Abstract
Isopropanol represents a widely-used commercial alcohol which is currently produced from petroleum. In nature, isopropanol is excreted by some strains of Clostridium beijerinckii, simultaneously with butanol and ethanol during the isopropanol butanol ethanol (IBE) fermentation. In order to increase isopropanol production, the gene encoding the secondary-alcohol dehydrogenase enzyme from C. beijerinckii NRRL B593 (adh) which catalyzes the reduction of acetone to isopropanol, was cloned into the acetone, butanol and ethanol (ABE)-producing strain C. acetobutylicum ATCC 824. The transformants showed high capacity for conversion of acetone into isopropanol (> 95%). To increase isopropanol production levels in ATCC 824, polycistronic transcription units containing, in addition to the adh gene, homologous genes of the acetoacetate decarboxylase (adc), and/or the acetoacetyl-CoA:acetate/butyrate:CoA transferase subunits A and B (ctfA and ctfB) were constructed and introduced into the wild-type strain. Combined overexpression of the ctfA and ctfB genes resulted in enhanced solvent production. In non-pH-controlled batch cultures, the total solvents excreted by the transformant overexpressing the adh, ctfA, ctfB and adc genes were 24.4 g/L IBE (including 8.8 g/L isopropanol), while the control strain harbouring an empty plasmid produced only 20.2 g/L ABE (including 7.6 g/L acetone). The overexpression of the adc gene had limited effect on IBE production. Interestingly, all transformants with the adh gene converted acetoin (a minor fermentation product) into 2,3-butanediol, highlighting the wide metabolic versatility of solvent-producing Clostridia.
Similar content being viewed by others
Introduction
The limited supply and the negative environmental effects of the use of petroleum-derived fuels and chemicals have stimulated efforts for the development of more environmentally-friendly processes. In this respect, the fermentation of carbohydrates into acetone, butanol and ethanol (ABE) or isopropanol, butanol and ethanol (IBE) is a promising way for the production of green chemicals and fuels. In the past, both ABE and IBE fermentations were performed worldwide at industrial scale until they were replaced by petrochemical processes (Jones and Woods1986; Rogers et al.2006). Many resources are currently being devoted to develop economically-viable fermentation processes based primarly on lignocellulosic biomass hydrolysates as substrates (Dürre20072008; López-Contreras et al.2010; Green2011).
For fuel applications, the IBE mixture appears to be more attractive than the ABE one. Isopropanol shows a higher energy density than acetone (23.9 MJ/L vs 22.6 MJ/L) and this mixture has already been used as an additive for gasoline or diesel oil (Peralta-Yahya and Keasling2010). Isopropanol can be catalytically condensed into di-isopropyl ether (DIPE) (Logsdon and Loke2000). DIPE displays good fuel properties and could substitute methyl tert-butyl ether (MTBE) as isooctane index enhancer in gasoline composition (Huang and Sorensen1990). Another important potential application of biologically-produced isopropanol is as a precursor for green propylene, which is the second most important chemical intermediate in the petrochemical industry after ethylene. Propylene is used in many chemical reactions for the synthesis of a wide variety of products, including plastic materials.
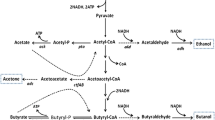
The clostridial species that produce neutral solvents (ABE or IBE) are strictly anaerobes, rod-shaped and spore-forming bacteria. Most of them, such as C. acetobutylicum ATCC 824, produce ABE but some others, such as C. beijerinckii NRRL B593, excrete IBE (George et al.1983; Chen and Hiu1986). ABE and IBE batch fermentations are similar, displaying a biphasic kinetic pattern (Jones and Woods1986; Girbal and Soucaille1998). After production of acetic and butyric acids in exponential growth, fermentation switches to formation of neutral solvents shortly before entering stationary phase. In the IBE fermentation, depending on the strain and the cultivation conditions, residual acetone may also be an end-product (Ismaiel et al.1993).
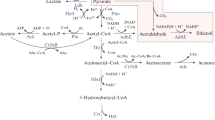
In C. beijerinckii NRRL B593, the reduction of acetone into isopropanol is catalyzed by a NADPH-dependent secondary-alcohol dehydrogenase (s-Adh), which has been extensively characterized (Yan et al.1988; Ismaiel et al.1993; Korkhin et al.1998; Goihberg et al.2010). Although the s-Adh was clearly distinct from clostridial primary-alcohol dehydrogenases (Chen1995) that reduce butyraldehyde into butanol, the s-Adh showed activity on both primary and secondary alcohols, with a preference for secondary ones (Ismaiel et al.1993). Kinetic studies confirmed that the physiological substrate was acetone.
Metabolic engineering has been used to create pathways for isopropanol production in Escherichia coli. Introduction of four genes from C. acetobutylicum (ctfA, ctfB, adc and thiolase (thl)) into E. coli generated a strain capable of producing acetone (Bermejo et al.1998). By introduction of the C. beijerinckii adh gene in combination with the aforementioned genes, isopropanol excretion by E. coli was achieved up to the concentrations of 4.9 g/L (Hanai et al.2007) and 13 g/L (Jojima et al.2008). The engineered E. coli strains surpassed the best reported wild-type clostridial strains, C. beijerinckii and C. isopropylicum, excreting approximately 4 g/L isopropanol (Groot and Luyben1986; Matsumura et al.1992). A major advantage of the engineered E. coli strains was the lack of important competing pathways for by-products. Recently, the adh gene from C. beijerinckii was cloned into the ABE-producing strain C. acetobutylicum ATCC 824. The resulting transformants excreted 6.1 g/L isopropanol and a minor amount of acetone (Lee et al.2012).
