Abstract
The aim of our research is the screening of extracts of marine sponges for their antifungal activity against phytopathogenic fungi. The in vitro screening of hydroalcoholic and organic extracts of ten marine sponges from Atlantic coast of Morocco against five phytopathogenic fungi (Fusarium oxysporum f.sp. melonis, Fusarium oxysporum f.sp. radicis-lycopersici, F usarium oxysporum f.sp. ciceris, Botrytis cinerea and Penicillium digitatum) showed that only two sponges (Haliclona viscosa and Cynachirella tarentina) are active against all phytopathogenic fungi studied.
Similar content being viewed by others
Introduction
Agriculture in Morocco is an important economic sector, with 40% of the population living on its revenues. The agricultural area is estimated to be 9.5 million hectares. Fungi are the main responsible agents for losses in agriculture and horticulture and can infect any part of the plant (Messiaen et al. 1991). The fight against these fungi is based on the use of chemical pesticides. However, chemical pesticides sprayed into the air or discharged into the soil can be harmful to the environment and to humans as well.
More than 15 000 natural products were isolated between 1965 and 2005 from marine organisms (Blunt et al. 2007). One of the main factors contributing to this trend is related to modern technology, and ocean biodiversity has become more accessible (Battershill et al. 2005).
Most marine invertebrates, which lack defence structures, have developed chemical defence systems in producing toxic secondary metabolites (Anderson et al. 1994; Aratake et al. 2009).
The sponges, which have a very primitive origin, adopted and developed a cemical very powerful defence (Sipkema et al. 2005) and are the source of many chemical compounds with various biological activities, including antitumor (Acosta and Rodriguez 1992; Carmely et al. 1989), antiviral (Carter and Rinehart Jr 1978) antialgal (Wright et al. 2011), anti-inflammatory (Randazzo et al. 2001), antiparasitic (Galeano et al. 2011; Kossuga et al. 2008), antibacterial (Ankisetty and Slattery 2012; El Amraoui et al. 2014) and antifungal activities (Clark et al. 2001; El-Amraoui et al. 2013; Sata et al. 1999). These compounds also show the chemical diversity, and are composed among others of unusual nucleosides (Bergmann and Feeney 1951; Wang et al. 2009), peptides (Sjogren et al. 2006), and fatty acids (Carballeira et al. 2007; Carballeira and Pagan 2001; Pham et al. 1999).
Sponges consist not only of sponge tissue but also of microorganisms, which represent 50% of their mass. So, is that the antifungal that is secreted by the sponge or a microorganism associated with this sponge? The isolation of microorganisms associated to sponges and screening of antifungal metabolites produced by these microorganisms may provide an answer.
In Morocco, the researches of the metabolism products of medicinal plants and other groups of terrestrial or marine organisms are intensified to explore the possible use of metabolites in different areas. Despite the richness and biodiversity of the Moroccan sea, invertebrates and algae from seabed are poorly studied.
In this study, we report the antifungal activity of ethanol and dichloromethane extracts of ten marine sponges collected from Coastal Atlantic of El Jadida (Morocco) to select the most active species, which could be utilized to purify antifungal compounds.
Results and discussion
The identification of sponge species and their sampling sites are summarized in Table 1.
The results of the screening of antifungal activity of sponge extracts against phytopathogenic fungi are summarized in Table 2.
Among 20 extracts tested, only three extracts (15%), showed antifungal activity against the studied phytopathogenic fungi.
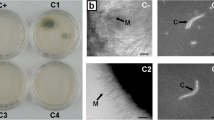
Organic and hydroalcoholic extracts of H. viscosa exhibit antifungal activity whereas in C. tarentina, only the organic extract is active. This inhibition’s effect can be shown in Figure 1.
Example of antifungal activity of extracts of H. viscosa and C. tarentina against Fusarium oxysporum f.sp. melonis (a), Botritis cinerea (b) and Penicillium digitatum (c). (C+ : positive control (Desogerme sp); 8C: dichloromethane extract of C. tarentina; 14C: dichloromethane extract of H. viscosa; 14B: ethanol extract of H. viscosa).
