Abstract
Fifty-four cows with left displacement of the abomasum (LDA) submitted to the hospital facility at Riverview Veterinary Clinic from February to July 2005 were treated by right flank laparotomy and omentopexy. Five cows died (a survival rate 90.7%) and one cow (1.8%) was culled due to recurrence of the LDA post-operatively. Forty-one cows (76%) returned to good production post-operatively. Thirty-nine cows (72%) were pregnant six months after corrective surgery.
Similar content being viewed by others
Introduction
Left displacement of the abomasum (LDA) mainly affects high yielding lactating dairy cows during the first month postpartum [12], and was first described in the 1950s [15]. Many techniques have been employed including: the left paralumbar abomasopexy or 'Utrecht' method [1]; the right paralumbar omentopexy or 'Hannover technique' [8]; the 'toggle pin' method (Sterner-Gymer, 1982); the ventral laparoscopic abomasopexy method [16]; and, more recently, the two-step laparoscopic-Janowitz technique [22]. LDA surgery was initially performed the authors' veterinary clinic in the late 1970s (Kelleher, pers. comm.). In this paper, the current approach to surgical correction of LDA at this clinic is described.
Materials and methods
All LDA cases were diagnosed after hearing classical resonant sounds (i.e., 'pings') on simultaneous auscultation and percussion of the left flank, especially over the last three ribs during the clinical examination.
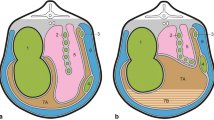
Following diagnosis, surgery was performed within 24 hours. Surgery involved a right paralumbar laparotomy and omentopexy. The right paralumbar fossa area was surgically prepared. A 2% lidocaine solution (Norocaine; Norbrook Laboratories, Newry, Northern Ireland) was infused in an inverted 'L-shape' fashion. A 20 cm vertical skin incision was made at the centre of the sublumbar fossa, 10 cm caudal to the most posterior aspect of the last rib. The external oblique muscle was incised in a similar fashion. The internal oblique was incised bluntly along its fibres, whilst the transversalis muscle and peritoneum were incised vertically. The surgeon's hand was placed inside the abdominal cavity, lightly touching the peritoneal surface of the body wall. The abomasum was located by moving the hand down the right abdominal wall to the floor and up the other side, under the rumen. Previously, the trapped gas would have been released using a needle and rubber tubing reaching to the exterior; however, in the last few years the gas has been released by pushing the abomasum down to the midline and replacing it into its correct position on the right hand side of the abdomen. The pyloric sphincter region and proximal duodenum were exteriorised so that the omentum adjacent to the sphincter region ('Sow's ear') could be visualised. An area of omentum, 2 cm × 2 cm within 5 cm of the pyloric sphincter, was stitched in a continuous 'through and through' pattern, using a polyamide suture (USP 3&4/EP 6) and a round-bodied needle. The two ends of the polyamide were sutured through the peritoneum and transversalis muscle and tied with a simple suture midway between the abdominal incision and the final rib. The peritoneum/transversalis layer and both abdominal muscles were sutured separately using chromic catgut (USP 3/EP 7) in a simple continuous fashion. The skin was closed using the aforementioned polyamide in a continuous Ford interlocking pattern.
Four cows in a shocked state were given hypertonic saline (5 ml/kg of 7.2% NaCL, 3l infusion for a 600 kg cow over five to six minutes) post-operatively. All cows were treated with penicillin and streptomycin (Pen-Strep; Norbrook Laboratories, Newry, Northern Ireland), propylene glycol (200 ml BID for the first day and 100 ml BID for three further days) and ruminal stimulants, 1 × 100 g BID (Stimulex; Biofac A/S, Denmark) for four days post-operatively. Any concurrent diseases were also treated. Metritis cases were treated with ceftiofur hydrochloride (Excenel RTU; Pfizer Animal Health, Ringaskiddy, Co. Cork) parenterally and intrauterine cephapirin (Metricure; Intervet, Boxmeer, the Netherlands). Mastitis cases were treated with trimethoprim sulphadiazine (Norodine 24%; Norbrook Laboratories, Newry, Northern Ireland).
Cows were discharged either on the day of surgery or on the following day, with a recommendation of once-daily milking and hay feeding for three to four days, with a gradual reintroduction to concentrate ration and to the rest of the herd.
On the day of diagnosis of LDA, details of each case were taken, including: length of time calved; lactation number; average annual yield of cow and herd; and, any concurrent diseases at or since calving. Several months later, clients were telephoned to determine whether cows survived post-surgery, how they had milked and whether they were going to continue in the herd.
Results
Seasonality
The majority of cases in this study occurred in March and April (Figure 1).
Post-partum interval to disease
Ten cases (25%) of LDA occurred in each of the first three weeks postpartum (Table 1).
Lactation number
The distribution of surgical cases by lactation number is shown in Table 2.
Milk yield
The predicted 305-day milk yield for the surgery cases is shown in Table 3.