In the present study, different IBE-producing transformants of C. acetobutylicum that showed high isopropanol excretion capacities have been constructed. The fermentation performances of the transformants were characterized in batch cultures using laboratory-scale bioreactors with or without pH-control and compared to those of the wild-type IBE or ABE- producing strains. In addition, formation of 2,3-butanediol by C. acetobutylicum transformant strains harbouring the adh gene was described and characterized for the first time.
Materials and methods
Strains and cultivation conditions
The microorganisms used are listed in Table1. E. coli culture stocks were stored at −80°C in 20% (v/v) glycerol. E. coli strains were cultivated at 37°C with agitation (250 rpm) in LB (lysogeny broth) medium (Bertani2004). For cultivation of E. coli BW25113 harbouring pMTL500E based plasmids, the LB medium was supplemented with 2% (w/w) glucose.
Clostridial wild-type and transformants were stored as spore suspensions at −20°C in 15% (v/v) glycerol. Prior to the inoculation of pre-cultures, each spore suspension (500 μL) was heat-shocked in a water bath for 10 min at 70°C (C. acetobutylicum ATCC 824 and its transformants) or 1 min at 100°C (C. beijerinckii NRRL B593). Culture media for Clostridia were made anaerobic by sparging with nitrogen gas. Cultures and pre-cultures were performed in CM1 medium (Kuit et al.2012) which contains, per liter: yeast extract, 5.0 g; KH2PO4, 1.0 g; K2HPO4, 0.76 g; ammonium acetate, 3.0 g; p-aminobenzoic acid, 0.10 g; MgSO4·7 H2O, 1.0 g; and FeSO4·7 H2O, 0.5 g, glucose, 90 g. Pre-cultures of C. beijerinckii were grown in medium containing 60 g/L glucose.
Batch fermentations were carried out anaerobically in 2-L (1-L working volume) Applikon glass bioreactors (Applikon, The Netherlands) using CM1 medium. When needed, pH was maintained at 5.0 by automatic addition of 4 M KOH solution. Static flask fermentations were carried out anaerobically in 120 mL serum bottles with 50 mL of CM1 medium.
For the preparation of clostridial competent cells, cells were grown on CG medium (Roos et al.1985), as described previously (Oultram et al.1988). When required, culture media were supplemented with ampicillin (100 μg/mL), chloramphenicol (30 μg/mL), erythromycin (50 μg/mL or 30 μg/mL for liquid cultures and plates, respectively).
Microbial growth was monitored by optical density measurements at 600 nm (Pharmacia Biotech Ultrospec 2000).
Plasmid construction and transformation
Genomic DNA from Clostridium strains was isolated using GenElute bacterial genomic DNA kit from Sigma-Aldrich. Plasmid DNA from E. coli strains was extracted using the GeneJet plasmid miniprep kit from Fermentas. PCR amplifications were done using high fidelity PCR master mix (Roche). DNA restrictions and ligations were performed using New England Biolabs restriction enzymes (Apa I, Fnu 4H1, Spe I, Sph I, Xba I and Xho I) and T4 DNA-Ligase enzyme, respectively. The oligonucleotides used are listed in Table2 and were synthesized by Eurogenetec. Chemically competent E. coli strains were prepared using the Z-competent kit from Zymo-research. Kits were used according to supplier protocols.
The constructs used for the C. acetobutylicum transformation were based on the E. coli/Clostridium shuttle vector pMTL500E (Table1). The thiolase promoter was PCR-amplified from genomic-DNA of C. acetobutylicum ATCC 824 using thlp_for and thlp_rev oligonucleotides and digested by Apa I and Sph I. The adh gene was PCR-amplified from C. beijerinckii NRRL B593 genomic-DNA using adh_for and adh_rev oligonucleotides and digested by Apa I and Xho I. The thl promoter and adh gene sequences were simultaneously cloned into pMTL500E plasmid (digested by Sph I and Xho I) to yield pFC002 construct. Genes from C. acetobutylicum ATCC 824 were amplified by PCR on genomic DNA using adc_for and adc_rev oligonucleotides for adc, ctfAB_for and ctfAB_rev oligonucleotides for ctfA and ctfB. PCR amplifications of adc gene and ctfA_ctfB genes were digested by Xho I and Spe I restriction enzymes and cloned into pFC002 digested Xho I and Xba I to yield pFC005 and pFC006, respectively. PCR amplification of adc gene was subsequently cloned into pFC006 digested Xho I and Xba I to yield pFC007.
