Abstract
Background
A myocardial infarction (MI) (‘heart attack’) can be intensely stressful, and the impact of this event can leave patients with clinically significant post-MI stress symptoms. Untreated stress can make heart disease worse. Few tools are available that screen for specific thoughts or beliefs that can trigger post-MI stress responses. In other life-threatening illnesses, fear of recurrence (FoR) of illness has been identified as a key stressor, and screening tools have been developed to identify this. The aim of this review is to identify FoR screening tools used in other common life-threatening diseases that report on the development of the tool, to assess if there are any that can be adapted for use in MI survivors so that those with high levels of FoR can be identified and helped.
Methods/Design
The review will evaluate full FoR screening tools and methods of measurement used in common life-threatening disease clinical populations. The Campbell and Cochrane Libraries, Cumulative Index of Nursing and Allied Health Literature (CINAHL), PsycINFO, MEDLINE, Embase, Applied Social Sciences Index and Abstracts (ASSIA), Published International Literature on Traumatic Stress (PILOTS), Social Services Abstracts, Sociological Abstracts, Web of Knowledge, Health and Psychosocial Instruments and SCOPUS databases will be searched for relevant studies published from database inception. Reference lists and published reviews/meta-analyses will also be searched. All titles and abstracts will be screened and relevant full-text versions retrieved by two reviewers, who will then extract all the data. Each will independently review all data extracted by the other. Selected studies will also be assessed by two independent researchers using the COnsensus-based standards for the Selection of health status measurement INstruments (COSMIN) checklist and other quality criteria. This will be done to evaluate the degree to which their measurement properties meet the standards for good methodological quality. Disagreement will be resolved through consensus.
Discussion
Untreated post-MI stress has a considerable psychological and physical impact on MI survivors. Therefore, there is a critical need to develop a screening tool to identify fear of recurrent MI so that those affected can be identified and directed to appropriate support interventions. This proposed research will enable a tool to be developed and adapted for use in the MI survivor patient population.
Systematic review registration
PROSPERO: CRD42014010500
Similar content being viewed by others
Background
In the UK, there are over 100,000 incidences of myocardial infarctions (MI) every year, with a post-MI 30-day discharge survival rate of over 90% [1]. Scotland has one of the highest rates of MI in the world, despite a reported 25% reduction in incidence between 2000 and 2009 [2]. Over the last 15 years, specific interventions (coronary angioplasty, drugs, devices and cardiac rehabilitation) have considerably reduced MI-related mortality rates [3, 4].
Yet despite the improvements in treatment strategies, the experience of having an MI can be intensely stressful and frightening for many patients and the psychological impact of this event can be long lasting [5–8]. Clinically significant post-MI stress symptoms are thought to be present in up to 12.5% (one in eight) patients, and evidence indicates that the post-trauma stress may increase patients risk for subsequent cardiac events and mortality [9]. Even transient stress and anxiety appear to be associated with poorer longer-term outcomes [10] and short-term cardiovascular post-stress recovery rates [11]. Moreover, even cardiac patients with less clinically obvious, mild to moderate low mood, stress and anxiety are at risk of adverse outcomes, with evidence indicating that it is not simply the case (as has been argued in the past), that these elements merely co-exist alongside the pathophysiological effects, but rather that these mild to moderate elements and adverse cardiovascular reactions may indeed share a common (but as yet unidentified) underlying pathophysiological mechanism [12, 13]. Therefore, screening for modifiable psychological triggers of stress in post-MI patients to identify patients at risk is crucial to improving longer-term physical and psychological recovery. However, to date, few tools are available which allow cardiology service providers to screen and identify MI survivors for specific thoughts or beliefs that can trigger modifiable stress responses. In contrast, in other life-threatening illnesses such as cancer, ‘fear of illness recurrence’ has been identified as a key trigger of stress in patients and screening tools to identify this specific stressor have been developed [14–17].
Given the risk and impact of untreated post-MI stress, the development of a similar screening tool which can identify fear of recurrent MI in post-MI patients is therefore critical. In order for such a tool to be developed, it is necessary to identify and evaluate the quality of screening tools used to detect fear of recurrent illness in other common life-threatening diseases to find out if any existing fear of recurrence (FoR) tools can be adapted for use in post-MI patients. This evaluation will inform the development of a theory and evidence-based screening tool which can assess fear of recurrence in MI survivors to enable patients at risk to be directed to appropriate support and intervention. This proposed systematic review of the fear of recurrent illness literature aims to do just this.
