventilation with low VTs (6 mL/kg) is still the standard support for those patients [5], although lower VTs might produce additional protection [6, 24]. Of note, one third of ARDS patients under protective ventilation still have lung hyperdistention, which is associated with increases in systemic inflammatory markers [25]. This subset of patients, usually more severely injured, could possibly benefit from further VT reductions [24].
Increasing the RR at constant alveolar ventilation, we obtained a progressive decrease in VTs reaching levels below 4 mL/kg. This finding challenges the paradigm - promulgated by the design of many clinical trials that RRs should be kept equal to or less than 35 breaths per minute [5, 26–28]. In our model of severe ARDS, the standard of care [5] settings of VTs at 6 mL/kg and a maximum RR of 35 breaths/min led to a median PaCO2 value of 81 mmHg with a median pH of 7.13. Targeting a PaCO2 of 60 mmHg, we were able to reduce VTs by 36% with a RR of 150 breaths per minute. Other authors have shown, in an experimental model of ARDS, that higher RRs allow for a reduction in VT when associated with a strategy to lower the dead space (aspiration of dead space) [29, 30]. Similarly, a recent study in patients with ARDS showed that protective VT around 4 mL/kg can be achieved with modest increments in RR, provided that care is taken to minimize the circuit dead space [31]. These studies combined increases in RR with other measures to decrease the dead space. Our findings on the isolated effect of RR on the reduction of tidal volume help understand the independent effect of manipulating the RR.
The increases in RR were not associated with significant changes in gas exchange. We did notice a not significant but progressive fall in the median PaO2/FiO2 (P/F) ratio with increases in RR above 30 breaths per minute amounting to a fall of 26% at a RR of 150 breaths per minute (Table 2). Concurrently, the Tinsp/Ttot ratio increased from 19% to 42% when RR increased from 30 to 150 (Table 2), due to the fixed inspiratory flow rate and the need for higher minute ventilation at high RR. These increases in the Tinsp/Ttot ratio would favor a change in the P/F ratio in the opposite direction of the trend we found. These observations emphasize that with our relatively small sample size, we might have been underpowered to detect some differences such as the P/F ratio variation. If such trend proved significant in a larger study, it is possible that the lower tidal volumes at higher RR have favored the development of absorption atelectasis, although we cannot exclude that hemodynamic factors may played a role.
HFOV, a more classical strategy than HFPPV to provide adequate gas exchange at very low VTs [32, 33], has been recently shown to provide no benefit or even cause harm to patients with ARDS [16, 17]. Our results showed that HFOV = 5 Hz could stabilize the PaCO2 with VTs 26% lower than HFPPV = 150, however, with a RR twice as high and a Pmean 30% higher [15]. This is illustrative of the disproportionate increases in RR to maintain alveolar ventilation at progressively lower VTs, especially when close to the dead space, and the need to increase Pmean, which may have deleterious hemodynamic effects. The consequence of this ventilation inefficiency might be an increased dissipation of energy in the lungs, potentially leading to more lung injury even at reduced stress and strain per breath. Therefore, reducing VT without increasing mean airway pressure might be of special interest. In that sense, HFPPV might offer a better compromise between VT and RR than HFOV.
Ventilation decreased more in the gravitation-dependent regions, a finding suggestive of reabsorption atelectasis, air trapping, or incomplete filling of those regions due to airway narrowing. Even after taking this ‘functional baby lung’ into account, the net result was likely a lesser degree of tidal lung stretch as suggested by the decrease in driving pressures and plateau pressures. Additionally, despite a preferential reduction in dependent ventilation (Figure 4), HFPPV could result in lower regional VT in non-dependent regions (Figure 3).
Limitations
Our study has several limitations. First, the arbitrary choice of the target CO2 level during HFPPV can be criticized. The CO2 value can be a confounding factor of the ventilatory settings during ARDS ventilation, with some studies showing a protective [34, 35] and others a potentially deleterious role [36, 37]. We chose a narrow range of 57 to 63 mmHg to avoid such potential confounding effect and to avoid significant acidosis (pH < 7.15), a goal we achieved in all experimental conditions. Likely, the main findings of the study would maintain had a normocapnia target been applied. Second, our study design, with sequential changes in the ventilator settings, was susceptible to carryover phenomena. We tried to avoid that effect through the randomization of sequences, the disconnection from the ventilator between the steps, and through a prolonged wait to the PaCO2 equilibrium. Third, the performance of conventional ventilators declines at very high RRs and low VTs, especially if low-compliance tubing is not employed [38]. Fourth, we did not rule out that histological damage to the lungs might have happened at those very high RRs. Fifth, HFOV was the last step of the study due to logistic issues and at this time the animals had significant positive fluid balances. This could be one explanation why HFOV was not associated with hemodynamic alterations, even with the use of higher Pmean. Finally, we cannot directly extrapolate these experimental findings to patients, who have longer time constants than pigs and might not tolerate RRs as high. Interestingly, those with the most severe lung injury tolerate better very high RR, because of their low time constants. Even so, in our experience, it is difficult to apply RR > 60 breaths per minute to patients without leading to intrinsic PEEP.