Plasmid DNA constructs were introduced into chemically competent E. coli DH10B harbouring pAN2 (Oultram et al.2007) for methylation prior to transformation into C. acetobutylicum as described earlier (Mermelstein and Papoutsakis1993). Correct methylation was checked by restriction analysis with Fnu 4HI. Methylated pFC002, pFC005, pFC006, pFC007 and methylated pMTL500E plasmids (Table1) were electroporated into C. acetobutylicum ATCC 824 as described by Oultram et al (Oultram et al.1988). Erythromycin-resistant colonies were cultivated in CGM liquid medium and total DNA was extracted as described above. The presence of the respective construct in the transformants obtained was confirmed by PCR on DNA extracted from the different colonies using specific oligonucleotides for the specific inserts. Transformant strains harbouring the right construct were found for all constructs (results not shown). Transformant strains were stored as spore suspensions and kept at −20°C.
The thiolase transcription unit was amplified from C. acetobutylicum genomic DNA using oligonucleotides thlp_for and thlp_rev (Table2). The amplificate was cloned into pCR blunt II Topo (Invitrogen) to yield pTHL. The plasmid pTHL was cotransformed with pFC002, pFC005, pFC006, pFC007 or pMTL500E in chimiocompetent E. coli BW25113.
Reduction of ketones by cell-free extracts
For preparation of cell-free extracts (CFEs) for enzymatic assays, C. acetobutylicum transformants and C. beijerinckii wild type strains were grown anaerobically in 100 mL of CM1 medium with 60 g/L glucose. After 15–20 h of culture, cells were harvested at 4°C by centrifugation at 15,000 g for 7 min (OD600: 1.5-2.0). Pellets were suspended in 20 mL of 50 mM sodium-HEPES buffer (pH 8.5) containing DTT (0.2 mM) and a set of protease inhibitors (Complete; Mini, Roche, 1 tablet in 50 mL) and washed twice. Pellets were then suspended in 3 mL of 50 mM sodium-HEPES buffer. Cell suspensions were frozen in liquid nitrogen and stored overnight at −80°C in anaerobic conditions. Cell suspensions were then slowly thawed, loaded in a French Press (Thermo Electron Corporation) and homogenized by two passes at 16,000 psi. When used, CFEs were kept on ice. Protein content in the CFEs was determined by the Bradford method (Biorad) with BSA as standard.
Reduction of acetone or racemic acetoin (D/L 3-hydroxy-2-butanone, Fluka) by s-Adh was carried out at 37°C in 50 mM of Tris buffer (pH 7.5) with 0.2 mM of NADPH and 50 mM of substrate. NADPH decrease was monitored by absorbance decrease at 340 nm using a Safire spectrophotometer (Tecan).
Analytical procedures
Samples taken during fermentation were centrifuged at 20,000 g for 5 min and supernatants were stored at −20°C. Metabolite concentrations (sugars, organic acids, solvents and 2,3-butanediol) were determined by HPLC as previously described (Gosselink et al.1995; Siemerink et al.2011). A solution of 4-methyl valeric acid (Sigma-Aldrich) at 30 mM was used as an internal standard.
Results
Construction of expression vectors
The well-studied ABE-producing strain C. acetobutylicum ATCC 824 was engineered to be an IBE producer. For this purpose, the coding sequence of the adh gene from C. beijerinckii NRRL B593 was cloned downstream of the promoter sequence of the thiolase gene (thl) from C. acetobutylicum ATCC 824 to form pFC002 plasmid (Table1). The promoter sequence of the thiolase gene was chosen in order to maximize expression of the adh gene, since the thiolase gene of C. acetobutylicum was reported to be constitutively expressed (Hartmanis and Gatenbeck1984; Tummala et al.1999; Alsaker and Papoutsakis2005). To up-regulate the acetone pathway in the host organism, genes encoding the enzymes active in acetoacetyl-CoA to acetone conversion i.e. acetoacetate decarboxylase (adc) and acetoacetyl-CoA : acetate/butyrate:CoA transferase subunits A and B (ctfA and ctfB) were cloned into pFC002, downstream of the adh gene, resulting in the construct pFC007 (Table1). Genes adc, ctfA and ctfB were expressed under the control of the thl-promoter. The role of each gene over expressed in pFC007 was subsequently assessed by constructing different combinations of adh, adc, ctfA and ctfB genes. The plasmid pFC005 contained adh and adc genes and pFC006 contained adh, ctfA and ctfB genes (Table1).
Expression of isopropanol pathway genes in E. coli
To assess their ability to promote isopropanol production, the constructs pFC002, pFC005, pFC006, pFC007 and pMTL500E were co-transformed with pTHL plasmid into E. coli BW25113. The pTHL plasmid contained the thiolase gene (thl) from C. acetobutylicum ATCC 824 (Stim-Herndon et al.1995) to allow sufficient formation of acetoacetyl-CoA by E. coli. Productions of solvents by E. coli transformants are shown in Figure1. All transformants produced ethanol, but only the transformants which, in addition to thiolase and adh genes, expressed ctfA and ctfB genes, i.e. transformants harbouring pFC006 (thl p _ adh; ctfA; ctfB) or pFC007 (thl p _ adh; adc; ctfA; ctfB) plasmids, produced isopropanol (3–8 mM). The E. coli transformants harbouring pTHL and pFC005 (thl p _ adh; adc) plasmids or harbouring pTHL and pMTL500E (control) plasmids did not produce isopropanol.
