Abstract
Background
The aim of this meta-analysis was to compare the long-term efficacy of diet plus exercise (D + E) vs. diet (D), D + E vs. exercise (E) and D vs. E on anthropometric outcomes and cardiovascular risk factors in overweight and obese participants.
Methods
Electronic searches were performed in MEDLINE and the Cochrane Central Register of controlled trials. Inclusion criteria were as follows: body mass index ≥25 kg/m2 and a minimum intervention period including follow-up of ≥12 months. Outcomes of interest were as follows: anthropometric parameters, blood lipids, blood pressure and cardiorespiratory fitness. Pooled effects were calculated using pairwise random effects and Bayesian random effects network meta-analysis. Results of the corresponding fixed effects models were compared in sensitivity analyses.
Results
Overall, 22 trials (24 reports) met the inclusion criteria and 21 (including 3,521 participants) of them were included in the quantitative analysis. As compared with D, D + E resulted in a significantly more pronounced reduction in body weight [mean differences (MD): -1.38 kg, 95% confidence interval (CI) -1.98 to -0.79], and fat mass (MD: -1.65 kg, 95% CI -2.81 to -0.49], respectively. When comparing D + E with E, MD in change of body weight (-4.13 kg, 95% CI -5.62 to -2.64), waist circumference (-3.00 cm, 95% CI -5.81 to -0.20), and fat mass (-3.60 kg, 95% CI -6.15 to -1.05) was in favour of combined diet and exercise, respectively. Comparing E vs. D, diet resulted in a significantly more pronounced decrease in body weight (MD: -2.93 kg, 95% CI -4.18 to -1.68), and fat mass (MD: -2.20 kg, 95% CI -3.75 to -0.66). D + E yielded also the greatest reductions with respect to blood lipids and blood pressure when compared to single applications of D and E, respectively. Results from the network meta-analyses confirmed these findings.
Conclusions
Moderate-quality evidence from the present network meta-analysis suggests that D + E can be highly recommended for long-term obesity management. Furthermore, the evidence suggests a moderate superiority of D over E with respect to anthropometric outcomes.
Systematic review registration
PROSPERO CRD42013003906
Similar content being viewed by others
Background
In 2008, an estimated 1.4 billion adults were overweight meaning that the prevalence of obesity has more than doubled since 1980. Of these, over 200 million men and nearly 300 million women were obese [1]. Overweight (body mass index (BMI): ≥25 kg/m2) and obesity (BMI: ≥30 kg/m2) are independent risk factors for non-communicable diseases, especially cardiovascular diseases (CVD) and several types of cancer [2, 3]. Exercise and diet are cornerstones in the prevention and management of overweight and obesity. Reductions of fat mass, primarily visceral adipose tissue, are major objectives. Energy expenditure increases with physical activity, especially with aerobic exercise or combined aerobic and resistance training [4, 5]. Caloric restriction induces weight loss by negative energy balance. Evidence from meta-analyses indicates that low-carbohydrate diets have slightly more favourable effects on body weight as compared to low-fat diets. Nevertheless, independent of macronutrient composition, the long-term health effects of diets are as yet unknown, and the observed outcomes appear of little clinical significance [6–9]. Regarding exercise training, results from recent network meta-analyses indicate that combined aerobic and resistance exercise is the most effective training modality in the treatment/prevention of overweight/obesity and type 2 diabetes mellitus [4, 10].
Previous meta-analyses by Shaw et al. [11] (including trials: ≥3 months length) as well as Wu et al. [12] (≥6 months) focused on intervention trials comparing diet plus exercise (D + E) vs. diet (D) on body weight and BMI as outcome parameters, but anthropometric outcomes such as waist circumference, fat mass, waist to hip ratio and cardiovascular risk factors (blood lipids, blood pressure and cardiorespiratory fitness) were not included. To the best of our knowledge, to date, no meta-analysis has compared the head-to-head and indirect long-term (≥12 months) effects of D + E vs. D vs. E on anthropometric parameters and cardiovascular risk factors. Therefore, the aim of this study was to conduct a systematic review with pairwise and network meta-analysis of randomized controlled trials to combine the direct and indirect evidence on the efficacy of different lifestyle long-term weight-reducing interventions on anthropometric parameters, blood lipids, blood pressure and cardiorespiratory fitness in participants with a BMI ≥25 kg/m2.
Methods
The review protocol has been registered in PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp Identifier: CRD42013003906).
Literature search
Queries of literature were performed using the electronic databases MEDLINE (between 1966 and June 2014) and the Cochrane Trial Register (until June 2014) with no restrictions to language and calendar date using the following search terms: (“lifestyle” OR “exercise” OR “diet”) AND (“body weight” OR “lipids”) AND (“randomized controlled trial” OR “randomized” OR “clinical trials as topic” OR “placebo” OR “randomly” OR “trial”) NOT (“animals” NOT “humans”). Moreover, the reference lists from retrieved articles and systematic reviews and meta-analyses were checked to search for further relevant studies. This systematic review was planned, conducted and reported in adherence to standards of quality for reporting meta-analyses [13]. Literature search was conducted independently by two authors (LS, GH), with disagreements resolved by consensus.
