Abstract
Porous alumina template was prepared by constant voltage in three different electrolytes (0.45 M sulfuric acid, 0.3 M oxalic acid, and mixed solution of 0.45 M sulfuric and 0.3 M oxalic acids). In this paper, the effects of the electrolyte type on the morphology is studied, so the anodization is carried out in an oxalic acid, sulfuric acid, and a mixture of oxalic and sulfuric acids. The effect of these three different electrolytes was investigated by scanning electron microscopy. Results show that in sulfuric acid, the pore structure is smaller than the oxalic and a mixture of oxalic and sulfuric acid. Density of holes in sulfuric acid was higher than other electrolytes; however, arrangement of pores in oxalic acid was more regular than that of the others.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The anodization of aluminum, a method of synthesis of ordered porous structures, seems to be a cheaper, simpler, and less time-consuming option for industrial purposes. The advantages of this method led to its further attraction in recent decades. One common method for nanofabrication is the use of membranes with nanosized holes [1–7]. The conditions could be controlled to achieve desired pore length and diameter, and they can be used in many different ways to prepare a variety of nanostructured materials, magnetic metals and alloys, semiconductor alloys, heterostructures, superconductors, carbon nanotubes, etc. A lot of alternative methods have been developed for the formation of nanopore and nanodot array on various substrates including sol-gel methods [8, 9], CVD based on the vapor-liquid-solid growth method [10], and template synthesis, in particular, using anodized aluminum oxide membranes [11–13]. The aim of the present study is to investigate the self-organized formation of ordered arrays of nanopores by a two-step anodizing process of aluminum. We investigated the effect of the type of electrolyte on the size and regularity of the pores of samples. For this purpose, the effects of three different electrolytes such as the solution of 0.45 M sulfuric acid, 0.3 M oxalic acid, and a mixture of the same volume of 0.45 M sulfuric and 0.3 M oxalic acid were studied.
Results and discussion
By the anodization process, fabrication of porous arrays of aluminum was successfully performed in three different electrolytes, and the results of surface morphology of the films were studied with a scanning electron microscope (SEM, Leo-440I, Leo Electron Microscopy, Cambridge, UK) and a field emission scanning electron microscope (FESEM, Hitachi-54160, Chiyoda-ku, Japan).
The situation during a steady-state pore growth must be considered to explain the effect of self-organization. By applying voltage between the anode electrode and cathode electrode on the aluminum surface, a layer of flat oxide surface is produced and proceeds with the dissolution of some oxide area (surface defects) within the electrolyte, and with the dissolution tendency of the oxide layer of primary hollow points, holes are created on the surface.
The electric field was focused in the hollow areas, and concentration of the electric field causes the formation of pores. It can be seen that the hole growths are the same, parallel to each other, and perpendicular to the surface. This is because the kind of conflict in relation to growth of the oxide layer and its dissolution and corrosion, simultaneously in millions of dots or holes, comes into existence, creating a kind of balance.
Another quantity of anodic alumina in the morphology is interpore distance which is the interval between two consecutive holes. In fact, an electric field exists in all the holes that caused the equal interpore distances.
In general, the shape and diameter of holes can be controlled by the anodization voltage, anodization temperature, and acid type and concentration. Sulfuric acid can produce the smallest diameter holes at lower voltages [14]. In the anodization process of porous alumina membranes, there is equilibrium between the dissolution of field-enhanced oxide in the interface electrolyte/oxide and oxide formation at the interface oxide/metal. This balance is critical for the formation of porous alumina because it causes the thickness of the barrier to remain constant throughout the anodization process [15].
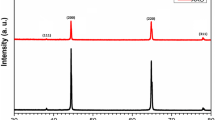
SEM images in Figure 1 show that fabricated holes in 0.45 M sulfuric acid baths are very tiny so the density of the holes is high. The fabricated pores are arranged in a hexagonal structure.