Effect of expression of the adh gene on the product pool of C. acetobutylicum
Plasmids pMTL500E, pFC002, pFC005, pFC006 and pFC007 were independently electroporated into C. acetobutylicum. The fermentation performance of the different C. acetobutylicum transformant strains was studied and compared with those of the wild-type strains (WT) in batch cultures performed in bioreactors with a 1 L-working volume. Cultures were performed with or without pH regulation. When pH regulated, the system was setup in such a way that once the pH had dropped to 5.0 it was kept at that level by the addition of KOH. Table3 shows final fermentation performances of C. acetobutylicum transformants with pH regulation at 5.0.
Expression of adh by strains ATCC 824(pFC002), ATCC 824(pFC005), ATCC 824(pFC006) and ATCC 824(pFC007) resulted in the reduction to isopropanol of about 95% of the acetone natively produced. In contrast to ATCC 824 WT or ATCC 824(pMTL500E) that produced between 0.5 and 1.1 g/L acetoin (3-hydroxy-2-butanone) (Jones and Woods1986; Xiao and Xu2007), very low concentrations of acetoin were detected in cultures of transformants expressing the adh gene. However, D and/or L 2,3-butanediol (2,3-BD) accumulated at 0.5-0.6 g/L when the pH was regulated at 5.0 and at 0.6-1.2 g/L when the pH was not regulated. No meso-2,3-BD was identified. Production of 2,3-BD was concomitant with production of IBE. It is worth noting that 2,3-BD was not detected in cultures of C. beijerinckii NRRL B593, probably because the organism does not produce acetoin. The enzymatic reduction of acetone and acetoin by cell-free extracts of ATCC 824(pMTL500E), ATCC 824(pFC002), ATCC 824(pFC007) and NRRL B593 WT was tested in vitro (Table4). The cell-free extracts of ATCC 824(pFC002), ATCC 824(pFC007) and NRRL B593 WT displayed significantly higher reduction activities towards acetone and acetoin than those of ATCC 824(pMTL500E) used as control (Table4).
Early isopropanol production in static flask culture
As the constitutive promoter of the thlgene was used to control gene expression (Tummala et al.1999), the isopropanol production by ATCC 824(pFC007) was expected to start concomitantly with the production of butyric acid. Product excretion in the first hours of fermentation was studied in static flask fermentations. Butyric acid was detected prior to any solvent in all cultures of ATCC 824 transformants. The transformants expressing ctfA and ctfB genes i.e. harbouring pFC006 and pFC007 excreted isopropanol earlier than the wild type strain or other transformants (data not shown) and prior to any other solvent.
Kinetics of IBE production by C. acetobutylicum transformants
The fermentation kinetics of ATCC 824 transformants were first investigated using a pH set-point of 5.0. The fermentation profile and performances of the control strain ATCC 824(pMTL500E) were similar to those of C. acetobutylicum ATCC 824 WT in the first 45 h of fermentation (Table3). The expression of only the adh gene in ATCC 824(pFC002) resulted in lower solvent production (15.1 g/L IBE of which 4.8 g/L isopropanol) than ATCC 824(pMTL500E) without the adh gene. Moreover, the productivity of ATCC 824(pFC002) at 30 h was 25% lower than that of ATCC 824(pMTL500E). In comparison to ATCC 824(pFC002), the wild-type IBE-producer C. beijerinckii NRRL B593 excreted less IBE (13.2 g/L of which 4.5 g/L isopropanol), but reassimilated more efficiently the acids previously excreted. Thus NRRL B593 displayed higher solvent yield (0.36 gIBE/gglc for NRRL B593 vs 0.29-0.30 gIBE/gglc for ATCC 824(pFC002)).
ATCC 824(pFC007) surpassed ATCC 824(pFC002) in the production of IBE, indicating that the overall metabolic activity was stimulated by the expression of pFC007 genes. ATCC 824(pFC007) produced more solvents (20.4 g/L IBE of which 7.3 g/L) and less acids than ATCC 824(pFC002) (15.1 g/L IBE of which 4.8 g/L isopropanol). In addition, the fermentation period was shorter and stopped about 10–15 hours earlier than for ATCC 824(pFC002) (Figure2). Consequently, the solvent productivity after 30 h by ATCC 824(pFC007) (0.67 g/L h) was 2.6 times higher than that of ATCC 824(pFC002).
Fermentation kinetics of C. beijerinckii NRRL B593 WT and C. acetobutylicum ATCC 824 WT and its transformants. Column a: pH regulated to 5.0; column b: pH not regulated. Glucose (Closed diamonds), acetic acid (open circles), butyric acid (open triangles), ethanol (crosses), isopropanol (closed circles), acetone (closed triangles) and butanol (closed squares).
Cultures of ATCC 824(pFC005) and ATCC 824(pFC006) were performed to evaluate the contribution of each gene to the improvement of the ATCC 824(pFC007) phenotype (Table3). Both strains produced more IBE than ATCC 824(pFC002). The combined overexpression of the ctfA and ctfB genes along with expression of adh conferred to ATCC 824(pFC006) a fermentation profile similar to that of ATCC 824(pFC007) (Table3). The final concentration of acids in the ATCC 824(pFC006) culture was slightly lower than that of ATCC 824(pFC002). As with ATCC 824(pFC007), fermentations with ATCC 824(pFC006) stopped 10–15 hours earlier than with the other transformants (Figure2). The resulting solvent productivity after 30 h (0.62 g/L h) was 2.0 times higher than ATCC 824(pFC002). In ATCC 824(pFC005), the overexpression of adc along with expression of adh gene had a more pronounced effect on the production of isopropanol (+27%) than on the production of butanol (+7%) when compared with ATCC 824(pFC002). The fermentation performances of ATCC 824(pFC005) were lower than those of ATCC 824(pFC006) or ATCC 824(pFC007) and no shortening of the fermentation period was observed. Besides, ATCC 824(pFC005), ATCC 824(pFC006) and ATCC 824(pFC007) exhibited the same solvent yield than ATCC 824(pFC002), ATCC 824(pMTL500E) and the WT.