Eligibility criteria
Studies were included in the meta-analysis if they met all of the following criteria: (i) randomized controlled design; (ii) minimum intervention period including follow-up of 12 months; (iii) body mass index: ≥25 kg/m2; (iv) comparing D + E vs. D or/and D + E vs. E or/and D vs. E; (v) assessment of “primary outcome” markers: body weight (BW), waist circumference (WC), waist-to-hip ratio (WHR), fat mass (FM) and “secondary outcome” markers: total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triacylglycerols (TG), diastolic blood pressure (DBP), systolic blood pressure (SBP) and cardiorespiratory fitness (VO2 max); (vi) participants with coronary heart disease were excluded; (vii) report post-intervention mean values (if not available change-from-baseline value scores were used) with standard deviation (or basic data to calculate these parameters: standard error or 95% confidence interval (CI)) according to the Cochrane Handbook [14]; and (viii) ≥19 years of age.
Risk of bias assessment
Full copies of studies were independently assessed for methodological quality by two authors (LS, GH) using the risk of bias assessment tool by the Cochrane Collaboration. The following sources of bias were detected: selection bias (random sequence generation, allocation concealment), performance/detection bias (blinding of participants and personnel, blinding of outcome assessment), attrition bias (incomplete data outcome) and reporting bias (selective reporting) (Figure 1) [14, 15].
Data extraction and statistical analysis
The following data were extracted from each study: the first author’s last name, publication year, study length (including follow-up), participant’s sex and age, BMI, sample size,% T2D, intervention type, characteristics of dietary intervention, characteristics of exercise intervention, dropout rates, post-intervention mean values or change-from-baseline value scores with corresponding standard deviation. Data extraction was performed by one author (LS).
Separate pairwise meta-analyses were first used to compare all lifestyle interventions. Network meta-analysis was then used to synthesize all the available evidence [16]. Network meta-analysis methods are extensions of the standard pairwise meta-analysis model which enable simultaneous comparison of multiple interventions whilst preserving the internal randomization of individual trials. They have the advantage of adequately accounting for the correlation in relative effect estimates from three-arm trials as well as providing a single coherent summary of all the evidence.
Pairwise meta-analyses
For each outcome measure of interest and for each pair of treatments, a random effects inverse variance meta-analysis was performed in order to determine the pooled effect of the intervention in terms of mean differences (MDs) between the post-intervention (or change-from-baseline) values of the different lifestyle interventions [14]. Data were pooled if outcomes were reported by at least three studies. Heterogeneity between trial results was tested with a standard χ 2 test. The I 2 parameter was used to quantify any heterogeneity: I 2 = [(Q - d.f.)]/Q × 100%, where Q is the χ 2 statistic and d.f. is its degrees of freedom. A value for I 2 > 50% was considered to represent substantial heterogeneity [17]. As study characteristics were expected to differ, statistical heterogeneity was also expected and a random effects model was used to estimate MDs with 95% CIs. In addition, sensitivity analyses were planned to further elucidate the potential influence of heterogeneity due to different study characteristics on the outcome of the pairwise meta-analysis (such as study length, age of participants, risk of bias). Forest plots were generated to illustrate the study-specific effect sizes along with a 95% CI. To determine the presence of publication bias, we assessed the symmetry of the funnel plots in which mean differences were plotted against their corresponding standard errors taking into account the recommendation by Sterne et al. [18], i.e. that testing for funnel plot asymmetry should only be conducted if the number of studies is ten or larger. Additionally, Begg’s and Egger’s regression tests were performed to detect small study effects [19, 20].
Network meta-analyses
To account for the expected between-study heterogeneity, random effects network meta-analysis models were used. For each outcome, a common between-study heterogeneity parameter was assumed to reflect the variability between studies of all interventions.
Model fit was assessed by comparing the number of data points to the posterior mean of the residual deviance [16] (these values should be similar in a well-fitting model). Pooled effect sizes from the network meta-analyses are presented as posterior medians and 95% credible intervals (CrI) (i.e. Bayesian equivalent of confidence intervals) in the appropriate units along with the estimated between-study heterogeneity and its 95% CrI. Treatments were ranked best, second best and third best based on their efficacy.
To assess sensitivity to the choice of random or fixed effects network meta-analysis models, the two models were compared using the deviance information criteria for each outcome [16, 21] which account for both model fit and complexity. The fixed effects model was considered adequate when its deviance information criterion (DIC) was lower than the random effects model (differences >3 or 5 are considered meaningful) [16, 21]. Mean differences for the fixed effects network meta-analysis (NMA) model are also presented for comparison.