Conclusions
In an animal model of severe ARDS, as compared to the standard protective ventilation, high-frequency positive-pressure ventilation delivered by a conventional ventilator allowed further reductions in tidal volume and in inspiratory pressures. As such, HFPPV could be a well-suited alternative in the treatment of severe ARDS with very low lung compliance, although its impact on lung inflammation still awaits evaluation.
Abbreviations
- ABPm:
-
mean systemic arterial blood pressure
- ARDS:
-
acute respiratory distress syndrome
- CcO2:
-
pulmonary capillary oxygen content
- Cdyn:
-
dynamic compliance
- CI:
-
cardiac index
- Cstatic:
-
static compliance
- CVP:
-
central venous pressure
- CxO2:
-
blood oxygen content
- EIT:
-
electrical impedance tomography
- EtCO2:
-
airway end-tidal pressure of carbon dioxide
- FiO2:
-
inspiratory fraction of oxygen
- HFOV:
-
high-frequency oscillatory ventilation
- HFPPV:
-
high-frequency positive-pressure ventilation
- I/E:
-
inspiratory/expiratory time ratio
- PaCO2:
-
partial arterial carbon dioxide pressure
- PaO2:
-
partial pressure of arterial oxygen
- PAOP:
-
pulmonary artery occlusion pressure
- PAPm:
-
mean pulmonary artery pressure
- PEEP:
-
positive end-expiratory pressure
- PEEPe:
-
extrinsic positive end-expiratory pressure
- PEEPi:
-
intrinsic positive end-expiratory pressure
- Pmean:
-
mean airway pressure
- Ppeak:
-
airway peak pressure
- Pplateau:
-
airway plateau pressure
- RR:
-
respiratory rate
- SvO2:
-
mixed venous blood gases
- Tinsp:
-
inspiratory time
- VT:
-
tidal volumes.
References
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD: Incidence and outcomes of acute lung injury. N Engl J Med 2005, 353(16):1685–1693. 10.1056/NEJMoa050333
Webb HH, Tierney DF: Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 1974, 110(5):556–565.
Ranieri VM, Giunta F, Suter PM, Slutsky AS: Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 2000, 284(1):43–44. 10.1001/jama.284.1.43
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR: Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998, 338(6):347–354. 10.1056/NEJM199802053380602
Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network N Engl J Med 2000, 342(18):1301–1308.
Fuchs H, Mendler MR, Scharnbeck D, Ebsen M, Hummler HD: Very low tidal volume ventilation with associated hypercapnia - effects on lung injury in a model for acute respiratory distress syndrome. PLoS One 2011, 6(8):e23816. 10.1371/journal.pone.0023816
Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, Vieillard-Baron A: Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med 2009, 35(11):1850–1858. 10.1007/s00134-009-1569-2
Otulakowski G, Kavanagh BP: Hypercapnia in acute illness: sometimes good, sometimes not. Crit Care Med 2011, 39(6):1581–1582. 10.1097/CCM.0b013e318215bb0a
Hager DN, Fessler HE, Kaczka DW, Shanholtz CB, Fuld MK, Simon BA, Brower RG: Tidal volume delivery during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2007, 35(6):1522–1529. 10.1097/01.CCM.0000266586.04676.55
Eriksson I, Sjostrand U: Effects of high-frequency positive-pressure ventilation (HFPPV) and general anesthesia on intrapulmonary gas distribution in patients undergoing diagnostic bronchoscopy. Anesth Analg 1980, 59(8):585–593.
Barzilay E, Lev A, Ibrahim M, Lesmes C: Traumatic respiratory insufficiency: comparison of conventional mechanical ventilation to high-frequency positive pressure with low-rate ventilation. Crit Care Med 1987, 15(2):118–121. 10.1097/00003246-198702000-00007
Barzilay E, Lev A, Lesmes C, Fleck R, Khourieh A: Combined use of HFPPV with low-rate ventilation in traumatic respiratory insufficiency. Intensive Care Med 1984, 10(4):197–200. 10.1007/BF00259437
Abu-Dbai J, Flatau E, Lev A, Kohn D, Monis-Hass I, Barzilay E: The use of conventional ventilators for high frequency positive pressure ventilation. Crit Care Med 1983, 11(5):356–358. 10.1097/00003246-198305000-00007
Iguchi N, Hirao O, Uchiyama A, Mashimo T, Nishimura M, Fujino Y: Evaluation of performance of two high-frequency oscillatory ventilators using a model lung with a position sensor. J Anesth 2010, 24(6):888–892. 10.1007/s00540-010-1032-0
Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, Barreau-Baumstark K, Castanier M, Papazian L, Roch A: Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2012, 40(5):1539–1545. 10.1097/CCM.0b013e3182451b4a
Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH: High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013, 368(9):806–813. 10.1056/NEJMoa1215716
Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO: High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013, 368(9):795–805. 10.1056/NEJMoa1215554
Sjostrand UH, Eriksson IA: High rates and low volumes in mechanical ventilation–not just a matter of ventilatory frequency. Anesth Analg 1980, 59(8):567–576.