Effect of pH control
In order to assess the effect of the culture mode, another set of fermentations was performed without pH control (Table5). For every strain tested, the minimum pH value reached was 4.7-4.8 (data not shown). The glucose consumptions were roughly similar to those without pH regulation. A better reassimilation of acetic and butyric acids was observed leading to better solvent titres, yields and productivities. The excretion profiles of metabolites for each strain were similar to the corresponding ones with pH regulation at 5.0. The highest solvent productions were obtained with ATCC 824(pFC007) that produced 24.4 g/L IBE (of which 8.8 g/L was isopropanol) and ATCC 824(pFC006) that produced 24.0 g/L IBE (of which 8.0 g/L was isopropanol). Lack of sporulation and extensive cell lysis were observed in cultures of ATCC 824(pFC006) or ATCC 824(pFC007) performed without pH control, highlighting the strong inhibitory effect of solvents in the early phase of cellular growth.
Discussion
In the latest developments related to the ABE fermentation process, acetone was considered to be indesirable co-product, whereas butanol is the main product of interest. Over the past few decades, various strategies have been developed to decrease the production of acetone and increase the production of butanol (Nair et al.1994; Nair and Papoutsakis1994; Harris et al.2000; Sillers et al.2008; Jiang et al.2009; Sillers et al.2009; Han et al.2011). The intracellular conversion of acetone into isopropanol was an attractive alternative to avoid acetone excretion and produce a valuable alcohol. The C. beijerinckii NRRL B593 strain reduced acetone naturally thanks to a secondary-alcohol dehydrogenase (s-Adh) but the final titres of solvents by the NRRL B593 (George et al.1983; Survase et al.2011) were lower than those of the best ABE producers (Monot et al.1982; Qureshi and Blaschek1999).
In this study, we have constructed four plasmids harbouring the adh gene from C. beijerinckii NRRL B593 and the genes from C. acetobutylicum ATCC 824 that are part of the metabolic pathway from acetoacetyl-CoA to acetone. The cloned genes were successfully expressed in both E. coli BW25113 and C. acetobutylicum ATCC 824. In E. coli, the expression of ctfA and ctfB along with adh and thl genes allowed for the production of isopropanol. The lack of the adc gene did not prevent the decarboxylation of acetoacetate by E. coli harbouring pFC006, probably because of the instability of the molecule in acidic conditions (Hay and Bond1967). The final concentration of isopropanol in cultures of E. coli strains expressing ctfA and ctfB genes (pTHL and pFC006 or pTHL and pFC007) was lower than those previously reported by other groups (Hanai et al.2007; Atsumi and Liao2008; Jojima et al.2008; Yoshino et al.2008). In our study, E. coli cultures were not optimised, but carried out with the purpose of checking the validity of each construct.
The plasmids were electroporated in C. acetobutylicum ATCC 824. The expression of adh gene allowed transformants to reduce natively produced acetoin and acetone to 2,3-BD and isopropanol, respectively. Either the D or L forms of 2,3-BD or a combination of both but no meso-2,3-BD was produced. The achiral HPLC used in the present study did not differentiate between the D and L enantiomers. Since the activity of s-Adh on acetoin had never been described, this result extends the range of substrates known for this enzyme (Ismaiel et al.1993). Recently, the production of 2,3-BD by C. acetobutylicum transformants expressing an acetoin reductase (acr) from C. beijerinckii NCIMB 8052 was reported (Siemerink et al.2011). The resulting strains also produced 2,3-BD but did not produced isopropanol. For future applications, the production of 2,3-BD by ATCC 824 transformants is still very far from that of Klebsiella pneumoniae (up to 150 g/L of 2,3-BD) (Ma et al.2009).
Each transformant of ATCC 824 was characterised in a batch culture either with pH regulation at 5.0 or without pH regulation. All transformants of ATCC 824 and the wild type displayed higher solvent production levels when grown without pH-regulation. The solvent yield based on glucose consumption did not depend on the genetic modifications, but rather on the culture conditions (pH control or not). Acid assimilation was improved in the cultures without pH regulation, as also suggested by the increase of the C3 compound (acetone or isopropanol) productions. When the pH was not regulated, the pH value of the culture dropped below 5.0, increasing the concentrations of the protonated form of the acids. This has been associated with the onset of solventogenesis (Monot et al.1984; Hüsemann and Papoutsakis1988). Therefore, the high level of protonated acid forms in pH not-regulated cultures of ATCC 824 transformants might trigger solventogenesis at a lower concentration of total acids (protonated plus ionized) and drive more the carbon flux towards butanol or ethanol formation.