Computation
For pairwise meta-analyses, data were analysed using the Review Manager 5.1 software, provided by the Cochrane Collaboration (http://ims.cochrane.org/revman). Network meta-analyses were conducted using Markov chain Monte Carlo (MCMC) simulation implemented with the open-source software WinBUGS, version 1.4.3 [22]. The WinBUGS code used is freely available online (program “TSD2-5aRE_Normal_id.odc” for the random effects models and “TSD2-5aFE_Normal_id.odc” for the fixed effects models) [16, 23]. Minimally informative normal priors (with mean zero and variance 10,000) were used for all treatment effect parameters, and a uniform (0, 150) prior was used for the between-study standard deviation (heterogeneity) parameter. These priors were considered non-informative over the expected range of data. Sensitivity to the prior on the between-study heterogeneity was assessed by varying the upper bound of the uniform distribution, but there was no meaningful change in relative effects or overall conclusions.
Three MCMC chains were used to assess convergence using Brooks-Gelman-Rubin plots and by inspection of the trace plots [24]. Convergence was achieved after 20,000 iterations for all outcomes. Posterior summaries were then obtained from further simulation of 50,000 iterations in each of the three chains (150,000 in total), resulting in a small Monte Carlo error.
Treatments were ranked at each iteration (post-convergence) according to their efficacy, where the “best” treatment was the one with the most favourable outcome (which could be described by a higher or lower MD, depending on the outcome).
The potential for inconsistency was assessed by inspection of the network plots. Where there was a potential for inconsistency, i.e. where there were independent sources of evidence informing direct and indirect estimates, Bayesian p values for the difference between direct and indirect evidence were calculated using the node split method [25, 26] implemented in R through GeMTC [27], and direct and indirect estimates were compared.
Results
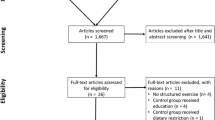
In order to ease interpretation of results, the following changes would be considered to be a benefit: BW, decrease; WC, decrease; FM, decrease; WHR, decrease; TC, decrease; LDL-C, decrease; HDL-C, increase; TG, decrease; DBP, decrease; SBP, decrease; VO2 max, increase; altogether, 22 trials (24 reports) met the inclusion criteria and 21 of them were included in the quantitative analysis [28–52]. The detailed steps of the meta-analysis article selection process are given as a flow chart in Figure 2, and full search strategy for PUBMED and the Cochrane Trial Register is given in Additional file 1.
All studies included were randomized controlled trials (RCTs) with a duration ranging between 12 and 72 months, published between 1988 and 2013 and enrolling a total of 3,521 participants, 680 of them being participants with T2D. The mean age varied between 35 and 70 years and the BMI between 25.6 and 38.2 kg/m2. Seventeen trials compared D + E vs. D, 11 compared D + E vs. E and 14 compared D vs. E. General study characteristics are summarized in Table 1. Regarding the dietary interventions, a major part of the included trials recommended energy-reduced low-fat diets (≤30% fat of total energy), low in saturated fat, and increased intakes of fruit, vegetables and fibre. Exercise prescription was partly supervised and included aerobic exercise (i.e. jogging, walking, flexibility, circuit training) and resistance training, overall 50%–85% of maximal heart rate.
The direct pairwise and network pooled estimate of effect size for the effects of D + E vs. D, D + E vs. E and D vs. E on anthropometric outcomes, blood lipids, blood pressure and cardiorespiratory fitness are summarized in Table 2.
Anthropometric outcomes/cardiorespiratory fitness
Diet + exercise vs. diet
The weighted mean difference in change of BW [MD: -1.38 kg (95% CI -1.98 to -0.79), I 2 = 0%], WC [MD: -1.68 cm (95% CI -2.66 to -0.70), I 2 = 0%], WHR [MD: -0.01 U (95% CI -0.02 to -0.01), I 2 = 0%] and FM [MD: -1.65 kg (95% CI -2.81 to -0.49), I 2 = 61%] was significantly more pronounced in the D + E group as compared to D, respectively. Furthermore, the D + E group revealed significantly more prominent increases in cardiorespiratory fitness (measured as VO2 max) [MD: 3.61 ml/kg/min (95% CI 2.07 to 5.14), I 2 = 88%] and HDL cholesterol [MD: 1.62 mg/dl (95% CI 0.28 to 2.95), I 2 = 51%] as well as decreases in TG [MD: -10.08 mg/dl (95% CI -17.38 to -2.79), I 2 = 0%] and DBP [MD: -1.20 mmHg (95% CI -2.26 to -0.15), I 2 = 28%]. In contrast, changes observed for TC, LDL-C and SBP did not differ significantly between both groups.
