Abstract
The use of microorganisms in the synthesis of nanoparticles emerges as an eco-friendly and exciting approach. In the present investigation, we report the biosynthesis of silver nanoparticles employing the bacterium Salmonella typhirium. Silver nanoparticles were synthesized through the reduction of aqueous Ag+ ion using the growth culture supernatants in the bright conditions. The synthetic process was fast, and silver nanoparticles were formed within 4 min of silver ion coming in contact with the cell filtrate. An absorption peak at 427 nm in a Uv-vis spectrophotometer was detected indicating the presence of Ag nanoparticles. The morphology of nanoparticles was observed by transmission electron microscopy. The size of the nanoparticles was determined to be 87 ± 30 nm, applying dynamic light scattering. Reduction degree of Ag+ was measured as 75% by atomic absorption spectrophotometry. The silver nanoparticles have been prepared from silver sulfate during this investigation for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Nanotechnology plays a very important role in many key technologies of the new millennium [1]. The application of nanoscale and nanostructure materials within the range of 1 to 100 nm is an emerging area of nanoscience and nanotechnology. Nanomaterials may provide solutions to technological and environmental challenges in the areas of solar energy conversion, catalysis, medicine, and water treatment [2].
Nanoparticles of silver have many important applications that include spectrally selective coating for solar energy absorption and intercalation material for electrical batteries, as optical receptors, polarizing filters, catalysts in chemical reaction, biolabelling, and as antimicrobial agents [3].
For the production of nanoparticles, one needs to know the physical and chemical principles of nanoscale materials and also know how to commercialize them. Generally, metal nanoparticles can be prepared and stabilized by chemical, physical, and biological methods; the chemical approach, such as chemical reduction, electrochemical techniques, photochemical reduction [2, 4], and pyrolysis [5], and physical methods, such as Arc-discharge and physical vapor condensation [6], are used. Living organisms have huge potentials for the production of nanoparticles of wide applications.
Studies show that the size, morphology, stability, and properties (chemical and physical) of the metal nanoparticles are strongly influenced by the experimental conditions, the kinetics of interaction of metal ions with reducing agents, and the adsorption processes of stabilizing agent with metal nanoparticles. Therefore, the design of a synthesis method in which the size, morphology, stability, and properties are under control is a major field of interest [2, 7].
Researchers prefer a biological synthesis because the distribution control of particles obtained from this method is better than other methods [8]. In addition, this method involves no environmental toxicity which is usually accompanied with other chemical methods [9].
The first of the synthesis of silver nanoparticles by a bacterium has been reported in 2000. Joerger et al. used P.stutzeri bacterium (AG259) for silver nanoparticle synthesis with a size smaller than 200 nm [10]. In another study, silver nanocrystal biosynthesis was performed using the bacterium Bacillus licheniformis. This was indicated by the change in color from whitish-yellow to brown [11].
In 2009, it was investigated that there was a different visible light irradiation effect on the formation of silver nanoparticles from silver nitrate using the culture supernatant of Klebsiella pneumonia. Also, the study experimentally investigated the liquid mixing process effect on silver nanoparticle synthesis by visible light irradiation [12].
Research shows that one of the most important factors in the production of silver nanoparticles is the time of its formation. In addition, biosynthesis of silver nanoparticles using microorganisms is rather slow. However, finding microorganisms to rapidly synthesize Ag nanoparticles is an important aspect. In this study, we used Salmonella typhirium as the bacterium to reduce Ag+ ions in an aqueous solution.
Results and discussion
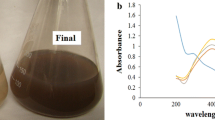
For the analytical study of the prepared sample, the amount of absorption within the wavelength of 350 to 600 nm was observed by UV-vis spectroscopy. This technique has proven to be very useful for analyzing nanoparticles [3, 7]. As illustrated in Figure 1, a strong surface plasmon resonance was centered at ca. 427 nm. Observation of this strong but broad surface plasmon peak has been well documented for various metal nanoparticles, with sizes ranging widely from 2 to100 nm [13, 14].
In order to carefully study the size of the produced silver nanoparticles, dynamic light scattering analysis was used. As seen in Figure 2, the average size of nanoparticles produced in the bioreactor is about 129.3 nm, and majority of the nanoparticles have the size of 87.18 nm.
The difference between the smallest and largest sizes of nanoparticles is 30.92 nm which indicates narrow distribution of the produced nanoparticles. By means of atomic absorption spectrometry, the amount of Ag+ reduction was measured and calculated. The amount of Ag+ reduction in the solution after 4 min was 75% which indicates high efficiency. From this amount of reduction, the largest nanoparticle volume (weight) was obtained at the size of 127 nm. In fact, silver nanoparticles with the size of 127 nm had the largest amount of reduction. Dynamic light scattering (DLS) volume analysis shows this fact (Figure 3).
The silver nanoparticles synthesized by Salmonella were studied by transmission electron microscopy (TEM), and images show and confirm silver nanoparticle production at nanosize. TEM images of the produced nanoparticles are shown in Figure 4.
