Abstract
This study was conducted in order to investigate the implications of the R72P polymorphism in the TP53 gene in breast cancer risk. The enlightenment of this matter might provide a piece of information about the potential implications of this polymorphism in patient risk. A meta-analysis was conducted considering a large sample size from studies with conflicting results on the R72P polymorphism in breast cancer patients. Relevant studies were selected from PubMed and SciELO databases for data extraction and statistical analysis. Database was built according to the continent and considering the genotype frequencies, sample size and genotyping methodology. The dominant models (RR vs RP + PP and RR + RP vs. PP), homozygous (RR vs. PP), heterozygous (RR vs. RP and RP vs. PP) and the allele (R vs. P) were used. Genotype frequencies were summarized and evaluated by χ2 test of heterogeneity in 2×2 contingency tables with 95% CIs. Odds Ratios (OR) were calculated with a fixed-effect model (Mantel-Haenszel) or a random-effect model (DerSimonian-Laird) if the studies were considered homogeneous (P > 0.05) or heterogeneous (P < 0.05), respectively, using BioEstat® 5.0 software. Supported by a large sample size composed by 25,629 cases and 26,633 controls from 41 studies, we found significant association between the R72P polymorphism in the TP53 gene and the breast cancer risk. The overall data shows an increased risk due to the P allele dominant model, but not in Asia where the risk was associated with the R allele and R dominant model.
Similar content being viewed by others
Introduction
The R72P polymorphism in the TP53 gene results of the transversion G → C in the second position of the codon 72 at exon 4. Both the polymorphic alleles vary among ethnic groups (Dokianakis et al. 2000) and geographic location, where the P allele is more frequent toward the equador line purportedly as a protective factor against UV rays (Damin et al. 2006; Olivier et al. 2002). P53 variant proteins have an arginine (R) or a proline (P) encoded by codon 72, which differ in structure and function, specially concerning cell cycle progress (Chang-Claude et al. 2009; Schmidt et al. 2009; Thomas et al. 1999; Petitjean et al. 2007; Dumont et al. 2003).
Breast cancer is an heterogeneous sporadic or hereditary disease (Lima et al. 2006). The hereditary syndrome affects 10% of patients, of which 5% has high penetrance mutations in genes like BRAC1 and BRCA2 (BRCA1/2) (Pinto et al. 2007). BRCA1/2 and TP53 are susceptibility genes that confer high-risk of breast cancer (Oluwagbemiga et al. 2012). Evidences that the R72P polymorphisms in the TP53 gene can differently promote the transcription of BRAC1/2 have widely supported studies on R72P role in breast tumorigenesis (Sinilnikova et al. 2009; Lum et al. 2008; Osorio et al. 2008; Gochhait et al. 2007; Cavallone et al. 2008; Baynes et al. 2007; Tommiska et al. 2005; Martin et al. 2003; Huang et al. 2003), e.g., (1) the P variant binds greater to transcriptional machinery (Thomas et al. 1999) and thus shows higher rates of G1 arrest than the R variant protein (Petitjean et al. 2007; Gochhait et al. 2007); (2) the decreased efficiency of the P variant at triggering apoptosis (Chang-Claude et al. 2009; Dumont et al. 2003), mainly due to its decreased ubiquination by MDM2 (Sinilnikova et al. 2009; Lum et al. 2008; Gochhait et al. 2007; Francisco et al. 2010) and to its increased efficiency to bind the inhibitor of apoptosis-stimulating protein of p53 (iASPP) (Schmidt et al. 2009; Bergamaschi et al. 2006).
In the present study, a meta-analysis was conducted considering a large sample size from studies with conflicting results on the R72P polymorphism in breast cancer patients. The enlightenment of this matter might provide a piece of information about the potential implications of this polymorphism in patient’s risk.
Material and methods
Identification and eligibility of relevant studies
A literature search was conducted in SciELO (Scientific Eletronic Library Online) and PubMed databases by using the keywords: p53, polymorphism, breast cancer. Additional studies were searched among the references surveyed in the databases. Eligible studies were selected regardless of sample size, but had to meet the following criteria of inclusion: (a) the studies were published from 2002 to 2012; (b) the association between the R72P polymorphism and breast cancer were investigated; (c) the studies were case–control design; (d) genotyping was carried out by molecular biology methods, such as PCR, RFLP-PCR and DNA sequencing; (e) the reference was published in English; (f) histological confirmation of breast cancer diagnosis was performed; and (g) the genotype distributions were available for estimating odds ratios (OR) and 95% confidence intervals (CI).