Study aims
The primary aim is to identify any fear of recurrent illness screening tools used in cancer, stroke, asthma and acute coronary syndrome patient populations and assess the quality of each tool to assess if there are any tools that can be adapted for FoR MI screening in MI survivors.
The secondary aim is to look at psychometric characteristics of FoR screening tools so that adaptations are only made to good quality and well-developed tools.
Methods/Design
Design
The review will evaluate FoR screening tools and methods of measurement in different clinical populations (cancer, stroke, asthma and acute coronary syndrome). Acute coronary syndrome encompasses a range of unstable coronary artery disease ranging from unstable angina to myocardial infarction [18]. These clinical populations are chosen because they are the most common life-threatening diseases in the UK, and survival from first occurrence and primary treatment has improved [19–22], but there is still risk of recurrence. Thus, FoR screening tools are important for these clinical populations.
Strategy
An evidence synthesis of literature focusing on FoR in the most common life-threatening diseases in the UK will be conducted. Although existing literature identifies FoR as a common problem in cancer survivors, to date, there appears to be a lack of consensual definition of the phenomenon in the existing research [17]. Furthermore, the theoretical concept of FoR is currently unexplored in the MI survivor population. Therefore, for the purpose of this review, which is to examine the psychometric characteristics of FoR screening tools and assess potential transferability of FoR items, domains and constructs to a different survivor population, only FoR screening tools with >3 items will be included. Tools with less than three items tend to psychometrically unstable as with fewer items, cross-validation of factor structures becomes difficult [23]. Also, more comprehensive tools (>3 items) should offer greater sensitivity and granularity for the purposes of assessing potential transferability of meaningful and clinically relevant domains and items to a different survivor population. Sub-scales will be excluded as in existing FoR research, understanding of what existing sub-scales are measuring may be limited at this time, particularly as the most widely used sub-scale screening tools used for measuring patients’ point of view are typically derived from earlier longer versions of the same tool [24] or have reduced complex phenomenon to single items and domains without clear rationale for the non-standardised characteristics [25]. And as shorter versions typically reduce the original multiple items within a domain to single items, sub-scales cover a narrower range of precision and concept sensitivity because of this aggregation [26].
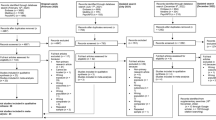
Systematic searches will be conducted across relevant health databases (Campbell and Cochrane Library, Cumulative Index of Nursing and Allied Health Literature (CINAHL), PsycINFO, MEDLINE, Embase, ASSIA, PILOTS, Social Services Abstracts, Sociological Abstracts, Web of Knowledge, Health and Psychosocial Instruments and SCOPUS) published in peer-reviewed journals from database inception (Table 1). Reference lists and published reviews/meta-analyses will also be searched. Studies involving the development of fear of illness recurrence screening or measurement tools will be evaluated. All titles and abstracts will be screened and relevant full-text versions retrieved by two reviewers.
Inclusion criteria
All titles and abstracts will be screened and relevant full-text versions retrieved and assessed by two reviewers (Table 2). The following inclusion criteria will be applied: 1) studies that report on the development of screening tools used to measure fear of recurrent illness in life-threatening diseases (must include >3 items); 2) the study population should be adults diagnosed with cancer, stroke, asthma or acute coronary syndrome over 18 years of age; and 3) published in a peer-reviewed journal. Disagreements will be resolved through consensus, and the level of agreement will be reported and limitations acknowledged. All exclusion decisions will be reported.
Data extraction
A data extraction form will standardise the data extrapolated from each paper (Table 3). As health measurement instrument development lacks consensus at present and often includes different ways to measure a given construct, screening tools can vary widely in content, method and quality [27]. This means the fragmented health measurement literature is often non-comparable. Therefore, for the purposes of this review, we will adopt a ‘holistic’ approach to contrasting and comparing identified relevant tools, and narratively report on relevant quality or methodological properties relevant to the aim of this review. This approach will take into account quality measurements so that useful comparisons of quality can be made. In addition, design data will be extracted and summarised so that adaptations are only made to good quality and well-developed tools. Two researchers will extract data independently, then each will review all data extracted by the other and agree, through consensus, the accuracy and completeness of the data. Where consensus is difficult to reach, a third researcher will be consulted to reach agreement.
