Abstract
Background
To determine if testosterone levels are influenced by dopaminergic therapy in Parkinson disease (PD) patients. Testosterone level has been reported to be low in patients with PD and other neurodegenerative diseases. In this study, we sought to determine whether dopaminergic therapy (i.e. levodopa and dopamine agonist) influenced testosterone levels. We used a cohort of consecutive male patients from the INSPECT trial--a multi-center, prospective, study that primarily investigated the effects of short-term treatment with pramipexole or levodopa on [123I] B-CIT SPECT imaging in early PD.
Methods
Testosterone levels were drawn on consenting male subjects with early PD who enrolled in the INSPECT trial at three study visits (baseline, 12 weeks post-treatment, and 8–12 weeks post-washout). Subjects were randomized to: no treatment, pramipexole (up to 3 mg) or levodopa (up to 600 mg). Testosterone levels were obtained twice (prior to 10 AM) and averaged for each of three study visits.
Results
Thirty two male patients participated in this sub-study and there were no significant differences in disease characteristics in the 3 groups at baseline. Twenty-nine patients completed the follow-up visits and were suitable for analysis. There were statistically significant differences in the change in free testosterone level, increased in both the levodopa group and pramipexole group but decreased in the untreated group at 12-weeks post-treatment. There were no significant differences in the changes of UPDRS total or motor scores, although there was a strong trend toward improvement in motor scores. The testosterone level persisted in its increase only in the pramipexole group at the end of the washout period.
Conclusion
These preliminary data support the premise that dopaminergic medications do not reduce testosterone levels in early PD patients.
Similar content being viewed by others
Background
There has been mounting evidence suggesting that inappropriately low plasma testosterone levels commonly exist in male patients with Parkinson disease (PD) [1–6]. The relevance and underlying cause of this endocrine disturbance remain unknown. Additionally, replacement of testosterone has not to date been proven to significantly improve the motor and non-motor symptoms of these patients, though there have been anecdotal successes [4–7]. Two main hypotheses have been proposed to explain the low testosterone levels: the levels are reduced by dopaminergic medications, or the testosterone level is a surrogate marker for pathology known to occur in the hypothalamus and in other relevant regions of the PD brain [4]. We sought to examine the former hypothesis that dopaminergics may lower testosterone. In this study we utilized the INSPECT cohort of early PD subjects.
Methods
Consecutive male PD patients (who fulfilled the UK Parkinson’s Disease Brain Bank Criteria [8]) enrolled in the INSPECT study had plasma free and total testosterone levels drawn. Patients had two testosterone levels (free and total) drawn prior to 10 AM during each study visit as described in a previous study [4]. The levels for each subject at each study interval were averaged. Three scans- [123I] -CIT and SPECT imaging were performed at baseline, 12 weeks following randomization to treatment group (levodopa [up to 600 mg/day], pramipexole [up to 3 mg/day], or no treatment), and 8–12 weeks following medication washout (see Figure 1). Baseline levels and descriptive clinical variables were recorded, and changes from baseline to 12 weeks post-treatment, and changes from baseline to 8–12 weeks post washout were compared between the three groups using analysis of variance (ANOVA).The University of Florida Institutional Review Board (IRB) through the parent INSPECT study approved the investigation, and each participant provided informed consent.
-CIT and SPECT imaging were performed at baseline, 12 weeks following randomization to treatment group (levodopa [up to 600 mg/day], pramipexole [up to 3 mg/day], or no treatment), and 8–12 weeks following medication washout (see Figure 1). Baseline levels and descriptive clinical variables were recorded, and changes from baseline to 12 weeks post-treatment, and changes from baseline to 8–12 weeks post washout were compared between the three groups using analysis of variance (ANOVA).The University of Florida Institutional Review Board (IRB) through the parent INSPECT study approved the investigation, and each participant provided informed consent.
Results
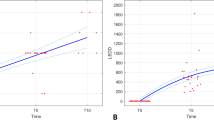
Thirty-two consecutive male patients enrolled in the study and no differences in disease characteristics between groups were seen at baseline (Table 1). Twenty nine patients completed the follow-up visits and were suitable for analysis. Figure 2 shows the mean and 95% confidence interval of the free testosterone level by time and group. The free testosterone level increased in both the levodopa group (change of 5.41 ± 16.18) and pramipexole group (8.95 ± 13.15), but decreased in the untreated group (−13.92 ± 23.12) from baseline to 12-week post-treatment. The between group difference reached a statistical significance of 0.019 with the use of an ANOVA test on the changes. There were no significant changes in UPDRS total or motor scores although there was a trend toward improvement in motor scores in both treatment groups compared to placebo. The increase in testosterone level persisted only the pramipexole group post-washout (see Tables 2 and 3).