Data extraction
Two investigators independently extracted data and reached a consensus on all of the items. A third investigator took part of data extraction in case of disagreement in any of the items. The data extracted regarded country of origin, first author, and year of publication, number of cases and controls, and genotype frequencies.
Statistical analysis
In the current meta-analysis, the dominant models (RR vs RP + PP and RR + RP vs. PP), homozygous (RR vs. PP), heterozygous (RR vs. RP and RP vs. PP) and the allele (R vs. P) were used. Genotype frequencies were summarized and evaluated by χ2 test of heterogeneity in 2×2 contingency tables with 95% CIs (Böhning et al. 2002). Odds Ratios (OR) were calculated with a fixed-effect model (Mantel-Haenszel) or a random-effect model (DerSimonian-Laird) if the studies were considered homogeneous (P > 0.05) or heterogeneous (P < 0.05), respectively. The OR and their corresponding 95% CI were used to test the association between the 72 codon polymorphism and breast cancer. All analyses were performed with BioEstat® 5.0 software. To estimate a combined effect, OR were calculated for both fixed and random effect analyses, by applying 95% CIs and individual or combined weights for the studies (Li et al. 2012; Conn et al. 2012; Manning et al. 2011; Higgins et al. 2008).
Results and discussion
Study inclusion and characteristics
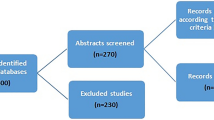
A total of 492 studies were screened, of which three were found in both PubMed and SciELO. From the 489 studies screened, 218 were published before 2002 and 20 were reviews or published in another language than English, or both. The remaining 271 records were assessed for eligibility and 41 fulfilled the criteria of inclusion (Figure 1). From the selected studies, a database was built considering the continent, genotype frequencies, sample size and genotype methodology. All together, the 41 studies that met the inclusion criteria and were identified as eligible article, yielding 25,629 cases and 26,633 controls.
In the last 10 years, eligible studies on R72P polymorphism in the TP53 gene in breast cancer were mostly from Europe with 19 articles, followed by Asia, America and Africa with 14, 6 and 2, respectively. Subject’s age was collected, when available, showing that mean age of patients was 51.9 y.o. and of control subjects 48.1 y.o. The genotyping for p53 codon 72 polymorphism was performed using Polymerase Chain Reaction (PCR), Allele-Specific PCR (AS-PCR), Amplifluor®, GoldenGate® Genotyping Assay (GGA), PCR-Denaturing Gradient Gel Electrophoresis (PCR-DGGE), PCR-Restriction Fragment Length Polymorphism (PCR-RFLP), sequencing and Taqman PCR. Breast cancer patients and controls subjects were mainly heterozygous in Asia (50.1%; 48.0%) and Africa (43.9%; 49.7%), while the RR homozygous was predominant in America (53.6%; 54.5%) and Europe (54.1%; 53.4%). The R allele was predominant in breast cancer patients from America and Europe (73.3%, each), Africa (63.2%) and Asia (58.3%).
Quantitative synthesis
The dominant models RR + RP vs. PP and RR vs. RP + PP had OR calculated using a random-effect model. No association between breast cancer risk and the dominant model RR + RP vs. PP (OR = 1.09; 95% CI 0.98-1.22) was found (Figure 2 and Table 1). By the other hand, our findings for RR vs. RP + PP (OR = 1.11; 95% CI 1.02-1.21) showed a markedly increased risk of breast cancer associated with the RP and PP genotypes, considering the P allele as dominant (Figure 3 and Table 1). In agreement with our results, the PP genotype was previously associated with higher risk for breast cancer (Huang et al. 2003; Rajkumar et al. 2008). Among unselected breast cancer patients, the PP genotype also predicted poor survival and a 2-fold increased risk of death (Tommiska et al. 2005).