The expression of only the adh gene lowered total solvent production by ATCC 824(pFC002) compared to the wild type and the transformant harbouring the empty vector (pMTL500E). The lower solvent excretion by ATCC 824(pFC002) could be explained by the higher toxicity of isopropanol compared to acetone, as suggested by the octane/water partition coefficients (logKow) values i.e. 0.05 for isopropanol and −0.25 for acetone (Yaws and Sachin1999). The logKow was reported to be a good estimation for solvent toxicity (Vermue et al.1993; Heipieper et al.1994), high logKow compounds are generally more toxic than compounds with lower value. It has to be noted that the final IBE concentration of ATCC 824(pFC002) cultures (16 g/L) was still higher than that of NRRL B593 cultures (13 g/L) suggesting that the solvent sensitivity is a strain-dependent characteristic.
Under all culture conditions tested, the overexpression of all genes encoding enzymes of the acetone route (ctfA, ctfB and adc), along with expression of adh gene, conferred to ATCC 824(pFC007) high solvent production rate and high final solvent titres. The use of thl promoter to control the expression of ctfA and ctfB genes initiated excretion of isopropanol before those of other solvents. Recently, (Lee et al.2012) have developed a transformant comparable with ATCC 824(pFC007) in which expression of isopropanol pathway genes were controlled by two adc promoters. In batch culture with pH regulation at 5.0, the maximal end-concentration of IBE was only 17.1 g/L of which 6.1 g/L was isopropanol. The difference in solvent productions observed in the two studies might result from the culture mode applied. Fed-batch with gas stripping was used and was found to improve IBE production by 35.6 g/L (Lee et al.2012) but this type of process has never been scaled up.
The role of each gene involved in the pathway from acetoacetyl-CoA to acetone in the enhancement of ATCC 824(pFC007) fermentation performances was clarified by expressing two derivative plasmids. The overexpression of the ctfA and ctfB genes increased both the speed and the extent of acid assimilation while the overexpression of the adc gene had a little effect (Table3).
This result indicates that decarboxylation of acetoacetate is not the real bottleneck. In a previous study on ABE production by ATCC 824, the overexpression of ctfA, ctfB and adc genes controlled by the adc promoter was studied at pH 5.5 (Mermelstein et al.1993). As with our results, the combined overexpression of ctfA, ctfB and adc increased the solvent production by transformants, whereas expression of adc gene alone had little effect. Unlike our results, the combined expression of ctfA and ctfB genes without adc was found to have a limited effect. Therefore, the impact of ctfA and ctfB overexpression observed in our study might have been supported by the chemically acid-calalysed decarboxylation of acetoacetate (Hay and Bond1967).
Conclusion
The expression of ctfA and ctfB genes along with the adh gene in C. acetobutylicum appears to be a promising way for constructing efficient isopropanol/ethanol producers. The transformants in the present study produce the highest total IBE concentration reported for clostridial batch cultures without online IBE removal (24.4 g /L). As the IBE alcohol mix is considered to be a valuable fuel additive, the transformants obtained represent a step forward towards the development of an industrial IBE process for the production of biofuels.
References
Alsaker KV, Papoutsakis ET: Transcriptional Program of Early Sporulation and Stationary-Phase Events in Clostridium acetobutylicum. J Bacteriol 2005, 187: 7103–7118. 10.1128/JB.187.20.7103-7118.2005
Atsumi S, Liao JC: Metabolic engineering for advanced biofuels production from Escherichia coli. Curr Opin Biotechnol 2008, 19: 414–419. 10.1016/j.copbio.2008.08.008
Bermejo LL, Welker NE, Papoutsakis ET: Expression of Clostridium acetobutylicum ATCC 824 genes in Escherichia coli for acetone production and acetate detoxification. Appl Environ Microbiol 1998, 64: 1079–1085.
Bertani G: Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 2004, 186: 595–600. 10.1128/JB.186.3.595-600.2004
Chen JS: Alcohol-dehydrogenase: multiplicity and relatedness in the solvent-producing Clostridia. FEMS Microbiol Rev 1995, 17: 263–273. 10.1111/j.1574-6976.1995.tb00210.x
Chen JS, Hiu SF: Acetone-butanol-isopropanol production by Clostridium beijerinckii (synonym, Clostridium butylicum). Biotechnol Lett 1986, 8: 371–376. 10.1007/BF01040869
Dürre P: Biobutanol: An attractive biofuel. Biotechnol J 2007, 2: 1525–1534. 10.1002/biot.200700168
Dürre P: Fermentative butanol production, Bulk chemical and biofuel. Ann N Y Acad Sci 2008, 1125: 353–362. 10.1196/annals.1419.009
Lee J, Yu-Sin J, Joon Choi S, Ae Im J, Song H, Hee Cho J, Seung DY, Papoutsakis ET, Bennett GN, Lee SY: Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. Appl Environ Microbiol 2012, 78: 1416–1423. 10.1128/AEM.06382-11
George HA, Johnson JL, Moore WEC, Holdeman LV, Chen JS: Acetone, isopropanol and butanol production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microbiol 1983, 45: 1160–1163.