The sponge genus Haliclona is known for its high chemical various secondary metabolites with interesting biological activities (Faulkner 2002) including the antifungal (Barrett et al. 1996; Clark et al. 2001; El-Wahidi et al. 2013), antileishmanial (Dube et al. 2007), antioxidant (Regoli et al. 2004), cytotoxic (Fusetani et al. 1989) and other activities (Lakshmi et al. 2009; Randazzo et al. 2001).
Up to now, the researches conducted on H. viscosa led to the isolation of a number of alkaloids. Fusetani et al. 1989 have isolated two cytotoxic compounds, haliclamine A and B from H. viscosa. Volk and Kock 2003 have isolated viscosamine, then viscosaline in Volk and Kock 2004. Recently, two forms of viscosaline have been isolated (Schmidt et al. 2012). In addition to this, two other alkaloids, haliclamine C and D, were isolated from H. viscosa (Volk et al. 2004).
Lately, we have isolated a new product called haliscosamine from H. viscosa (El-Amraoui et al. 2013). This product is active against yeasts involved in human pathology. The chemistry of Cinachyrella tarentina sponge is rarely studied (El-Amraoui et al. 2010; El-Wahidi et al. 2011). This sponge was discovered in Italy (Pulitzer-Finali 1983), and now, we have collected this sponge from the Deauville beach, El Jadida, Morocco (El-Amraoui et al. 2010).
Conclusion
Preliminary results have shown that Moroccan sponges constitute a potential source of compounds, which can be used for crop protection. Haliclona viscosa and Cinchyrella tarentina have an interesting antifungal potential. Thus, these sponges provide a potential source of antifungal compound to fight against plant diseases and should be investigated for isolation of this natural compound.
Materials and methods
Phytopathogenic strains
Strain of Fusarium oxysporum f.sp. melonis (FOM 20474 CECT) was obtained from Coleccion Espanola de Cultivos Tipo (Suárez-Estrella et al. 2007), Fusarium oxysporum f.sp. radicis-lycopersici (FORL), F usarium oxysporum f.sp. ciceris (FOC) and were obtained from the laboratory of Plant Pathology, Faculty of Sciences (El Jadida, Morocco), Botrytis cinerea (BC630) was obtained from biology-biochemistry department, Reims Champagne-Ardenne University, France and Penicillium digitatum (PD001), isolated from an infected orange, were used throughout this study.
Sponge materials
Ten marine sponges were collected from five sites of the littoral Atlantic coast of El Jadida (Morocco). Figure 2 shows the locations and depths of sampling sponges. All the sponges were identified by Dr. Maria-Jesús Uriz, Research Professor at the Centro de Estudios Avanzados de Blanes (CEAB) and Consejo Superior de Investigaciones Cientificas (CSIC) Spain by morphological characteristics and molecular methods (El-Amraoui et al. 2010). The collected materials were immediately frozen at −4°C for one night prior to extraction.
Preparation of the extracts
Each sponge (100 g wet weight) was thawed, and extracted with ethanol (3 × 100 ml). The ethanol was evaporated at reduced pressure. The suspension was completed with sterile water to 100 ml and extracted with CH2Cl2 (3 × 100 ml).
The CH2Cl2 extract was dried on anhydrous sodium sulphate (Na2SO4), then filtered and concentrated at reduced pressure to give a C extract.
The aqueous phase was lyophilised and dissolved twice in absolute ethanol, then filtered and concentrated at reduced pressure to give a B extract (El-Amraoui et al. 2010).
DESOGERME SP VEGETAUX®
DESOGERME SP VEGETAUX® (LAKORALE, Morocco), used in this study as a positive control, is an algaecide, fungicide and bactericide product used in Morocco both to remove algae, fungi and bacteria in irrigation systems and also to disinfect soil. It consists of 20 g/L of polyhexamethyle bioguanidine hydrochlorique, and 50 g/L of N-alkyl dimethyl benzyl ammonium chloride.