The P allele has been associated with earlier breast cancer onset in BRCA1/2 mutation carriers (Tommiska et al. 2005; Martin et al. 2003), probably due to penetrance modification in BRCA1 (Martin et al. 2003) and to the lower ability of the P variant to induce apoptosis in genotoxic stress (Chang-Claude et al. 2009; Dumont et al. 2003). However, most of the studies selected for this meta-analysis have failed to detect any implication of R72P to breast cancer risk. Among most of the selected references, no risk modification by R72P was found in wild type BRCA1/2 and mutation carriers, even if the age of diagnosis or tumor stage were regarded (Sinilnikova et al. 2009; Lum et al. 2008; Cavallone et al. 2008; Baynes et al. 2007; Tommiska et al. 2005). Our overall data showed an association of risk increase with PP genotype, but not with the alleles alone (R vs. P; OR = 1.02; 95% CI 1.00-1.05), as described in Table 1. The lack of implication concerning P allele alone might be explained by the R allele in the heterozygous, because the R variant may act in a codominant mode to decrease breast cancer risk and to detain the onset in sporadic cases (Lum et al. 2008). Although our overall data show no association with the alleles alone, the analysis of 2,570 cases and 2,833 controls from Asia demonstrated a markedly increase of the R allele frequency in breast cancer patients (R vs. P; OR = 1.09; 95% CI 1.01-1.17), as detailed in Table 1.
Ethnic and geographical nonspecific factors, further to allele frequencies variations in different health populations, have been argued as the reason to the controversial data on R72P role in breast cancer (Dokianakis et al. 2000; Lum et al. 2008; Huang et al. 2003). Worth of note in this regard is that, in our meta-analysis, R allele was the most frequent in patients and control subjects, featuring the allele frequencies as a potential ethnic or geographical risk factor. By pooling all studies per continent, we performed the analyses of the dominant models and the genotypes using the fixed-effect model. Our overall results showed no association of the R72P polymorphism with breast cancer, but Asian patients had an increased risk associated with the dominant model RR + RP vs. PP (OR = 1.23; 95% CI 1.07-1.41), as described in Table 2. These remarkable data concerning RR + RP genotypes and R allele in Asia are in agreement with the reports that R variant increased breast risk in patients from China (Weston & Godbold 1997; Li et al. 2002) and India (Gochhait et al. 2007). In contrast, previous meta-analysis designed studies failed to correlate the R72P polymorphism with breast cancer (Ma et al. 2006; Zhuo et al. 2009), even when subjects were stratified by ethnicity or source of controls (Ma et al. 2011).
In conclusion, we found significant association between the R72P polymorphism in the TP53 gene and the breast cancer risk. The overall data showed an increased risk due to the P allele dominant model, but not in Asia where the risk was associated with the R allele and R dominant model. The present meta-analysis is supported by a large sample size composed by 25,629 cases and 26,633 controls from 41 studies.
References
Akkiprik M, Sonmez O, Gulluoglu BM, Caglar HB, Kaya H, Demirkalem P, Abacioglu U, Sengoz M, Sav A, Ozer A: Analysis of p53 gene polymorphisms and protein over-expression in patients with breast cancer. Pathol Oncol Res 2009, 15: 359-368. 10.1007/s12253-008-9129-6
Alawadi S, Ghabreau L, Alsaleh M, Abdulaziz Z, Rafeek M, Alkhalaf M: P53 gene polymorphisms and breast cancer risk in Arab women. Med Oncol 2011, 3: 709-715.
Aoki MN, da Silva AHAC, Amarante MK, Do Val Carneiro JL, Fungaro MH, Watanabe MA: CCR5 and p53 codon 72 gene polymorphisms: implications in breast cancer development. Int J Mol Med 2009, 23: 429-435.