Wattwil LM, Sjostrand UH, Borg UR: ) Comparative studies of IPPV and HFPPV with PEEP in critical care patients. I: a clinical evaluation. Crit Care Med 1983, 11(1):30–37. 10.1097/00003246-198301000-00009
Costa EL, Chaves CN, Gomes S, Beraldo MA, Volpe MS, Tucci MR, Schettino IA, Bohm SH, Carvalho CR, Tanaka H, Lima RG, Amato MB: Real-time detection of pneumothorax using electrical impedance tomography. Crit Care Med 2008, 36(4):1230–1238. 10.1097/CCM.0b013e31816a0380
Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, Tanaka H, Sipmann FS, Santos DC, Barbas CS, Carvalho CR, Amato MB: Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med 2004, 169(7):791–800. 10.1164/rccm.200301-133OC
Gomes S, Belmino R, Hirota A, Costa ELC, Barbeiro DF, Tucci MR, Gregores GB, Beraldo MA, Timenetsky K, Borges JB, Carvalho CRR, Amato MBP: A New Experimental Model of Acute Lung injury. Am J Respir Crit Care Med 2009, 179: A3568.
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS: Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003, 289(16):2104–2112. 10.1001/jama.289.16.2104
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM: Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 2009, 111(4):826–835. 10.1097/ALN.0b013e3181b764d2
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM: Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007, 175(2):160–166. 10.1164/rccm.200607-915OC
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE: Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008, 299(6):637–645. 10.1001/jama.299.6.637
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004, 351(4):327–336.
Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L: Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008, 299(6):646–655. 10.1001/jama.299.6.646
Uttman L, Ogren H, Niklason L, Drefeldt B, Jonson B: Computer simulation allows goal-oriented mechanical ventilation in acute respiratory distress syndrome. Crit Care 2007, 11(2):R36. 10.1186/cc5719
Uttman L, Bitzen U, De Robertis E, Enoksson J, Johansson L, Jonson B: Protective ventilation in experimental acute respiratory distress syndrome after ventilator-induced lung injury: a randomized controlled trial. Br J Anaesth 2012, 109(4):584–594. 10.1093/bja/aes230
Retamal J, Libuy J, Jimenez M, Delgado M, Besa C, Bugedo G, Bruhn A: Preliminary study of ventilation with 4 ml/kg tidal volume in acute respiratory distress syndrome: feasibility and effects on cyclic recruitment - derecruitment and hyperinflation. Crit Care 2013, 17(1):R16. 10.1186/cc12487
Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J: High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002, 166(6):801–808. 10.1164/rccm.2108052
Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, Adhikari NK: High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ 2010, 340: c2327. 10.1136/bmj.c2327
Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK, Post M, Lindsay T, Kavanagh BP: Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med 2000, 162(6):2287–2294. 10.1164/ajrccm.162.6.2003066
Nichol AD, O’Cronin DF, Howell K, Naughton F, O’Brien S, Boylan J, O’Connor C, O’Toole D, Laffey JG, McLoughlin P: Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit Care Med 2009, 37(11):2953–2961. 10.1097/CCM.0b013e3181b028ce
Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, Beitel GJ, Hauser AR, Sznajder JI, Sporn PH: Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am J Respir Cell Mol Biol 2013, 49(5):821–828. 10.1165/rcmb.2012-0487OC
Doerr CH, Gajic O, Berrios JC, Caples S, Abdel M, Lymp JF, Hubmayr RD: Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator-injured lungs. Am J Respir Crit Care Med 2005, 171(12):1371–1377. 10.1164/rccm.200309-1223OC
Lyazidi A, Thille AW, Carteaux G, Galia F, Brochard L, Richard JC: Bench test evaluation of volume delivered by modern ICU ventilators during volume-controlled ventilation. Intensive Care Med 2010, 36(12):2074–2080. 10.1007/s00134-010-2044-9
Acknowledgements
The authors are grateful to the Research and Education Institute, Hospital Sírio-Libanês, São Paulo, Brazil; Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, Brazil; and Financiadora de Estudos e Projetos (FINEP), Brazil, for the grant offered to the study development. We gratefully thank Marcelo do Amaral Beraldo, Mauro Roberto Tucci, Roberta Ribeiro de Santis Santiago, and Takeshi Yoshida, whose contribution to the animal experiments was very important.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RLC designed the study, participated in the research protocols, analyzed and interpreted the data, and drafted the manuscript. MP designed the study, participated in the research protocols, helped interpret the data, and drafted the manuscript. ELVC helped design the study, participated in the research protocols, analyzed and interpreted the data, and helped draft the manuscript design. SG participated in the research protocols and analyzed the data. LB participated in the discussion, helped interpret the data, and drafted the manuscript. MBPA designed the study, analyzed and interpreted the data, and reviewed the manuscript. LCPA designed and conceived the study, participated in the research protocols, and reviewed the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cordioli, R.L., Park, M., Costa, E.L.V. et al. Moderately high frequency ventilation with a conventional ventilator allows reduction of tidal volume without increasing mean airway pressure. ICMx 2, 13 (2014). https://doi.org/10.1186/2197-425X-2-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2197-425X-2-13