Diet + exercise vs. exercise
Comparing D + E vs. E, a significantly more distinctive reduction in BW [MD: -4.13 kg (95% CI -5.62 to -2.64), I 2 = 77%], WC [MD: -3.00 cm (95% CI -5.81 to -0.20), I 2 = 69%], WHR [MD: -0.01 U (95% CI -0.02 to -0.00), I 2 = 15%] and FM [MD: -3.60 kg (95% CI -6.15 to -1.05), I 2 = 92%] could be observed in the D + E group. Rise of VO2 max was significantly more pronounced in the D + E group as well [MD: 2.13 ml/kg/min (95% CI 1.52 to 2.74), I 2 = 9%]. With respect to blood lipids, TC [MD: -11.36 mg/dl (95% CI -15.93 to -6.79), I 2 = 0%], LDL-C [MD: -10.03 mg/dl (95% CI -14.28 to -5.78), I 2 = 8%], DBP [MD: -2.06 mmHg (95% CI -3.39 to -0.72), I 2 = 0%] and SBP [MD: -2.84 mmHg (95% CI -4.54 to -1.13), I 2 = 0%] were reduced more substantially following combined D + E when compared to single E interventions. No significant differences could be observed for HDL-C and TG.
Diet vs. exercise
Following D vs. E comparisons, reductions in BW [MD: -2.93 kg (95% CI -4.18 to -1.68), I 2 = 74%], FM [MD: -2.20 kg (95% CI -3.75 to -0.66), I 2 = 82%], HDL-C [MD: -0.96 mg/dl (95% CI -1.88 to -0.04), I 2 = 0%] and SBP [MD: -2.19 mmHg (95% CI -4.23 to -0.15), I 2 = 25%] were significantly more pronounced in the D group. WC, WHR, TC, LDL-C and cardiorespiratory fitness were not affected in a different fashion by either D or E.
Network meta-analysis
Additional file 1: Figure S1 shows the network of included trials. The pooled estimates of effect size for the comparison of D + E vs. D vs. E using both direct and indirect evidence on anthropometric outcomes, blood lipids, blood pressure and cardiorespiratory fitness are summarized in Table 2.
Both D + E and D were significantly more effective in reducing BW and FM when compared to E alone. D + E turned out to be the most effective lifestyle intervention with respect to reduction of BW, WC, FM, TC, LDL-C, TG, DBP, SBP and increasing HDL-C. D + E resulted in a high (>75%) probability to be the best for most outcomes. There is greater uncertainty regarding which treatment is the best for HDL-C, although again D + E yielded the highest probability of being the best. D turned out be the second effective lifestyle intervention for BW, WC, FM, TC and DBP (>75% probability).
There was potential for inconsistency in the networks for all outcomes except WHR (Additional file 1: Figure S1). There was some evidence of inconsistency for the outcome LDC (p value = 0.02) although this might be due to chance since several p values for inconsistency are being calculated (Additional file 1: Table S8). After inspection of the evidence on this outcome we did not identify a reason for this apparent inconsistency.
Risk of bias
The dropout rates ranged from 0% to 65%, with 11 out of 22 trials reporting dropout rates <10% (Table 1). Pairwise meta-analysis resulted in no significant dropout differences (p = 0.37) between D + E (122/1,257) vs. D (111/1,152), D + E (79/714) vs. D (82/715) (p = 0.96) and D + E (87/701) vs. D (84/706) (p = 0.34). Eight trials (nine reports) reported random sequence generation [34, 35, 42, 43, 46, 47, 50–52], and only two trials reported allocation concealment [42, 52]. None of the studies reported blinding of volunteers towards mode of intervention (not possible in diet/exercise trials), and four trials (five reports) appear to have adequate blinding of outcome assessment [46, 47, 50–52]. High risk of bias was defined as fewer than three out of a maximum yield of six low risk of bias items using the risk of bias assessment tool from the Cochrane Collaboration. Only seven low risk of bias trials (eight reports) were identified [34, 35, 42, 46, 50–52], and sensitivity analyses were performed for studies with a low risk of bias (Additional file 1: Table S1).
Sensitivity analysis
Sensitivity analyses (pairwise meta-analysis) were performed for obese, study length ≥24 months, older participants (age: ≥50 years) and low risk of bias studies. Comparison of long-term vs. short-term trials resulted in smaller reductions in body weight (Additional file 1: Tables S2-S3). The results of the primary analysis (D + E vs. D and D vs. E) could be confirmed including only obese participants (Additional file 1: Table S4), except for WC and VO2 max. Including only participants ≥50 years of age (Additional file 1: Table S5), results of the primary analysis could not be confirmed regarding anthropometric outcomes when comparing D + E vs. D (only four to eight trials available), whilst the results of the main analysis for D vs. E were not affected. Sensitivity analyses excluding trials with a high risk of bias changed some summary estimates (D + E vs. D) for anthropometric outcomes and became statistically non-significant (with the exception of D vs. E comparisons).