Study quality
Selected studies will also be assessed by two independent researchers using the COnsensus-based Standards for the selection of health status measurement INstruments (COSMIN) checklist to evaluate the degree to which their measurement properties meet the standards for good methodological quality. Disagreement will be resolved through consensus. The COSMIN checklist contains standards for design requirements and preferred statistical methods of studies on the measurement properties of health measurement instruments. The checklist can be used to determine if a study on measurement properties meets the standards for good methodological quality and has been developed by international experts in the field of health status measurement [28]. It is designed to evaluate the methodological quality of studies that report on psychometric properties for inclusion in a systematic review, even if the studies have conducted different validity and reliability tests. Each item of the COSMIN checklist data extraction form offers four possible response options (‘excellent’, ‘good’, ‘fair’, ‘poor’) in relation to methodological quality and the score obtained by taking the lowest rating of any item (Figure 1) [29]. This will allow us to report on the overall methodological quality of each study included in the review if sufficient data is reported in each publication.
Data analysis
As study methods and development processes are anticipated to be heterogeneous, pooling of measurement tool properties is not possible. Therefore, synthesis of data will be primarily reported in words and text where appropriate, to summarise and explain the findings and content of multiple studies in narrative format. This synthesis will be based on the general framework and tools outlined in the ESRC Guidance on the Conduct of Narrative Synthesis in Systematic Reviews [30]. Relevant extracted study data including COSMIN ratings will be tabulated in a hierarchical rating of quality and individual instruments will be categorised in terms of relevant and comparable design features or characteristics [31].
Discussion
Given the risk and consequences of untreated post-MI stress, the impact of this planned systematic review of fear of recurrent illness screening tools in common life-threatening diseases is expected to be high, as there is currently no fear of recurrent MI screening tool available. Therefore, there is a critical need to develop a screening tool to identify fear of recurrent MI in the MI survivor population so that post-MI patients with this fear can be identified and directed to appropriate support interventions. This proposed research will provide the best possible evidence to provide a foundation for the development of a fear of recurrent MI screening tool, by systematically and ‘holistically’ evaluating which fear of recurrence tools work, why they work and for whom. Thus, this research will enable an evidence and theory-based screening tool to be developed and adapted for use in the MI survivor patient population. This systematic review is timely and will make a valuable contribution to improving post-MI patient care.
Abbreviations
- MI:
-
Myocardial infarction
- ACS:
-
Acute coronary syndrome
- FoR:
-
Fear of recurrence
- FoRMI:
-
Fear of recurrent myocardial infarction.
References
ISD Scotland: Acute Myocardial Infarction (AMI) Incidence by Year, Health Board, Age Group and Sex 2002/03–2011/12, Numbers, Crude Rates and Age-Sex Standardised Rates). 2012.
British Heart Foundation: Incidence. 2014. [online]. Available at: (accessed on 24–05–2014) http://www.bhf.org.uk/research/heart-statistics/morbidity/incidence.aspx
Smolina K, Wright LF, Rayner M, Goldacre MJ: Long-term survival and recurrence after acute myocardial infarction in England, 2004–2010.Circ Cardiovasc Qual Outcomes 2010, 5:532–40.
Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC: Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis.J Am Coll Cardiol 2008,51(18):1247–54.
Tedstone JE, Tarrier N: Posttraumatic stress disorder following medical illness and treatment.Clin Psychol Rev 2003, 23:409–48.
Whitehead DL, Strike P, Perkins-Porras L, Steptoe A: Frequency of distress and few of dying during acute coronary syndromes and consequences for adaptation.Am J Cardiol 2005,96(11):1512–16.
Wearden AJ: Illness perception interventions for heart attack patients and their spouses: invited commentary.J Psychosom Res 2009, 67:25–7.
Von Kanel R, Hari R, Schmid J-P, Saner H, Begre S: Distress related to myocardial infarction and cardiovascular outcome: a retrospective observational study.BMC Psychiatry 2011, 11:1–8. 98
Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y: Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review.PLoS One 2012,7(6):e38915.
Frasure-Smith N, Lesperance F, Talajic M: Depression and 18-month prognosis after myocardial infarction.Circulation 1995, 91:999–1005.
Papousek I, Nauschenegg K, Paechter M, Lackner HK, Goswami N, Schulter G: Trait and state positive affect and cardiovascular recovery from experimental and academic stress.Biol Psychol 2009, 83:108–15.
Emani S, Binkley PF, Mind-Body Medicine in Chronic Heart Failure: A translational science challenge.Circ Heart Fail 2010, 3:715–25.
Smith DF: Negative emotions and coronary artery disease: causally related or merely coexistent? A review.Scand J Psychol 2001, 42:57–69.
Townend E, Deborah T, Kwan J, Sharpe M: Fear of recurrence and beliefs about preventing recurrence in persons who have suffered a stroke.J Psychosom Res 2006, 61:747–55.