Baynes C, Healey CS, Pooley KA, Scollen S, Luben RN, Thompson DJ, Pharoah PD, Easton DF, Ponder BA, Dunning AM: SEARCH breast cancer study: common variants in the ATM, BRCA1, BRCA2, CHEK2 and TP53 cancer susceptibility genes are unlikely to increase breast cancer risk. Breast Cancer Res 2007, 9(2):R27. 10.1186/bcr1669
Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, Del Sal G, Syed N, Smith P, Gasco M, Crook T, Lu X: iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet 2006, 38(10):1133-1141. 10.1038/ng1879
Bisof V, Salihović MP, Narancić NS, Skarić-Jurić T, Jakić-Razumović J, Janićijević B, Turek S, Rudan P: TP53 gene polymorphisms and breast cancer in Croatian women: a pilot study. Eur J Gynaecol Oncol 2010, 31(5):539-544.
Böhning D, Malzahn U, Dietz E, Schlattmann P, Viwatwongkasem C, Biggeri A: Some general points in estimating heterogeneity variance with the DerSimonian–Laird estimator. Biostatistics 2002, 3(4):445-457. 10.1093/biostatistics/3.4.445
Buyru N, Tigli H, Dalay N: P53 codon 72 polymorphism in breast cancer. Oncol Rep 2003, 10: 711-714.
Buyru N, Altinisik J, Demokan S, Dalay N: p53 genotypes and haplotypes associated with risk of breast cancer. Cancer Detect Prev 2007, 31: 207-213. 10.1016/j.cdp.2007.04.004
Cavallone L, Arcand SL, Maugard C, Ghadirian P, Mes-Masson AM, Provencher D, Tonin PN: Haplotype analysis of TP53 polymorphisms, Arg72Pro and Ins16, in BRCA1 and BRCA2 mutation carriers of French Canadian descent. BMC Cancer 2008, 8: 96. 10.1186/1471-2407-8-96
Chang-Claude J, Ambrosone CB, Lilla C, Kropp S, Helmbold I, von Fournier D, Haase W, Sautter-Bihl ML, Wenz F, Schmezer P, Popanda O: Genetic polymorphisms in DNA repair and damage response genes and late normal tissue complications of radiotherapy for breast cancer. Br J Cancer 2009, 100(10):1680-1686. 10.1038/sj.bjc.6605036
Conn VS, Ruppar TM, Phillips LJ, Chase J-AD: Using meta-analyses for comparative effectiveness research. Nurs Outlook 2012, 60: 182-190. 10.1016/j.outlook.2012.04.004
Costa S, Pinto D, Pereira D, Rodrigues H, Cameselle-Teijeiro J, Medeiros R, Schmitt F: Importance of TP53 codon 72 and intron 3 duplication 16 bp polymorphisms in prediction of susceptibility on breast cancer. BMC Cancer 2008, 8: 32. 10.1186/1471-2407-8-32
Cox DG, Deer D, Guo Q, Tworoger SS, Hankinson SE, Hunter DJ, De Vivo I: The p53 Arg72Pro and MDM2-309 polymorphisms and risk of breast cancer in the nurses’ health studies. Cancer Causes Control 2007, 18: 621-625. 10.1007/s10552-007-9004-x
Damin AP, Frazzon AP, Damin DC, Roehe A, Hermes V, Zettler C, Alexandre CO: Evidence for an association of TP53 codon 72 polymorphism with breast cancer risk. Cancer Detect Prev 2006, 30: 523-529. 10.1016/j.cdp.2006.09.007
Denisov EV, Cherdyntseva NV, Litvyakov NV, Slonimskaya EM, Malinovskaya EA, Voevoda MI, Belyavskaya VA, Stegniy VN: TP53 mutations and Arg72Pro polymorphism in breast cancers. Cancer Genet Cytogenet 2009, 192(2):93-95. 10.1016/j.cancergencyto.2009.03.014
Dokianakis DN, Koumantaki E, Billiri K, Spandidos DA: P53 codon 72 polymorphism as a risk factor in the development of HPV-associated non-melanoma skin cancers in immunocompetent hosts. Int J Mol Med 2000, 5: 405-409.
Dumont P, Leu J, Della Pietra AC 3rd, George D, Murphy M: The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet 2003, 33(3):357-365. 10.1038/ng1093
Francisco G, Menezes PR, Neto JE, Chammas R: Arg72Pro TP53 polymorphism and cancer susceptibility: a comprehensive meta-analysis of 302 case–control studies. Int J Cancer 2010, 129: 920-930.