Discussion and conclusions
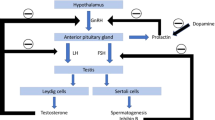
These results suggest that neither levodopa nor pramipexole decrease testosterone level in early PD. The observation that the untreated group experienced further lowering of free testosterone levels lends support to the hypothesis that testosterone decline in PD may be a result of disease-specific factors, and that the decline is less likely iatrogenically induced by dopaminergic medications. It is not entirely clear why the increase in free testosterone levels persisted in the dopamine agonist group post-washout. Dopamine agonists have been used in the treatment of prolactinoma, because dopamine is a natural inhibitor of prolactin [9, 10]. Prolactin lowers leutenizing hormone (LH) which in turn lowers testosterone level [11–16]. Thus, dopamine agonists may theoretically increase testosterone levels by inhibiting prolactin. This point will need further clarification.
Sinhamahapatra and Kirschner in 1971 published a detailed analysis of the effect of levodopa on testosterone level [17]. They sought in their study to answer the question as to whether levodopa stimulated LH production and Leydig cell activity. They utilized an electron capture gas liquid chromatographor, an older technique now considered less accurate when compared to more modern techniques for measuring testosterone. Seven PD men staged using an older system devised by Leon were included in the analysis . Very high doses of levodopa were used (2–6 grams/day). There were other methodological limitations including baseline normal levels in all patients enrolled (>325 ng/ml), and 2/7 PD patients who did not improve on levodopa. One interesting aspect of their study was the calculation of testosterone production and metabolism, as well as the measurement of LH. The study concluded that levodopa did not have an impact on plasma testosterone or LH [17]. Despite methodological limitations this data is supportive of our findings.
We suspect based on our results that the finding of low testosterone in PD patients is indicative of intrinsic PD pathology. It is well known that Lewy Body pathology is present in PD patients at post-mortem examination, and lesions includes hypothalamic involvement [18–22]. Braak has shown that this hypothalamic pathology may be present relatively early in the course of PD [18–21]. We propose this as a plausible explanation for the low testosterone levels.
There were several limitations in this study that should be addressed in future investigations. These limitations included a small sample size, the lack of non-PD controls, the use of early PD patients, differences between free and total testosterone levels, and observed changes in testosterone level were small and not likely clinically relevant. There were changes seen between free testosterone and total testosterone and this highlighted difficulties in laboratory measurements. Most experts use a free or bioavailable testosterone level as the gold standard rather than utilizing both a free and total testosterone level [23–25]. Despite these limitations we conclude as did Sinhamahapatra, that dopaminergics are probably not the cause of low testosterone in PD. Clinicians should not assume that low testosterone levels are an effect of PD medications. There is currently no evidence that checking a testosterone level prior to dopaminergic therapy will be clinically useful. We suggest that future research on this topic should focus on disease related factors as the potential culprits in the low testosterone PD story, however a larger study of the effects of medication can confirm our results.
References
Chou KL, Moro-De-Casillas ML, Amick MM, Borek LL, Friedman JH: Testosterone not associated with violent dreams or REM sleep behavior disorder in men with Parkinson’s. Mov Disord 2007, 22: 411–414. 10.1002/mds.21339
Okun MS, Crucian GP, Fischer L, Walter BL, Testa CM, Vitek JL, DeLong MR, Hanfelt J, Huang X: Testosterone deficiency in a Parkinson’s disease clinic: results of a survey. J Neurol Neurosurg Psychiatry 2004,75(1):165–166.