As shown in Figure 1, pores are more regular in oxalic acid, but their sizes are bigger in comparison with other electrolytes. By considering a rectangle with 300-nm × 600-nm sides, the average diameter of pores was almost 34.5, 20.26, and 25 nm, respectively, for samples which were anodized in 0.3 M oxalic acid, 0.45 M sulfuric acid, and a mixed solution of 0.3 M oxalic and 0.45 M sulfuric acids (Table 1).
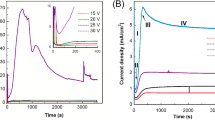
Variations of current with the applied anodizing potential are very important. So, the time evolutions of the current which pass through the electrolytes with different solutions in the first and second anodization processes were shown in the Figure 2.
In all of the curves, the current stabilized after enough time has passed (Figure 1A). Current during anodization is a key factor of the self-ordering process. So, variations of current with time are very important. When the rate of aluminum oxide dissolution occurring at the base of pores equals the rate of oxide formation at the metal-oxide interface, from that moment, a stable growth of the porous oxide layer on anodized aluminum starts.
Conclusions
By the anodization process, porous alumina was fabricated in different electrolytes. The pore diameter could be controlled by conditions of anodization such as temperature, applied voltage, annealing time, electrolyte, etc. In three different electrolytes which we investigated, the sizes of produced pores in sulfuric acid were smaller than the sizes of pores in the oxalic acid and a mixture of oxalic and sulfuric acids. Density of holes in sulfuric acid was higher than other electrolytes. Regularity of pores in oxalic acid was more than other electrolytes.
Methods
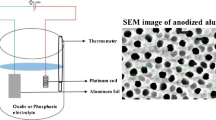
An aluminum sheet with 2-mm thickness and 1-cm × 5-cm size was prepared. The sample was washed with acetone and distilled water in order to wash the impureness from the surface. The morphology of anodized alumina has direct relation to the annealing time, so the Al sheet was annealed at 450°C for 2 h in air [16]. In order to remove an oxide layer of the substrate, the samples were etched in a 1 M NaOH solution for 3 min. The Al sheet was electropolished in a mixture of perchloric acid and ethanol with a value ratio of 25:75 for 3 min to decrease the roughness of the substrate.
In the first process of anodization, the aluminum sheet was selected as anode and an aluminum electrode as cathode with a 2.5-cm separation. Then, a DC voltage of 40 V was applied to the electrodes. The solution was stirred by a magnetic stirrer during the anodization process. After that, the alumina film was removed by putting it in a mixture of 0.2 M of chromic acid and 0.5 M of phosphoric acid for 12 h. Then, the second step of anodization was performed under the same condition as the first step (for 1 h). This experiment was implemented at 17°C.
Authors' information
AK was born in Shiraz, Iran. He received his B.S. degree in Atomic Physics from Shiraz University in 1995, M.S. degree in Atomic and Molecular Physics from Kerman University in 1997, and the Ph.D. degrees in Physics (Laser and Optics) from Shiraz University in 2004. He has worked on much scientific research areas such as nonlinear optics and laser, nanophotonics and nanophysics. He joined the faculty of basic sciences in Shiraz University of Technology in 2004. He became an Associate Professor in 2012. At present, he is actively involved in optics and photonics and has M.S. and Ph.D. students in these fields. ZP was born in 1978, in Kuwait. She received her B.S. degree in Physics from Shiraz University, Iran, in 2001, and her M.S. degree from Islamic Azad University, Research and Science Branch, Tehran, Iran, in 2005, under the supervision of Dr. Dorranian and Dr. Ghorannevis. She received her Ph.D. degree in Physics (Nano) at the same university under the supervision of Dr. Alireza Keshavarz and Dr. Mohammad Elahi. She is now teaching Physics in Islamic Azad University, Shiraz Branch, Iran. She is interested in nanoscience especially bimetallic nanoparticles and nanostructures. AN was born in 1981, in Shiraz. He completed her B.S. in Applied Solid State Physics from Yasuj University, Iran in 2004 and M.S. in Atomic and Molecular Physics, Payam-e-noor University of Shiraz, Iran. He is an experimental officer at the nanophysics laboratory of Shiraz University of Technology, Iran.