Girbal L, Soucaille P: Regulation of solvent production in Clostridium acetobutylicum. Trends Biotechnol 1998, 16: 11–16. 10.1016/S0167-7799(97)01141-4
Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon L, Frolow F, Burstein Y: Biochemical and structural properties of chimeras constructed by exchange of cofactor-binding domains in alcohol dehydrogenases from thermophilic and mesophilic microorganisms. Biochem 2010, 49: 1943–1953. 10.1021/bi901730x
Gosselink RJA, van Dam JEG, Zomers FHA: Combined HPLC analysis of organic acids and furans formed during organosolv pulping of fiber hemp. J Wood Chem Technol 1995, 15: 1–25. 10.1080/02773819508009497
Green E: Fermentative production of butanol: the industrial perspective. Curr Opin Biotechnol 2011, 22: 337–343. 10.1016/j.copbio.2011.02.004
Groot WJ, Luyben KCAM: In situ product recovery by adsorption in the butanol isopropanol batch fermentation. Appl Microbiol Biotechnol 1986, 25: 29–31.
Han B, Gopalan V, Ezeji TC: Acetone production in solventogenic Clostridium species: new insights from non-enzymatic decarboxylation of acetoacetate. Appl Microbiol Biotechnol 2011, 91: 565–576. 10.1007/s00253-011-3276-5
Hanai T, Atsumi S, Liao JC: Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl Environ Microbiol 2007, 73: 7814–7818. 10.1128/AEM.01140-07
Harris LM, Desai RP, Welker NE, Papoutsakis ET: Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: Need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol Bioeng 2000, 67: 1–11. 10.1002/(SICI)1097-0290(20000105)67:1<1::AID-BIT1>3.0.CO;2-G
Hartmanis MGN, Gatenbeck S: Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 1984, 47: 1277–1283.
Hay RW, Bond MA: Kinetics of decarboxylation of acetoacetic acid. Australian J Chem 1967, 20: 1823–1828. 10.1071/CH9671823
Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP: The ClosTron: A universal gene knock-out system for the genus Clostridium. J Microbiol Meth 2007, 70: 452–464. 10.1016/j.mimet.2007.05.021
Heipieper HJ, Weber FJ, Sikkema J, Keweloh H, Debont JAM: Mechanisms of resistance of whole cells to toxic organic-solvents. Trends Biotechnol 1994, 12: 409–415. 10.1016/0167-7799(94)90029-9
Huang TJ, Sorensen CM, Varghese P: Process for the production of ethers. USA, 4,906,787. 1990.
Hüsemann MHW, Papoutsakis ET: Solventogenesis in Clostridium acetobutylicum fermentations related to carboxylic-acid and proton concentrations. Biotechnol Bioeng 1988, 32: 843–852. 10.1002/bit.260320702
Ismaiel AA, Zhu CX, Colby GD, Chen JS: Purification and characterization of a primary-secondary alcohol dehydrogenase from two strains of Clostridium beijerinckii. J Bacteriol 1993, 175: 5097–5105.
Jiang Y, Xu CM, Dong F, Yang YL, Jiang WH, Yang S: Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 2009, 11: 284–291. 10.1016/j.ymben.2009.06.002
Jojima T, Inui M, Yukawa H: Production of isopropanol by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 2008, 77: 1219–1224. 10.1007/s00253-007-1246-8
Jones DT, Woods DR: Acetone-butanol fermentation revisited. Microbiol Rev 1986, 50: 484–524.
Korkhin Y, Kalb AJ, Peretz M, Bogin O, Burstein Y, Frolow F: NADP-dependent bacterial alcohol dehydrogenases: Crystal structure, cofactor-binding and cofactor specificity of the ADHs of Clostridium beijerinckii and Thermoanaerobacter brockii. J Mol Biol 1998, 278: 967–981. 10.1006/jmbi.1998.1750
Kuit W, Lopez Contreras AM, Eggink G: Disruption of the acetate kinase (ack) gene of Clostridium acetobutylicum results in delayed acetate production. Appl Biochem Biotechnol 2012, 9: 729–741.
Logsdon JE, Loke A: Isopropyl alcohol. Kirk-Othmer Encyclopedia of Chemical Technology. 2000.
López-Contreras AM, Kuit W, Siemerink MAJ, Kengen SWM, Springer J, Claassen PAM: Production of longer-chain alcohols from lignocellulosic biomass: butanol, isopropanol and 2,3-butanediol. Woodhead Publishing Ltd. In Bioalcohol production: Biochemical conversion of lignocellulosic biomass. Edited by: Waldron KW. Abington, Cambridge (UK); 2010:415–460.
Ma C, Ailong W, Qin J, Li L, Ai X, Jiang T, Tang H, Xu P: Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl Microbiol Biotechnol 2009, 82: 49–57. 10.1007/s00253-008-1732-7
Matsumura M, Takehara S, Kataoka H: Continuous butanol/isopropanol fermentation in down-flow column reactor coupled with pervaporation using supported liquid membrane. Biotechnol Bioeng 1992, 39: 148–156. 10.1002/bit.260390205
Mermelstein LD, Papoutsakis ET: Invivo methylation in Escherichia coli by the Bacillus subtilis phage Phi-3 T-I methyl transferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 1993, 59: 1077–1081.