Okun MS, DeLong MR, Hanfelt J, Gearing M, Levey A: Plasma testosterone levels in Alzheimer and Parkinson diseases. Neurology 2004, 62: 411–413. 10.1212/01.WNL.0000106840.72938.84
Okun MS, Fernandez HH, Rodriguez RL, Romrell J, Suelter M, Munson S, Louis ED, Mulligan T, Foster PS, Shenal BV, Armaghani SJ, Jacobson C, Wu S, Crucian G: Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol 2006,63(5):729–735. 10.1001/archneur.63.5.729
Okun MS, McDonald WM, DeLong MR: Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch Neurol 2002, 59: 807–811. 10.1001/archneur.59.5.807
Okun MS, Walter BL, McDonald WM, Tenover JL, Green J, Juncos JL, DeLong MR: Beneficial effects of testosterone replacement for the nonmotor symptoms of Parkinson disease. Arch Neurol 2002,59(11):1750–1753. 10.1001/archneur.59.11.1750
Mitchell E, Thomas D, Burnet R: Testosterone improves motor function in Parkinson’s disease. J Clin Neurosci 2006, 13: 133–136. 10.1016/j.jocn.2005.02.014
Hughes AJ, Daniel SE, Kilford L, Lees AJ: Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992, 55: 181–184. 10.1136/jnnp.55.3.181
Molitch ME: Pharmacologic resistance in prolactinoma patients. Pituitary 2005, 8: 43–52. 10.1007/s11102-005-5085-2
Shimon I, Benbassat C, Hadani M: Effectiveness of long-term cabergoline treatment for giant prolactinoma: study of 12 men. Eur J Endocrinol 2007, 156: 225–231. 10.1530/EJE-06-0646
Treatment of androgen deficiency in the aging male Fertil Steril 2006, 86: S236–240.
Haren MT, Kim MJ, Tariq SH, Wittert GA, Morley JE: Andropause: a quality-of-life issue in older males. Med Clin North Am 2006, 90: 1005–1023. 10.1016/j.mcna.2006.06.001
Harman SM: Testosterone in older men after the Institute of Medicine Report: where do we go from here? Climacteric 2005, 8: 124–135. 10.1080/13697130500118001
Kaku H, Saika T, Tsushima T, Ebara S, Senoh T, Yamato T, Nasu Y, Kumon H: Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate 2006,66(4):439–444. 10.1002/pros.20341
Morley JE: Androgens and aging. Maturitas 2001, 38: 61–71. discussion 71–63 10.1016/S0378-5122(00)00192-4
Morley JE, Haren MT, Kim MJ, Kevorkian R, Perry HM 3rd: Testosterone, aging and quality of life. J Endocrinol Invest 2005, 28: 76–80.
Sinhamahapatra SB, Kirschner MA: Effect of L-dopa on testosterone and luteinizing hormone production. J Clin Endocrinol Metab 1972, 34: 756–758. 10.1210/jcem-34-4-756
Braak H, Braak E: Pathoanatomy of Parkinson’s disease. J Neurol 2000,247(Suppl 2):II3–10.
Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U: Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 2002,249(Suppl 3):III/1–5.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E: Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003, 24: 197–211. 10.1016/S0197-4580(02)00065-9
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K: Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004, 318: 121–134. 10.1007/s00441-004-0956-9
Jellinger KA: Post mortem studies in Parkinson’s disease–is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 1999, 56: 1–29. 10.1007/978-3-7091-6360-3_1
Morley JE, Kim MJ, Haren MT: Frailty and hormones. Rev Endocr Metab Disord 2005, 6: 101–108. 10.1007/s11154-005-6722-9
Tan RS, Salazar JA: Risks of testosterone replacement therapy in ageing men. Expert Opin Drug Saf 2004, 3: 599–606. 10.1517/14740338.3.6.599
Vermeulen A: Androgen supplementation in elderly males: is dihydrotestosterone to be preferred? Aging Male 2004, 7: 325–327. 10.1080/13685530400016672
Acknowledgements
We would like to acknowledge the support of the National Parkinson Foundation Center of Excellence at the University of Florida, the UF Foundation, and the NIH NS044997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, Quantia, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. The other authors report no other relevant competing interests.
Authors’ contributions
MSO participated in study conception. MSO, SSW, DK, JM, RLR and HHF participated in study design. MSO wrote the first draft of the manuscript. MSO, SSW, DK, JM, RLR and HHF added critical revisions to the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Okun, M.S., Wu, S.S., Jennings, D. et al. Testosterone level and the effect of levodopa and agonists in early Parkinson disease: results from the INSPECT cohort. J Clin Mov Disord 1, 8 (2014). https://doi.org/10.1186/2054-7072-1-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2054-7072-1-8