Garcia-Closas M, Kristensen V, Langerod A, Qi Y, Yeager M, Burdett L, Welch R, Lissowska J, Peplonska B, Brinton L, Gerhard DS, Gram IT, Perou CM, Børresen-Dale AL, Chanock S: Common genetic variation in TP53 and its flanking genes, WDR79 and ATP1B2, and susceptibility to breast cancer. Int J Cancer 2007, 121: 2532-2538. 10.1002/ijc.22985
Gaudet MM, Gammon MD, Bensen JT, Sagiv SK, Shantakumar S, Teitelbaum SL, Eng SM, Neugut AI, Santella RM: Genetic variation of TP53, polycyclic aromatic hydrocarbon-related exposures, and breast cancer risk among women on Long Island, New York. Breast Cancer Res Treat 2008, 108: 93-99. 10.1007/s10549-007-9573-0
Gochhait S, Bukhari SI, Bairwa N, Vadhera S, Darvishi K, Raish M, Gupta P, Husain SA, Bamezai RN: Implication of BRCA2– 26GA 50 untranslated region polymorphism in susceptibility to sporadic breast cancer and its modulation by p53 codon 72 Arg- Pro polymorphism. Breast Cancer Res 2007, 9: R71. 10.1186/bcr1780
Henrıquez-Hernandez LA, Murias-Rosales A, Hernandez GA, Cabrera DLA, Dıaz-Chico BN, Rosales AM: Gene polymorphisms in TYMS, MTHFR, p53 and MDR1 as risk factors for breast cancer: a case–control study. Oncol Rep 2009, 22: 1425-1433.
Higgins JPT, White IR, Wood AM: Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials 2008, 5: 225-239. 10.1177/1740774508091600
Huang XE, Hamajima N, Katsuda N, Matsuo K, Hirose K, Mizutani M, Iwata H, Miura S, Xiang J, Tokudome S, Tajima K: Association of p53 codon Arg72Pro and p73 G4C14-to-A4T14 at exon 2 genetic polymorphisms with the risk of Japanese breast cancer. Breast Cancer 2003, 10: 307-311. 10.1007/BF02967650
Johnson N, Fletcher O, Palles C, Rudd M, Webb E, Sellick G, dos Santos SI, McCormack V, Gibson L, Fraser A, Leonard A, Gilham C, Tavtigian SV, Ashworth A, Houlston R, Peto J: Counting potentially functional variants in BRCA1, BRCA2 and ATM predicts breast cancer susceptibility. Hum Mol Genet 2007, 16: 1051-1057. 10.1093/hmg/ddm050
Kalemi TG, Lambropoulos AF, Gueorguiev M, Chrisafi S, Papazisis KT, Kotsis A: The association of p53 mutations and p53 codon 72, Her 2 codon 655 and MTHFR C677T polymorphisms with breast cancer in Northern Greece. Cancer Lett 2005, 222: 57-65. 10.1016/j.canlet.2004.11.025
Kara N, Karakus N, Ulusoy AN, Ozaslan C, Gungor B, Bagci H: P53 codon 72 and HER2 codon 655 polymorphisms in Turkish breast cancer patients. DNA Cell Biol 2010, 29(7):387-389. 10.1089/dna.2009.0995
Katiyar S, Thelma BK, Murthy NS, Hedau S, Jain N, Gopalkrishna V, Husain SA, Das BC: Polymorphism of the p53codon 72 Arg/Pro and the risk of HPV type 16/18-associatedcervical and oral cancer in India. Mol Cell Biochem 2003, 252: 117-124. 10.1023/A:1025546610920
Kazemi M, Salehi Z, Chakosari RJ: TP53 Codon 72 polymorphism and breast cancer in Northern Iran. Oncol Res 2009, 18(1):25-30. 10.3727/096504009789745629
Khadang B, Fattahi MJ, Talei A, Dehaghani AS, Ghaderi A: Polymorphism of TP53 codon 72 showed no association with breast cancer in Iranian women. Cancer Genet Cytogenet 2007, 173: 38-42. 10.1016/j.cancergencyto.2006.09.010
Li T, Lu ZM, Guo M, Wu QJ, Chen KN, Xing HP, Mei Q, Ke Y: p53 codon 72 polymorphism (C/G) and the risk of human papillomavirus-associated carcinomas in China. Cancer 2002, 95(12):2571-2576. 10.1002/cncr.11008
Li J, Y-f G, Pei Y, Deng H-W: The impact of imputation on meta-analysis of genome-wide association studies. PLoS One 2012, 7(4):e34486. 10.1371/journal.pone.0034486
Lima JM, Serafim PVP, Silva IDCG, Forones NM: Estudo do polimorfismo genético no gene p53 (códon 72) em câncer colorretal. Arq Gastroenterol 2006, 43(1):8-13. 10.1590/S0004-28032006000100005