Ghazali N, Cadwallader E, Lowe D, Humphris G, Ozakino B, Rogers SN: Fear of recurrence among head and neck cancer survivors: longitudinal trends.Psychooncology 2012. doi:10.1002/pon.3069
Humphris G, Ozankinci G: The AFTER intervention: a structured psychological approach to reduce fears of recurrence in patients with head and neck cancer.Br J Health Psychol 2008, 00:1–9.
Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, et al.: Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies.Journal of Cancer Survivorship 2013. doi:10.1007/s11764–013–0272-z (Accessed 03–12–2013)
Scottish Intercollegiate Guidelines Network: Acute Coronary Syndromes. Guideline No. 93. ISBN 1 899893 74 1. 2013. 2007. updated February 2013. SIGN, [Online], (accessed 16/10/2014) http://www.sign.ac.uk/pdf/sign93.pdf
NHS National Services Scotland, Information Services Division: Publication Summary: Cancer Mortality in Scotland (2012). 2012. Publication date – 26 November 2013. [online] (accessed 24–03–2014) https://isdscotland.scot.nhs.uk/Health-Topics/Cancer/2014-01-/2013-11-26/2013-11-26-CancerMortality-Summary.pdf?37160891295
NHS National Services Scotland: Information Services Division: Publication Summary: Stroke Statistics Update Year Ending 31 March 2013. 2014. Publication date – 28 January 2014. [online] (accessed 24–03–2014) https://isdscotland.scot.nhs.uk/Health-Topics/Stroke/Publications/2014-01-28/2014-01-28-Stroke-Summary.pdf?1864260436
NHS National Services Scotland: Information Services Division: Publication Summary: Heart Disease Statistics Update, Year Ending 31 March 2013. 2014. Publication date 20 January 2014. [online] (accessed 24–03–2014) https://isdscotland.scot.nhs.uk/Health-Topics/Heart-Disease/Publications/2014-01-28/2014-01-28-Heart-Disease-Summary.pdf?6000918150
NHS National Services Scotland: Information Services Division: Publication Summary: ScotPHO Routine Quarterly Web Updates 31/1/2012. 2012. [online] (accessed 24–03–2014) http://www.isdscotland.org/Health-Topics/Public-Health/Publications/2012-01-31/2012-01-31-ScotPHO-Summary.pdf
Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ: Evaluating the use of exploratory factor analysis in psychological research.Psychol Methods 1999,4(3):272.
Medical Outcomes Trust: Quality Metrics, Medical Outcomes Trust. 2014. SF Materials Available. 2014. [Online}. Available at: . Accessed on 14–07–2914 http://www.sf-36.org/wantsf.aspx?id=1
Stanton AL, Danoff-Burg S, Huggins M: The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment.Psychooncology 2002, 11:93–102.
Luckett T, King MT: Choosing patient-reported outcome measures for cancer clinical research—practical principles and an algorithm to assist non-specialist researchers.Eur J Cancer 2010, 46:3149–57.
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al.: The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study.Qual Life Res 2010,19(4):539–49.
Terwee CB, Mokkink LB, Knol DL, Ostelo RWJG, Bouter LM, de Vet HCW: Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist.Qual Life Res 2012, 21:651–7.
Mokkink LB, Terwee CB, Knol DL, Stratford PW, Alonso J, Patrick DL, et al.: Protocol of the COSMIN study: COnsensus-based Standards for the selection of health Measurement INstruments.BMC Med Res Methodol 2006, 6:2.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al.: Guidance On the Conduct of Narrative Synthesis in Systematic Reviews, ESRC Methods Programme. England: Lancaster University; 2006.
Squires JE, Estabrooks CA, O’Rourke HM, Gustavsson P, Newburn-Cook CV, Wallin L: A systematic review of the psychometric properties of self-report research utilization measures used in healthcare.Implement Sci 2011,6(83):1–18.
Acknowledgements
We would like to acknowledge the contribution of Ian Mitchell, who freely shared his first-hand experience and perspective of the impact of having a myocardial infarction and whose invaluable input helped to shape the project idea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests and no specific funding has been allocated for the study.
Authors’ contributions
JJ and GH conceived and designed the study. JJ, GH, RP, PK, SJL and NHW devised the search strategies, drafted the inclusion selection form and drafted the manuscript. GO and SS participated in the study design and revision of the manuscript. All authors have read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Jones, J., Kane, P., Polson, R. et al. Protocol for a systematic review of screening tools for fear of recurrent illness in common life-threatening diseases. Syst Rev 4, 10 (2015). https://doi.org/10.1186/2046-4053-4-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2046-4053-4-10