In vitro antifungal activity
The antifungal activity was assessed in vitro by agar disc-diffusion test.
Agar disc-diffusion test
This test uses Potato Dextrose Agar (PDA) as medium [Difco]. Conidial suspension was prepared from a 5-d-old fungal culture and adjusted with Malassez’s cellule in sterile water in order to obtain a final concentration of 105conidia/mL. Each disk received 100 μg of sponge extract (20 μL of each extract at 5 mg/mL were added to each cellulose disc) and was applied on the test media which were previously inoculated with each test strain (El-Amraoui et al. 2010). Plates were first kept at 4°C for at least two hours to allow the diffusion of chemicals, and then incubated at 28°C. Inhibition zones were measured after 24 h of incubation (Galeano and Martınez 2007). Standard disks of DESOGERME SP VEGETAUX® (100 μg) served as positive antifungal control. All the assays were carried out in triplicate.
References
Acosta AL, Rodriguez AD: 11-oxoaerothionin: a cytotoxic antitumor bromotyrosine-derived alkaloid from the Caribbean marine sponge Aplysina lacunosa . J Nat Prod 1992, 55(7):1007-1012. 10.1021/np50085a031
Anderson AP, Beveridge AA, Capon R: Pharmacological properties of the natural marine product furospongin-1. Clin Exp Pharmacol Physiol 1994, 21(12):945-953. 10.1111/j.1440-1681.1994.tb02656.x
Ankisetty S, Slattery M: Antibacterial secondary metabolites from the cave sponge Xestospongia sp . Mar Drugs 2012, 10: 1037-1043. 10.3390/md10051037
Aratake S, Trianto A, Hanif N, de Voogd NJ, Tanaka J: A new polyunsaturated brominated fatty acid from a Haliclona sponge. Mar Drugs 2009, 7(4):523-527. 10.3390/md7040523
Barrett AG, Boys ML, Boehm TL: Total synthesis of (+)-papuamine: an antifungal pentacyclic alkaloid from a marine sponge, Haliclona sp . J Org Chem 1996, 61(2):685-699. doi:jo951413z 10.1021/jo951413z
Battershill C, Jaspars M, Lon P: Marine biodiscovery: new drugs from the ocean depths. Biologist 2005, 52: 107-114.
Bergmann W, Feeney RJ: Contributions to the study of marine products. The nucleosides of sponges. J Org Chem 1951, 16: 981-987. 10.1021/jo01146a023
Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR: Marine natural products. Nat Prod Rep 2007, 24(1):31-86. 10.1039/b603047p
Carballeira NM, Pagan M: New methoxylated fatty acids from the Caribbean sponge Callyspongia fallax . J Nat Prod 2001, 64(5):620-623. doi:np000537q 10.1021/np000537q
Carballeira NM, Montano N, Vicente J, Rodriguez AD: Novel cyclopropane fatty acids from the phospholipids of the Caribbean sponge Pseudospongosorites suberitoides . Lipids 2007, 42(6):519-524. doi:10.1007/s11745-007-3047-3 10.1007/s11745-007-3047-3
Carmely S, Roll M, Loya Y, Kashman Y: The structure of eryloside A, a new antitumor and antifungal 4-methylated steroidal glycoside from the sponge Erylus lendenfeldi . J Nat Prod 1989, 52(1):167-170. 10.1021/np50061a022
Carter GT, Rinehart KL Jr: Acarnidines, novel antiviral and antimicrobial compounds from the sponge Acarnus erithacus (de Laubenfels). J Am Chem Soc 1978, 100: 4302-4304. 10.1021/ja00481a049
Clark RJ, Garson MJ, Hooper JN: Antifungal alkyl amino alcohols from the tropical marine sponge Haliclona n. sp. J Nat Prod 2001, 64(12):1568-1571. doi:np010246x 10.1021/np010246x