Lum SS, Chua HW, Li H, Li WF, Rao N, Wei J, Shao Z, Sabapathy K: MDM2 SNP309 G allele increases risk but the T allele is associated with earlier onset age of sporadic breast cancers in the Chinese population. Carcinogenesis 2008, 29: 754-761. 10.1093/carcin/bgn024
Ma H, Hu Z, Zhai X, Wang S, Wang X, Qin J, Chen W, Jin G, Liu J, Gao J, Wang X, Wei Q, Shen H: Joint effects of single nucleotide polymorphisms in P53BP1 and p53 on breast cancer risk in a Chinese population. Carcinogenesis 2006, 27: 766-771.
Ma Y, Yang J, Liu Z, Zhang P, Yang Z, Wang Y, Qin H: No significant association between the TP53 codon 72 polymorphism and breast cancer risk: a meta-analysis of 21 studies involving 24,063 subjects. Breast Cancer Res Treat 2011, 125: 201-205. 10.1007/s10549-010-0920-1
Mabrouk I, Baccouche S, El-Abed R, Mokdad-Gargouri R, Mosbah A, Saïd S, Daoud J, Frikha M, Jlidi R, Gargouri A: No evidence of correlation between p53 codon 72 polymorphism and risk of bladder or breast carcinoma in Tunisian patients. Ann N Y AcadSci 2003, 1010: 764-770. 10.1196/annals.1299.137
Manning AK, Valley ML, Liu C-T, Rice K, An P, Liu Y, Miljkovic I, Rasmussen-Torvik L, Harris TB, Province MA, Borecki IB, Florez JC, Meigs JB, Cupples LA, Dupuis J: Meta-analysis of Gene-Environment interaction: joint estimation of SNP and SNP × Environment regression coefficients. Genet Epidemiol 2011, 35: 11-18. 10.1002/gepi.20546
Martin A-M, Kanetsky PA, Amirimani B, Colligon TA, Athanasiadis G, Shih HA, Gerrero MR, Calzone K, Rebbeck TR, Weber BL: Germline TP53 mutations in breast cancer families with multiple primary cancers: is TP53 a modifier of BRCA1? Med Genet 2003, 40: e34. 10.1136/jmg.40.4.e34
Menzel HJ, Sarmanova J, Soucek P, Berberich R, Grünewald K, Haun M, Kraft HG: Association of NQO1 polymorphism with spontaneous breast cancer in two independent populations. Br J Cancer 2004, 90: 1989-1994. 10.1038/sj.bjc.6601779
Noma C, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S: Association of p53 genetic polymorphism (Arg72Pro) with estrogen receptor positive breast cancer risk in Japanese women. Cancer Lett 2004, 210: 197-203. 10.1016/j.canlet.2004.03.031
Ohayon T, Gershoni-Baruch R, Papa MZ, Distelman Menachem T, Eisenberg Barzilai S, Friedman E: The R72P P53 mutation is associated with familial breast cancer in Jewish women. Br J Cancer 2005, 92: 1144-1148. 10.1038/sj.bjc.6602451
Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P: The IARC tp53 database: New online mutation analysis and recommendations to users. Hum Mutat 2002, 19(6):607-614. 10.1002/humu.10081
Oluwagbemiga LA, Oluwole A, Kayode AAR: Seventeen years after BRCA1: what is the BRCA mutation status of the breast cancer patients in Africa? – a systematic review. Springer Plus 2012, 1: 83. 10.1186/2193-1801-1-83
Osorio A, Pollán M, Pita G, Schmutzler RK, Versmold B, Engel C, Meindl A, Arnold N, Preisler-Adams S, Niederacher D, Hofmann W, Gadzicki D, Jakubowska A, Hamann U, Lubinski J, Toloczko-Grabarek A, Cybulski C, Debniak T, Llort G, Yannoukakos D, Díez O, Peissel B, Peterlongo P, Radice P, Heikkinen T, Nevanlinna H, Mai PL, Loud JT, McGuffog L, Antoniou AC, et al.: An evaluation of the polymorphisms Ins16bp and Arg72Pro in p53 as breast cancer risk modifiers in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2008, 99: 974-977. 10.1038/sj.bjc.6604624
Petitjean A, Mi A, Al B-D, Hainaut P, Olivier M: TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 2007, 26(15):2157-2165. 10.1038/sj.onc.1210302
Pinto Y, Ibáñez M, Rangel N, Ramírez S, Sánchez W, Vanegas D: Polimorfismos del gen P53 em câncer mamário familiar en una población colombiana. Rev Colomb Cir 2007, 22: 17-26.