Fixed vs. random effects models
Results of the pairwise meta-analyses with a fixed effects model are presented in Additional file 1: Table S6. Overall, the results of the main analysis could be confirmed in the fixed pairwise sensitivity analyses. However comparing D vs. E resulted in a significant reduction in WC, TC and LDL-C in the fixed effects model, which was not observed in the random effects model, that were more pronounced in the D group. However, we note that the between-study heterogeneity was moderate to high for these outcomes and therefore the fixed effects model may not be appropriate.
In the NMA, comparing the DIC for fixed and random effects models suggests that the fixed effects model might be appropriate for DBP, SBP, TC, TG and WC (Additional file 1: Table S7). The estimated treatment effects using fixed effects network meta-analyses are given in Additional file 1: Table S6. Some differences could be observed between the random and fixed effects models. In the fixed effects model, comparing D vs. E resulted in a significant reduction in WC, TC, LDL-C, SBP and VO2 max, favouring D (with the exception of VO2 max), compared to the random effects model. Furthermore, the fixed effects model showed a significant reduction in WHR, comparing D + E vs. D/E, and increase in HDL-C compared to E. Again, we note that the fixed effects model was not always considered suitable (Additional file 1: Table S7).
Publication bias
Begg's and Egger's regression tests provided no evidence of substantial publication bias (data not shown). Funnel plots were generated only if specific outcome measures were provided by at least ten different trials (some trials provide more than one data point for analysis as they report results for subgroups: men and women) [18]. The plot with respect to effect size change for body weight in response to D + E vs. D vs. E indicates little asymmetry (Additional file 1: Figures S2-S3). Thus, publication bias cannot be completely excluded as a factor affecting the results of the present meta-analysis.
Discussion
The primary findings of this systematic review and meta-analysis of long-term trials are that a combination of D + E is more effective in reducing BW compared to either D or E in overweight and obese participants. Furthermore, improvements in WC, WHR and FM were significantly more pronounced following D + E compared to either D or E. The combination of D + E is also the most powerful lifestyle intervention to reduce blood lipids and blood pressure. Compared to E, a dietary intervention resulted in significantly greater reductions in body weight, fat mass and systolic blood pressure. Pooling both direct and indirect evidence on D + E, D and E via network meta-analysis demonstrated that D + E was the most efficacious exercise intervention regarding its impact on BW, WC, FM, TC, LDL-C, TG, DBP and VO2 max.
The findings of this meta-analysis, i.e. diet being significantly more effective in reducing BW than exercise, are supported by results of a 2006 Cochrane review [11] and are consistent with results of another meta-analysis [53]. Therefore, caloric restriction appears the most powerful method for achieving weight loss in overweight and obese people. The amount of weight loss yielded by diet and exercise can be compared to the corresponding results of the most effective pharmacological interventions such as orlistat (4.12/3.1 kg at 12 months) [54, 55]. A recent systematic review concluded that orlistat, lorcaserin and phentermine/topiramate ER when used as an adjunct to lifestyle intervention, could induce a clinically relevant (≥5%) 12-month weight loss [56]. The interpretation of our network meta-analysis is restricted by the fact that none of the trials evaluated the impact of their interventions on clinical outcomes. It should be noted, however, that no anti-obesity drugs have been shown to have a favourable effect on CVD morbidity and mortality as well [57]. Furthermore anti-obesity drugs were associated with increased risk of total adverse events, tachycardia, gastrointestinal disease, hypertension and mouth dryness [58].
Previous studies reported that a 5% reduction of BW is associated with a reduced risk of T2D incidence and other metabolic disorders. A 5-kg weight loss over time could account for a 55% reduction in the risk of diabetes over the mean of 3.2 years of follow-up in a high-risk population [59]. Regarding visceral adipose tissue, a comprehensive meta-analysis of cohort studies showed that a 1-cm increase in WC and a 0.01-U increase in WHR are associated with a 2%–5% increase in risk of future CVD [60]. Applying this data to the results of the present meta-analysis, D + E would be associated with a CVD risk reduction of ~3%–6%. Improvements on anthropometric outcomes were more distinct in younger participants compared with older which might be explained by the fact that younger participants were able to perform more intense exercise sessions. However, since not all trials applied supervised exercise, no definitive explanation can be given.
With respect to blood lipids, a meta-analysis of 70 studies indicate that each kilogram of weight loss was associated with a 1.9 mg/dl decrease in TC and a 0.77 mg/dl decrease in LDL-C, respectively [61]. Furthermore, RCTs showed that especially aerobic exercise was associated with an increase in HDL-C [62]. These associations could be confirmed in the present meta-analysis.