Mermelstein LD, Papoutsakis ET, Petersen DJ, Bennett GN: Metabolic engineering of Clostridium acetobutylicum ATCC 824 for increased solvent production by enhancement of acetone formation enzyme activities using a synthetic acetone operon. Biotechnol Bioeng 1993, 42: 1053–1060. 10.1002/bit.260420906
Monot F, Martin JR, Petitdemange H, Gay R: Acetone and butanol production by Clostridium acetobutylicum in a synthetic medium. Appl Environ Microbiol 1982, 44: 1318–1324.
Monot F, Engasser J-M, Petitdemange H: Influence of pH and undissociated butyric acid on the production of acetone and butanol in batch cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 1984, 19: 422–426. 10.1007/BF00454381
Nair RV, Papoutsakis ET: Expression of plasmid encoded AAD in Clostridium acetobutylicum M5 restores vigorous butanol production. J Bacteriol 1994, 176: 5843–5846.
Nair RV, Bennett GN, Papoutsakis ET: Molecular characterisation of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. J Bacteriol 1994, 176: 871–885.
Oultram JD, Loughlin M, Swinfield TJ, Brehm JK, Thompson DE, Minton NP: Introduction of plasmids into whole cells of Clostridium acetobutylicum by electroporation. FEMS Microbiol Lett 1988, 56: 83–88. 10.1111/j.1574-6968.1988.tb03154.x
Peralta-Yahya PP, Keasling JD: Advanced biofuel production in microbes. Biotechnol J 2010, 5: 147–162. 10.1002/biot.200900220
Qureshi N, Blaschek HP: Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol Prog 1999, 15: 594–602. 10.1021/bp990080e
Rogers P, Chen J-S, Zidwick M: Organic acid and solvent production. Part III; butanol, acetone, isopropanol;1,3- and 1,2-propanediol production; and 2,3-butanediol production. In The prokaryotes A handbook on the biology of bacteria, vol 1. 3rd edition. Edited by: Dworkin M. Springer, New York, USA; 2006:672–755.
Roos JW, McLaughlin JK, Papoutsakis ET: The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol Bioeng 1985, 27: 681–694. 10.1002/bit.260270518
Siemerink MAJ, Kuit W, Lopez Contreras AM, Eggink G, van der Oost J, Kengen SWM: D-2,3-butanediol production due to heterologous expression of an acetoin reductase in Clostridium acetobutylicum. Appl Environ Microbiol 2011, 77: 2582–2588. 10.1128/AEM.01616-10
Sillers R, Chow A, Tracy B, Papoutsakis ET: Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab Eng 2008, 10: 321–332. 10.1016/j.ymben.2008.07.005
Sillers R, Hinai AMA, Papoutsakis ET: Aldehyde-Alcohol Dehydrogenase and/or Thiolase Overexpression Coupled With CoA Transferase Downregulation Lead to Higher Alcohol Titers and Selectivity in Clostridium acetobutylicum Fermentations. Biotechnol Bioeng 2009, 102: 38–49. 10.1002/bit.22058
Stim-Herndon KP, Petersen DJ, Bennett GN: Characterisation of an acetyl-CoA C-acetyl transferase (Thiolase) gene from Clostridium acetobutylicum ATCC 824. Gene 1995, 154: 81–85. 10.1016/0378-1119(94)00838-J
Survase SA, Jurgens G, Heiningen A, Granström T: Continuous production of isopropanol and butanol using Clostidium beijerinckii DSMZ 6423. Appl Microbiol Biotechnol 2011, 91: 1305–1313. 10.1007/s00253-011-3322-3
Tummala SB, Welker NE, Papoutsakis ET: Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 1999, 65: 3793–3799.
Vermue M, Sikkema J, Verheul A, Bakker R, Tramper J: Toxicity of homologous series of organic-solvents for the gram positive bacteria Arthrobacter and Nocardia sp and the gram negative bacteria Acinetobacter and Pseudomonas sp. Biotechnol Bioeng 1993, 42: 747–758. 10.1002/bit.260420610
Xiao ZJ, Xu P: Acetoin metabolism in bacteria. Crit Rev Microbiol 2007, 33: 127–140. 10.1080/10408410701364604
Yan RT, Zhu CX, Golemboski C, Chen JS: Expression of solvent-forming enzymes and onset of solvent production in batch cultures of Clostridium beijerinckii ("Clostridium butylicum"). Appl Environ Microbiol 1988, 54: 642–648.
Yaws CL, Sachin N: Solubitlity in water and octanol-water partition coefficient. In Chemical properties handbook Edited by: Hill MG. 1999.
Yoshino S, Kawabe T, Ishibashi Y: Aerobic isopropanol fermentation by genetically engineered Escherichia coli. In Clostridium 10; 28 September 2008. Wageningen, The Netherlands; 2008.
Acknowledgements
This work was supported by the program EnerBio of the French Tuck foundation. The authors wish to thank Ms Hetty van der Wal, Ms Miriam Budde for their assistance in fermentation work and Rachel Licht for her critical reading of the manuscript and improvement of the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Collas, F., Kuit, W., Clément, B. et al. Simultaneous production of isopropanol, butanol, ethanol and 2,3-butanediol by Clostridium acetobutylicum ATCC 824 engineered strains. AMB Expr 2, 45 (2012). https://doi.org/10.1186/2191-0855-2-45
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-0855-2-45