Rajkumar T, Samson M, Rama R, Sridevi V, Mahji U, Swaminathan R, Nancy NK: TGFbeta1 (Leu10Pro), p53 (Arg72Pro) can predict for increased risk for breast cancer in south Indian women and TGFb1 Pro (Leu10Pro) allele predicts response to neo-adjuvant chemo-radiotherapy. Breast Cancer Res Treat 2008, 112: 81-87. 10.1007/s10549-007-9821-3
Schmidt MK, Reincke S, Broeks A, Braaf LM, Hogervorst FB, Tollenaar RA, Johnson N, Fletcher O, Peto J, Tommiska J, Blomqvist C, Nevanlinna HA, Healey CS, Dunning AM, Pharoah PD, Easton DF, Dörk T, Van't Veer LJ: Breast cancer association consortium: Do MDM2 SNP309 and TP53 R72P interact in breast cancer susceptibility? A large pooled series from the breast cancer association consortium. Cancer Res 2007, 67: 9584-9590. 10.1158/0008-5472.CAN-07-0738
Schmidt MK, Tommiska J, Broeks A, Leeuwen FEV, Veer LJV, Pharoah PD, Easton DF, Shah M, Humphreys M, Dörk T, Reincke SA, Fagerholm R, Blomqvist C, Nevanlinna H: Combined effects of single nucleotide polymorphisms TP53 TP53ARG P and MDM2 SNP309, and p53 expression on survival of breast cancer patients. Breast Cancer Res 2009, 11(6):R89. 10.1186/bcr2460
Siddique MM, Balram C, Fiszer-Maliszewska L, Aggarwal A, Tan A, Tan P, Soo KC, Sabapathy K: Evidence for selective expression of the p53 codon 72 polymorphs: implications in cancer development. Cancer Epidemiol Biomarkers Prev 2005, 14: 2245-2252. 10.1158/1055-9965.EPI-05-0153
Singh V, Rastogi N, Mathur N, Singh K, Singh MP: Association of polymorphism in MDM-2 and p53 genes with breast cancer risk in Indian women. Ann Epidemiol 2008, 18: 48-57. 10.1016/j.annepidem.2007.06.006
Sinilnikova OM, Antoniou AC, Simard J, Healey S, Léoné M, Sinnett D, Spurdle AB, Beesley J, Chen X, KConFab , Greene MH, Loud JT, Lejbkowicz F, Rennert G, Dishon S, Andrulis IL OCGN, Domchek SM, Nathanson KL, Manoukian S, Radice P, Konstantopoulou I, Blanco I, Laborde AL, Durán M, Osorio A, Benitez J, Hamann U, Hogervorst FB, van Os TA, Gille HJ HEBON, et al.: The TP53 Arg72Pro and MDM2 309G4T polymorphisms are not associated with breast cancer risk in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2009, 101: 1456-1460. 10.1038/sj.bjc.6605279
Sprague BL, Trentham-Dietz A, Garcia-Closas M, Newcomb PA, Titus-Ernstoff L, Hampton JM, Chanock SJ, Haines JL, Egan KM: Genetic variation in TP53 and risk of breast cancer in apopulation-based case control study. Carcinogenesis 2007, 28: 1680-1686. 10.1093/carcin/bgm097
Suresh K, Venkatesan R, Chandirasekar R, Kumar BL, Sasikala K: Association of Trp53 arg72pro polymorphic variants with breast cancer – a case control study in south Indian population. Biol Med 2011, 3(1):15-22.