Dube A, Singh N, Saxena A, Lakshmi V: Antileishmanial potential of a marine sponge, Haliclona exigua (Kirkpatrick) against experimental visceral leishmaniasis. Parasitol Res 2007, 101(2):317-324. 10.1007/s00436-007-0469-z
El Amraoui B, El Amraoui M, Cohen N, Fassouane A: Antifungal and antibacterial activity of marine microorganisms. Ann Pharm Fr 2014, 72: 107-111. 10.1016/j.pharma.2013.12.001
El-Amraoui B, Biard JF, Uriz MJ, Rifai S, Fassouane A: Antifungal and antibacterial activity of Porifera extracts from the Moroccan Atlantic coasts. J Mycol Med 2010, 20(1):70-74.http://dx.doi.org/10.1016/j.mycmed.2009.11.001 10.1016/j.mycmed.2009.11.001
El-Amraoui B, Biard JF, Fassouane A: Haliscosamine: a new antifungal sphingosine derivative from the Moroccan marine sponge Haliclona viscosa . Springerplus 2013, 2: 252. doi:10.1186/2193- 1801-2-252 363 10.1186/2193-1801-2-252
El-Wahidi M, El-Amraoui B, Biard JF, Uriz MJ, Fassouane A, Bamhaoud T: Variation saisonnière et géographique de l’activité antifongique des extraits de deux éponges marines récoltées sur le littoral atlantique d’El Jadida, Maroc. Journal de Mycologie Médicale/Journal of Medical Mycology 2011, 21(1):28-32.http://dx.doi.org/10.1016/j.mycmed.2010.11.005 10.1016/j.mycmed.2010.11.005
El-Wahidi M, El-Amraoui B, Fassouane A, Bamhaoud T: Isolement bio-dirigé d’un antifongique à partir de Haliclona enamela récoltée du port de Jorf Lasfar, Maroc. Journal de Mycologie Médicale/J Med Mycol 2013. doi:10.1016/j.mycmed.2013.04.006
Faulkner DJ: Marine natural products. Nat Prod Rep 2002, 19: 1-48.
Fusetani N, Yasumuro K, Matsunaga S, Hirota H: Haliclamines A and B, cytotoxic macrocyclic alkaloids from a sponge of the genus Haliclona . Tetrahedron Lett 1989, 30: 6891-6894. 10.1016/S0040-4039(01)93381-7
Galeano E, Martınez A: Antimicrobial activity of marine sponges from Uraba Gulf, Colombian Caribbean region. J Mycol Med 2007, 17: 21-24. 10.1016/j.mycmed.2006.12.002
Galeano E, Thomas OP, Robledo S, Munoz D, Martinez A: Antiparasitic bromotyrosine derivatives from the marine sponge Verongula rigida . Mar Drugs 2011, 9(10):1902-1913. doi:10.3390/md9101902 marinedrugs-09-01902
Kossuga MH, Nascimento AM, Reimao JQ, Tempone AG, Taniwaki NN, Veloso K, Ferreira AG, Cavalcanti BC, Pessoa C, Moraes MO, Mayer AM, Hajdu E, Berlinck RG: Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J Nat Prod 2008, 71(3):334-339. doi:10.1021/np0705256 10.1021/np0705256
Lakshmi V, Srivastava S, Kumar Mishra S, Misra S, Verma M, Misra-Bhattacharya S: In vitro and in vivo antifilarial potential of marine sponge, Haliclona exigua (Kirkpatrick), against human lymphatic filarial parasite Brugia malayi: antifilarial activity of H. exigua . Parasitol Res 2009, 105(5):1295-1301. 10.1007/s00436-009-1555-1
Messiaen CM, Blancard D, Rouxel F, Lafon R: Les maladies des plantes maraîchères. INRA, Paris; 1991:552.
Pham NB, Butler MS, Hooper JN, Moni RW, Quinn RJ: Isolation of xestosterol esters of brominated acetylenic fatty acids from the marine sponge Xestospongia testudinaria . J Nat Prod 1999, 62(10):1439-1442. doi:np9901635 10.1021/np9901635
Pulitzer-Finali G: A collection of Mediterranean Demospongiae (Porifera) with, in appendix, a list of the Demospongiae hitherto recorded from the Mediterranean sea. Annali del Museo Civico di Storia Naturale di Genova 1983, 84: 445-621.