The predominant dietary intervention implemented in the included trials was either an energy-restricted low-fat diet or an energy-balanced moderate-fat diet. In general, the composition of diets was approximately at least 500 kcal below the estimated energy need, and fat intake was ≤30% of total energy content. In the D + E and D trials, 1,200 kcal for women and 1,500 kcal for men were generally prescribed. In the dietary intervention trials, general guidelines for physical activity were recommended. However, in the D + E trials, specific goals for physical activity/exercise were implemented. Network meta-analyses provides evidence that a combination of aerobic and resistance training should be recommended in the prevention and treatment of overweight, obesity and associated diseases [4, 10].
Cardiorespiratory fitness is associated with cardiovascular mortality and cancer in men and women [63, 64]. A pooled analysis investigating the effects of cardiorespiratory fitness on all-cause mortality and cardiovascular events demonstrated that a 1-unit increase in metabolic equivalents was associated with a 13% and 15% reduction in risk of all-cause mortality and coronary heart disease (CHD)/CVD, respectively [65]. Transferring this data to the results of the present meta-analysis, D + E reduced the risk of all-cause mortality by 14% and of CVD/CHD by 16% and approximately 8% (mortality) and 9% (CVD/CHD) following applications of single lifestyle interventions, respectively. A possible explanation of the superior effect of D + E on cardiorespiratory fitness could be the greater weight loss induced by caloric restriction.
The present systematic review has several limitations. A major limitation is that no search for unpublished studies and any additional data sources and strategies (author contacts, trial registers) was performed. Another limitation is the fact that not all potential effect modifiers were accounted for. Often participants in different arms of trials did not receive equal numbers of contacts. It could be argued that contact time rather than the specific elements of the intervention affected participant’s weight and cardiovascular risk factor outcomes. Heterogeneity could be observed for some outcome parameters, probably introduced by differences between trials, including different D + E regimens. Publication bias cannot be excluded to affect the results on any meta-analysis; however, formal statistical testing did not suggest publication bias for the current analysis. Had there been evidence of publication bias and if more studies were available, regression techniques could be used to adjust for this [66].
Although many studies included in our analysis had a substantial dropout rate (see Table 1), intention-to-treat analyses were generally not conducted. However, dropouts were generally similar for all intervention groups. Taken together, 2/3 of the included trials were judged as being at high risk of bias. Therefore, the results should be interpreted in a conservative manner. Strengths of this research include the application of the network meta-analysis, as well as the fact that there was no evidence of inconsistency for most outcomes, and an overall sample size of 3,521 participants.
Conclusions
The present network meta-analysis provides moderate-quality evidence that D + E induces moderate long-term weight loss and reduces blood lipids and blood pressure when compared to E or D as single interventional measures, respectively. In addition, the evidence suggests moderate superiority of D over E regarding anthropometric outcome parameters. The current findings seem to be clinically relevant for public health, in particular for encouraging a combination of diet and exercise for primary prevention of overweight and obesity. Future trials should investigate the long-term effects of different training modalities (aerobic, resistance or combined training) in combination with dietary interventions for the prevention and treatment of overweight and obesity.
References
WHO: Obesity and Overweight. Fact sheet No. 311. 2011, [http://www.who.int/mediacentre/factsheets/fs311/en/]
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH: The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009, 9: 88-10.1186/1471-2458-9-88.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M: Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008, 371 (9612): 569-578. 10.1016/S0140-6736(08)60269-X.
Schwingshackl L, Dias S, Strasser B, Hoffmann G: Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One. 2013, 8 (12): e82853-10.1371/journal.pone.0082853.
Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC: Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999, 281 (4): 335-340. 10.1001/jama.281.4.335.
Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T: Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013, 110 (7): 1178-1187. 10.1017/S0007114513000548.
Schwingshackl L, Hoffmann G: Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013, 113 (12): 1640-1661. 10.1016/j.jand.2013.07.010.
Santos FL, Esteves SS, da Costa PA, Yancy WS, Nunes JP: Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012, 13 (11): 1048-1066. 10.1111/j.1467-789X.2012.01021.x.
Schwingshackl L, Hoffmann G: Comparison of the long-term effects of high-fat v. low-fat diet consumption on cardiometabolic risk factors in subjects with abnormal glucose metabolism: a systematic review and meta-analysis. Br J Nutr. 2014, 111 (12): 2047-2058. 10.1017/S0007114514000464.
Schwingshackl L, Missbach B, Dias S, Konig J, Hoffmann G: Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. 2014, 57 (9): 1789-1797. 10.1007/s00125-014-3303-z.
Shaw K, Gennat H, O’Rourke P, Del Mar C: Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006, 4: CD003817-
Wu T, Gao X, Chen M, van Dam RM: Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009, 10 (3): 313-323. 10.1111/j.1467-789X.2008.00547.x.
Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6 (7): e1000097-10.1371/journal.pmed.1000097.
Higgins JP, Green S: The Cochrane Collaboration. Cochrane Handbook of Systematic Reviews, Version 5.1.0.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA: The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011, 343: d5928-10.1136/bmj.d5928.
Dias S, Sutton AJ, Ades AE, Welton NJ: Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013, 33 (5): 607-617. 10.1177/0272989X12458724.
Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ. 2003, 327 (7414): 557-560. 10.1136/bmj.327.7414.557.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP: Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011, 343: d4002-10.1136/bmj.d4002.
Begg CB, Mazumdar M: Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994, 50 (4): 1088-1101. 10.2307/2533446.
Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997, 315 (7109): 629-634. 10.1136/bmj.315.7109.629.
Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A: Bayesian measures of model complexity and fit. J R Stat Soc (B). 2002, 64: 583-616. 10.1111/1467-9868.00353.
Lunn DJ, Thomas A, Best N, Spiegelhalter D: WinBUGS - a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000, 10: 325-337. 10.1023/A:1008929526011.
Dias S, Welton NJ, Sutton AJ, Ades AE: NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011, [http://www.nicedsu.org.uk]
Brooks SP, Gelman A: Alternative methods for monitoring convergence of iterative simulations. J Comput Graphical Stat. 1998, 7: 434-455.
Dias S, Welton NJ, Caldwell DM, Ades AE: Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010, 29 (7–8): 932-944.
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE: Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013, 33 (5): 641-656. 10.1177/0272989X12455847.
van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ: Automating network meta-analysis. Res Synth Method. 2012, 3: 285-299. 10.1002/jrsm.1054.
Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, Superko HR, Fortmann SP, Albers JJ, Vranizan KM, Ellsworth NM, Terry RB, Haskell WL: Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988, 319 (18): 1173-1179. 10.1056/NEJM198811033191801.
Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W: Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998, 21 (3): 350-359. 10.2337/diacare.21.3.350.
Wing RR, Epstein LH, Paternostro-Bayles M, Kriska A, Nowalk MP, Gooding W: Exercise in a behavioural weight control programme for obese patients with type 2 (non-insulin-dependent) diabetes. Diabetologia. 1988, 31 (12): 902-909.
Williams PT, Krauss RM, Stefanick ML, Vranizan KM, Wood PD: Effects of low-fat diet, calorie restriction, and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism. 1994, 43 (5): 655-663. 10.1016/0026-0495(94)90210-0.
Wadden TA, Vogt RA, Foster GD, Anderson DA: Exercise and the maintenance of weight loss: 1-year follow-up of a controlled clinical trial. J Consult Clin Psychol. 1998, 66 (2): 429-433.
Volpe SL, Kobusingye H, Bailur S, Stanek E: Effect of diet and exercise on body composition, energy intake and leptin levels in overweight women and men. J Am Coll Nutr. 2008, 27 (2): 195-208. 10.1080/07315724.2008.10719691.
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K: Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011, 364 (13): 1218-1229. 10.1056/NEJMoa1008234.
Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD: Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med. 1998, 339 (1): 12-20. 10.1056/NEJM199807023390103.
Snel M, van Diepen JA, Stijnen T, Pijl H, Romijn JA, Meinders AE, Voshol P, Jazet IM: Immediate and long-term effects of addition of exercise to a 16-week very low calorie diet on low-grade inflammation in obese, insulin-dependent type 2 diabetic patients. Food Chem Toxicol. 2011, 49 (12): 3104-3111. 10.1016/j.fct.2011.09.032.
Skender ML, Goodrick GK, Del Junco DJ, Reeves RS, Darnell L, Gotto AM, Foreyt JP: Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc. 1996, 96 (4): 342-346. 10.1016/S0002-8223(96)00096-X.
Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO: One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006, 61 (9): 943-950. 10.1093/gerona/61.9.943.
Pritchard JE, Nowson CA, Wark JD: A worksite program for overweight middle-aged men achieves lesser weight loss with exercise than with dietary change. J Am Diet Assoc. 1997, 97 (1): 37-42. 10.1016/S0002-8223(97)00015-1.
Anderssen SA, Hjermann I, Urdal P, Torjesen PA, Holme I: Improved carbohydrate metabolism after physical training and dietary intervention in individuals with the "atherothrombogenic syndrome'. Oslo Diet and Exercise Study (ODES). A randomized trial. J Intern Med. 1996, 240 (4): 203-209. 10.1046/j.1365-2796.1996.22848000.x.
Reseland JE, Anderssen SA, Solvoll K, Hjermann I, Urdal P, Holme I, Drevon CA: Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr. 2001, 73 (2): 240-245.
Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, Jackson N, Fitzsimons K, Bright J, Coulman K, England CY, Gorton J, McLenaghan A, Paxton E, Polet A, Thompson C, Dayan CM: Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011, 378 (9786): 129-139. 10.1016/S0140-6736(11)60442-X.