Suspitsin EN, Buslov KG, Grigoriev MY, Ishutkina JG, Ulibina JM, Gorodinskaya VM, Pozharisski KM, Berstein LM, Hanson KP, Togo AV, Imyanitov EN: Evidence against involvement of P53 polymorphism in breast cancer predisposition. Int J Cancer 2003, 103: 431-433. 10.1002/ijc.10834
Thomas M, Kalira A, Labrecque S, Pim D, Banks L, Matlashewski G: Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol 1999, 19(2):1092-1100.
Tommiska J, Eerola H, Heinonen M, Salonen L, Kaare M, Tallila J, Ristimäki A, von Smitten K, Aittomäki K, Heikkilä P, Blomqvist C, Nevanlinna H: Breast cancer patients with p53 Pro72 homozygous genotype have a poorer survival. Clin Cancer Res 2005, 11: 5098-5103. 10.1158/1078-0432.CCR-05-0173
Trifa F, Karray-Chouayekh S, Mabrouk I, Baccouche S, Khabir A, Sellami-Boudawara T, Gargouri A, Mokdad-Gargouri R: Haplotype analysis of p53 polymorphisms: Arg72Pro, Ins16bp and G13964C in Tunisianpatients with familial or sporadic breast cancer. Cancer Epidemiol 2010, 34(2):184-188. 10.1016/j.canep.2010.02.007
Wang-Gohrke S, Becher H, Kreienberg R, Runnebaum IB, Chang-Claude J: Intron 3 16 bp duplication polymorphism of p53 is associated with an increased risk for breast cancer by the age of 50 years. Pharmacogenetics 2002, 12: 269-272. 10.1097/00008571-200204000-00012
Weston A, Godbold JH: Polymorphisms of H-ras-1 and p53 in breast cancer and lung cancer: a meta-analysis. Environ Health Perspect 1997, 105: 919-926. 10.1289/ehp.97105s4919
Zhuo W, Zhang Y, Xiang Z, Cai L, Chen Z: Polymorphisms of TP53 codon 72 with breast carcinoma risk: evidence from 12226 cases and 10782 controls. J Exp Clin Cancer Res 2009, 28: 115. 10.1186/1756-9966-28-115
Acknowledgements
The authors gratefully acknowledge the financial support of Brazilian Ministry of Education (MEC) through University for Everyone Program (PROUNI) fellowship to Meire Luzia Gonçalves and Foundation for the Support of Research in the State of Goiás (FAPEG) and Coordination for the Advancement of Higher Education Staff (CAPES) through fellowship to Jacqueline Andréia Bernardes Leão Cordeiro and Cesar Augusto Sam Tiago Vilanova-Costa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests. There are no financial or personal interests that might be viewed as inappropriate influences on the work presented herein. This manuscript was completely financed by governmental and nonprofit institutions, Foundation for the Support of Research in the State of Goiás (FAPEG), Coordination for the Advancement of Higher Education Staff (CAPES) and Brazilian Ministry of Education (MEC).
Authors’ contributions
The work presented here was carried out in collaboration between all authors. AMTCS, MLG and SMB conceived and designed the study. MLG, SMB and AMTCS reviewed the papers for inclusion in the meta-analysis. MLG, SMB, JABLC and AMTCS coded the studies for the moderation tests. AMTCS performed the effect size calculations and moderation tests. AMTCS analyzed the data. MLG, SMB, AMTCS and VAS wrote the paper. FMA and CASTVC reviewed the manuscript. All authors have contributed to, seen and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonçalves, M.L., Borja, S.M., Cordeiro, J.A.B.L. et al. Association of the TP53 codon 72 polymorphism and breast cancer risk: a meta-analysis. SpringerPlus 3, 749 (2014). https://doi.org/10.1186/2193-1801-3-749
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-3-749