References
Murray CB, Kagan CR, Bawendi MG: Self-organization of CdSe nanocrystallites into three-dimensional quantum dot superlattices. Science 1995, 270: 1335–1338. 10.1126/science.270.5240.1335
Andres RP, Bielefeld JD, Henderson JI, Janes DB, Kolagunta VR, Kubiak CP, Mahoney WJ, Osifchin RG: Self-Assembly of a two-dimensional superlattice of molecularly linked metal clusters. Science 1996, 273: 1690–1693. 10.1126/science.273.5282.1690
Shi J, Gider S, Babcock K, Awschalom DD: Magnetic clusters in molecular beams, metals, and semiconductors. Science 1996, 271: 937. 10.1126/science.271.5251.937
Billas IML, Becker JA, Chatelain A, de Heer WA: Magnetic momentsof iron clusters with 25 to 700 atoms and their dependence on temperature. Phys. Rev. Lett. 1993, 71: 4067–4070. 10.1103/PhysRevLett.71.4067
White RL, New RMH, Pease RFW: Magnetic domains: the analysis of magnetic microstructures. IEEE Trans. Magn. 1997, 33: 990–995. 10.1109/20.560144
Chou SY, Wei MS, Krausse PR, Fischer PB: Single-domain magnetic pillar array of 35 nm diameter and 65 Gbits/in2 density for ultrahigh density quantum magnetic storage. J. Appl. Phys 1994, 76: 6673. 10.1063/1.358164
Ross CA, Smith HI, Savas T, Schattenburgh M, Farhoud M, Hwang M, Walsh M, Abraham MC, Ram RJ: Fabrication of patterned media for high density magnetic storage. J. Vac. Sci. Technol. B 1999, 17: 3168. 10.1116/1.590974
Yang P, Deng T, Zhao D, Feng P, Pine D, Chmelka BF, Whitesides GM, Stucky GD: Hierarchically ordered oxides. Science 1998, 282: 2244–6.
Pavasupree S, Suzuki Y, Pivsa-Art S, Yoshikawa S: Synthesis and characterization of nanoporous, nanorods, nanowires metal oxides. Sci. Tech. Adv. Mater. 2005, 6: 224–229. 10.1016/j.stam.2005.02.001
Chik H, Xu JM: Nanometric superlattices: non-lithographic fabrication, materials, and prospects. Mater Sci Eng R 2004, 43: 103. 10.1016/j.mser.2003.12.001
Jain K, Lakshmikumar ST: Porous alumina template based nanodevices. IETE Technical Rev 2002, 19: 293–306.
Peng X, Chen A: Electrochemical fabrication of novel nanostructures based on anodic alumina. Nanotechnology 2004, 15: 743–748. 10.1088/0957-4484/15/7/005
Piao Y, Lim H, Chang JY, Lee W-Y, Kim H: Nanostructured materials prepared by use of ordered porous alumina. Electrochim. Acta 2005, 50: 2997–3013. 10.1016/j.electacta.2004.12.043
Jenny M: Nunn S, pang Y T: Electrochemical synthesis of ordered alumina nanowire arrays. J Solid State Electrochem. 2005, 7: 344–347.
John S, Balasubramanian V, Shenoi AB: Hard anodizing of aluminum and its Alloys at 30 to 35°C. Metal Finishing 1985, 83: 23–26.
Almasi Kashi M, Ramazani A: The effects of temperature and concentration on the self-organized pore formation in anodic alumina. J. Phys. 2005, 38: 2396–2399.
Acknowledgment
This project was supported by the University of Technology of Shiraz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZP carried out the preparation of nanoporous alumina. ZP and AN participated in the study of pores' structure. AK, as the supervisor, participated in this work. AK, ZP, and AN carried out the manuscript preparation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Keshavarz, A., Parang, Z. & Nasseri, A. The effect of sulfuric acid, oxalic acid, and their combination on the size and regularity of the porous alumina by anodization. J Nanostruct Chem 3, 34 (2013). https://doi.org/10.1186/2193-8865-3-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-34