Borg P, Kukkonen-Harjula K, Fogelholm M, Pasanen M: Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes Relat Metab Disord. 2002, 26 (5): 676-683. 10.1038/sj.ijo.0801962.
Brekke HK, Jansson PA, Lenner RA: Long-term (1- and 2-year) effects of lifestyle intervention in type 2 diabetes relatives. Diabetes Res Clin Pract. 2005, 70 (3): 225-234. 10.1016/j.diabres.2005.03.027.
Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M: Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch Intern Med. 2000, 160 (14): 2177-2184. 10.1001/archinte.160.14.2177.
Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, McTiernan A: Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012, 20 (8): 1628-1638. 10.1038/oby.2011.76.
Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, Palla S, Bleecker E, Pahor M: Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004, 79 (4): 544-551.
Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997, 20 (4): 537-544. 10.2337/diacare.20.4.537.
Wood PD, Stefanick ML, Williams PT, Haskell WL: The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991, 325 (7): 461-466. 10.1056/NEJM199108153250703.
Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF: Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013, 310 (12): 1263-1273. 10.1001/jama.2013.277669.
Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD: Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004, 50 (5): 1501-1510. 10.1002/art.20256.
Christensen P, Frederiksen R, Bliddal H, Riecke BF, Bartels EM, Henriksen M, Juul SRT, Gudbergsen H, Winther K, Astrup A, Christensen R: Comparison of three weight maintenance programs on cardiovascular risk, bone and vitamins in sedentary older adults. Obesity (Silver Spring). 2013, 21 (10): 1982-1990. 10.1002/oby.20413.
Curioni CC, Lourenco PM: Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond). 2005, 29 (10): 1168-1174. 10.1038/sj.ijo.0803015.
Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP: Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007, 107 (10): 1755-1767. 10.1016/j.jada.2007.07.017.
Gray LJ, Cooper N, Dunkley A, Warren FC, Ara R, Abrams K, Davies MJ, Khunti K, Sutton A: A systematic review and mixed treatment comparison of pharmacological interventions for the treatment of obesity. Obes Rev. 2012, 13 (6): 483-498. 10.1111/j.1467-789X.2011.00981.x.
Yanovski SZ, Yanovski JA: Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014, 311 (1): 74-86. 10.1001/jama.2013.281361.
Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A: Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013, 369 (2): 145-154.
Zhou YH, Ma XQ, Wu C, Lu J, Zhang SS, Guo J, Wu SQ, Ye XF, Xu JF, He J: Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2012, 7 (6): e39062-10.1371/journal.pone.0039062.
Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J: Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006, 29 (9): 2102-2107. 10.2337/dc06-0560.
de Koning L, Merchant AT, Pogue J, Anand SS: Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007, 28 (7): 850-856. 10.1093/eurheartj/ehm026.
Dattilo AM, Kris-Etherton PM: Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992, 56 (2): 320-328.
Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA: Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002, 347 (19): 1483-1492. 10.1056/NEJMoa020194.
Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW, Blair SN: Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011, 124 (23): 2483-2490. 10.1161/CIRCULATIONAHA.111.038422.
Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW: Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989, 262 (17): 2395-2401.
Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H: Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009, 301 (19): 2024-2035. 10.1001/jama.2009.681.
Moreno SG, Sutton AJ, Turner EH, Abrams KR, Cooper NJ, Palmer TM, Ades AE: Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ. 2009, 339: b2981-10.1136/bmj.b2981.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LS extracted the data. LS, SD and GH conducted the data analysis, interpreted the results and drafted and finalized the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
13643_2014_298_MOESM1_ESM.docx
Additional file 1: Full search strategy: PUBMED. Table S1: sensitivity analysis (low risk of bias). Table S2: sensitivity analysis (study length: ≥24 months). Table S3: sensitivity analysis (study length, <24 months). Table S4: sensitivity analysis (obese participants). Table S5: sensitivity analysis (participants ≥50 years of age). Table S6: sensitivity analyses (direct pairwise and network meta-analysis, fixed effects models). Table S7: model fit statistics for the random and fixed effects network meta-analysis models. Table S8: estimates of the effects of diet + exercise vs. exercise from direct and indirect evidence in node split models. Figure S1: network graph. Figure S2: funnel plot for body weight (diet + exercise vs. diet). Figure S3: funnel plot for body weight (diet + exercise vs. exercise). Figure S4: funnel plot for body weight (diet vs. exercise). (DOCX 101 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Schwingshackl, L., Dias, S. & Hoffmann, G. Impact of long-term lifestyle programmes on weight loss and cardiovascular risk factors in overweight/obese participants: a systematic review and network meta-analysis. Syst Rev 3, 130 (2014). https://doi.org/10.1186/2046-4053-3-130
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2046-4053-3-130