Randazzo A, Bifulco G, Giannini C, Bucci M, Debitus C, Cirino G, Gomez-Paloma L: Halipeptins A and B: two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J Am Chem Soc 2001, 123(44):10870-10876. 10.1021/ja010015c
Regoli F, Nigro M, Chierici E, Cerrano C, Schiapparelli S, Totti C, Bavestrello G: Variations of antioxidant efficiency and presence of endosymbiotic diatoms in the Antarctic porifera Haliclona dancoi . Mar Environ Res 2004, 58(2–5):637-640.
Sata NU, Matsunaga S, Fusetani N, Van Soest RW: Aurantosides D, E, and F: new antifungal tetramic acid glycosides from the marine sponge Siliquariaspongia japonica . J Nat Prod 1999, 62(7):969-971. doi:10.1021/np9900021 np9900021 10.1021/np9900021
Schmidt G, Timm C, Grube A, Volk CA, Kock M: Viscosalines B(1,2) and E(1,2): challenging new 3-alkyl pyridinium alkaloids from the marine sponge Haliclona viscosa . Chemistry 2012, 18(26):8180-8189. doi:10.1002/chem.201101362 10.1002/chem.201101362
Sipkema D, Franssen MC, Osinga R, Tramper J, Wijffels RH: Marine sponges as pharmacy. Mar Biotechnol (NY) 2005, 7: 142-162. 10.1007/s10126-004-0405-5
Sjogren M, Johnson AL, Hedner E, Dahlstrom M, Goransson U, Shirani H, Bergman J, Jonsson PR, Bohlin L: Antifouling activity of synthesized peptide analogs of the sponge metabolite barettin. Peptides 2006, 27(9):2058-2064. doi:S0196-9781(06)00159-8 10.1016/j.peptides.2006.03.027
Suárez-Estrella F, Vargas-Garcýa C, Lopeza MJ, Capelb C, Morenoa J: Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f.sp. melonis . Crop Prot 2007, 26: 46-53. 10.1016/j.cropro.2006.04.003
Volk CA, Kock M: Viscosamine: the first naturally occurring trimeric 3-alkyl pyridinium alkaloid. Org Lett 2003, 5(20):3567-3569. 10.1021/ol035006i
Volk CA, Kock M: Viscosaline: new 3-alkyl pyridinium alkaloid from the Arctic sponge Haliclona viscosa . Org Biomol Chem 2004, 2(13):1827-1830. 10.1039/b403413a
Volk CA, Lippert H, Lichte E, Köck M: Two New Haliclamines from the Arctic Sponge Haliclona viscosa . Eur J Org Chem 2004, 14: 3154-3158.
Wang B, Dong J, Zhou X, Lee KJ, Huang R, Zhang S, Liu Y: Nucleosides from the marine sponge Haliclona sp. Z Naturforsch C 2009, 64(1–2):143-148.
Wright AD, McCluskey A, Robertson MJ, MacGregor KA, Gordon CP, Guenther J: Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi . Org Biomol Chem 2011, 9: 400-407. 10.1039/c0ob00326c
Acknowledgements
We thank Dr. Maria-Jesús Uriz, Research Professor at the Centro de Estudios Avanzados de Blanes (CEAB), Spain for sponge identification, F. Suárez-Estrella of Alméria University, Spain who provide us with a Fusarium oxysporum f.sp. melonis strains, Mr. Aziz Aziz Professor at the Reims Champagne-Ardenne University, France who provide us with a Botritis cinerea strains and M. Mouchene, director of the agricultural department in LACORALE society who provided us with a DESOGERME SP VEGETAUX®.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
El Amraoui, B., El Wahidi, M. & Fassouane, A. In vitro screening of antifungal activity of marine sponge extracts against five phytopathogenic fungi. SpringerPlus 3, 629 (2014). https://doi.org/10.1186/2193-1801-3-629
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-3-629