Conclusions
Here, we described an inexpensive approach for reducing silver sulfate solution to produce nanoparticles. Silver nanoparticles in the range of approximately 50 to 150 nm are synthesized by the supernatant of Salmonella typhirium when it is added to silver sulfate. It seems that this method is the fastest biological method to form Ag nanoparticles. The time required to complete the silver ion reduction was obtained about 4 min. According to previous reports, the reductase enzymes released by the bacteria play the most important role in the reduction of silver ions. It seems the reductase enzymes released by Salmonella typhirium can reduce silver ions quickly; although, more research should take place in this area. This methodology could be used for synthesizing a number of metallic nanoparticles involving other metals with good size and shape morphology. This study would therefore lead to an easy (and rapid) procedure for producing silver nanoparticles with the added advantage of biosafety.
Methods
Organism
Salmonella typhirium was provided by the Department of Veterinary Medicine, Tehran University of Iran. The bacterium after growth was maintained on an agar plate (Macconkey agar) at 4°C temperature.
Growth medium
The Trypti casein soy broth medium (Company Merck, Whitehouse Station, NJ, USA) was used for bacterial culture in this study. The medium was prepared, and after autoclaving, it was maintained in order to be used at later stages.
Preparation of inoculum
A loopful of colony from an agar plate was picked up and aseptically transferred into a 100-cm3 medium in a 250-cm3 conical flask. The flask was cultured overnight at 37°C in order to prepare a suspension of the Salmonella bacterium.
Ag nanoparticle synthesis
The culture was centrifuged at 5,000 rpm for 15 min, and the supernatant was used for the synthesis of silver nanoparticles. Distilled water was used as solvent in the synthesis of silver nanoparticles. One milliliter of supernatant was added separately to the reaction vessel containing 100 ml Ag2SO4 at a concentration of 0.0005M. The reaction between this supernatant and Ag+ ions were carried out in bright conditions for 4 min. After 4 min, the colorless solution of silver sulfate in the container turns into brown color (Figure 5). This color change indicates a possibility of silver nanoparticle production. In order to prove the existence of nanoparticles, product efficiency, and their size, Uv-vis spectroscopy, atomic absorption spectroscopy, transmission electron microscopy, and DLS analysis were used.
References
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P: The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol 2006, 69: 485–492. 10.1007/s00253-005-0179-3
Sharma VK, Yngard RA, Lin Y: Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interfac 2009, 145: 83–96. 10.1016/j.cis.2008.09.002
Ahmad A, Mukherjee P, Senapati S, Mandal D, Islam Khan M, Kumar R, Sastry M: Extracellular biosynthesis of silver nanoparticles using the fungus Fusariumoxysporum. Colloid Surfaces B 2003, 28: 313–318. 10.1016/S0927-7765(02)00174-1
Jacob J, Kapoor S, Biswas N, Mukherjee T: Size tunable synthesis of silver nanoparticles in water–ethylene glycol mixtures. Colloid Surfaces A 2007, 301: 329–334. 10.1016/j.colsurfa.2006.12.070
Qiaoxin Z, Hao L, Xiaohui W, Xiaoliang S, Xinglong D: Fabrication and characterization of nano silver powder prepared by Spray Pyrolysis. J. Wuhan Univ. Technol 2009, 24: 871–874. 10.1007/s11595-009-6871-x
Tavakoli A, Sohrabi M, Kargari A: A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap 2007, 61: 151–170. 10.2478/s11696-007-0014-7
Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH: Biological synthesis of silver nanoparticles using the fungus Aspergillusflavus. Mater. Lett 2007, 61: 1413–1418. 10.1016/j.matlet.2006.07.042
Durán N, Marcato PD, De Souza G, Alves O, Esposito E: Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol 2007, 3: 203–208. 10.1166/jbn.2007.022
Kumar SA, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, Khan MI: Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO 3 . Biotechnol. Lett 2007, 29: 439–445. 10.1007/s10529-006-9256-7
Joerger R, Klaus T, Granqvist CG: Biologically produced silver-carbon composite materials for optically functional thin film coatings. Adv. Mater 2000, 12: 407–409. 10.1002/(SICI)1521-4095(200003)12:6<407::AID-ADMA407>3.0.CO;2-O
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S: Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloid Surface B 2008, 65: 150–153. 10.1016/j.colsurfb.2008.02.018
Mokhtari N, Daneshpajouh S, Seyedbagheri S, Atashdehghan R, Abdi K, Sarkar S, Minaian S, Shahverdi HR, Shahverdi AR: Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater. Res. Bull 2009, 44: 1415–1421. 10.1016/j.materresbull.2008.11.021
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N: Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mater. Lett 2007, 61: 3984–3987. 10.1016/j.matlet.2007.01.018
Pörtner R, Giese C: An Overview on Bioreactor Design, Prototyping and Process Control for Reproducible Three-Dimensional Tissue Culture. In Marx and Sandig: Drug Testing In Vitro – Breakthroughs and Trends in Cell Culture Technology. Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim; 2007.
Acknowledgements
We are thankful to the University of Qaemshahr Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author has no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ghorbani, H.R. Biosynthesis of silver nanoparticles using Salmonella typhirium. J Nanostruct Chem 3, 29 (2013). https://doi.org/10.1186/2193-8865-3-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-29