Concurrent disease
On clinical examination, concurrent disease was found in 33 of 54 cases (61.1%) of LDA (Figure 2). Three-quarters (76%) of all cases of concurrent disease were related to the reproductive tract, including retained foetal membranes (15%), metritis (37%) and vaginitis secondary to trauma following dystocia (24%).
Analysis of surgical intervention
Two cows were inoperable and were euthanased because extensive adhesions throughout the abdominal cavity were found during surgery. Three cows died following surgical intervention (Table 4). One cow died as a consequence of fibrinous peritonitis diagnosed at surgery. However, two cows died unexpectedly after routine surgery; one cow had early fibrinous peritonitis at necropsy. The survival rate was 100% from one week post-operatively. Forty-one of the 49 cows (83.7%) were still milking four to five months later and 39 (79.6%) were pregnant.
Discussion
The prevalence of LDA on high-producing dairy farms varies from 0-7% per annum [2] with an increasing incidence due to rising average milk yields [6], higher genetic merit cows, inadequate nutritional management of high yielding cows and increasing awareness amongst farmers and veterinary surgeons. The temporal occurrence of LDA, with a peak in the months of March and April in our study, coincides with the early postpartum period and peak springtime calving.
An important finding of this study was that 75% of LDA cases occurred within the first three weeks postpartum. This occurrence is higher than some American studies which show only a 65% occurrence by 50 days postpartum [17]. However, in other studies, 80% of cases occurred within 30 days of calving [18, 13]) and 57% in the first two weeks, 80% in the first month and 91% by six weeks [9].
It is possible that many of the cases of LDA in our study that were diagnosed during the third week postpartum could have been present earlier because the case history obtained from the client reported that the cow "had not been right" since parturition.
In addition the peak in LDA occurrence associated with high milk yielding cows (≥9,000 litres) may be explained by the increased metabolic stress on these individuals. The peak at the 5,900 litre average milk yield may be explained by the fact that the majority of animals included in this study were young cows in their first and second lactations. These young cows naturally milk less than older cows and thus their expected average milk yield would be lower.
Our finding of 32.5% of LDA cases in primiparous animals contrasts with American research [18, 13] that reported that first-calvers had an incidence of only 18.9%, second lactation an incidence of 21.7% and third lactation an incidence of 59.4%. Fubini and Ducharme [9] reported that LDA was most common in cows between four and seven years of age.
The present study finding that 61.1% of LDA cases had concurrent illness is supported by other studies whereby cows with LDA were twice as likely to have another ailment as those that did not [7].
The pathogenesis of LDA involves three factors: rumen fill; post-partum abdominal void; and, abomasal atony [3]. For left abomasal displacement, space in the abdominal cavity must be created. This naturally occurs postpartum with the delivery of the calf, expulsion of the placenta and subsequent involution of the uterus. Holstein cows may be particularly predisposed to LDA due to the larger abdominal cavity [6]. This increase in abdominal space is exacerbated when the rumen is small, thus leaving space for the abomasum to displace to the left side [8]. Poor rumen fill in its own right is also a major contributory factor [3]. Reduced appetite after calving directly leads to reduced rumen fill and size. Also, these animals may not have access to adequate quantities of long fibre, resulting in reduced rumen function and appetite suppression. Cows that are fed finely ground rations [6] or an excess of short fibre forages such as maize [21] are more likely to develop an LDA due to reduced rumen fill and increased fermentation.
The final contributory influence is abomasal atony [3]. Endotoxins and mediators of inflammation (e.g., histamine) released after metritis, mastitis and laminitis can lead to a reduction in abomasal motility [4]. Such a hypomotile abomasum filled with gas due to excess volatile fatty acid (VFA) production displaces from its normal position to the dorso-cranial aspect of the left abdominal cavity.
In this study, two cases were inoperable as there were extensive adhesions throughout the abdominal cavity making reduction and relocation of the abomasum impossible. On consultation with the owner, both cows were euthanased.
One cow died post-operatively due to the presence of a fibrinous peritonitis that appeared to be well advanced. Although the position of the abomasum was corrected and the cow was treated accordingly, the prognosis remained extremely guarded. No abomasal ulcer was detected and no other obvious aetiology was observed. This represented a rate of 1.9%, compared with a rate of 1.5% achieved in a 400-case study by Verhoef [22].
In the present study, two of 54 cows (3.8%) died unexpectedly. Postmortem examination of one of the cows, two days post-surgery, displayed changes in peritoneal fluid and a presumptive diagnosis of peritonitis was made; no abomasal ulcer was present. This mortality rate compares with a rate of 7% in the study by Kelton et al. [11] which compared the paramedian approach with the toggle pin technique. Single deaths were recorded from mesenteric volvulus, salmonellosis, metabolic disease and suspected pyloric stenosis [11].
One case of LDA treated in the current study recurred (1.8%). This may have been associated with the tearing of the polyamide suture through the fat in the omentum. This recurrence compares to 0.25% in Verhoef's two-step laparoscopic-Janowitz technique (2005). An earlier study into the rolling method found re-occurrence rates of up to 50-70% [23]. A recurrence rate of 1.4% was reported for both the paramedian and toggle-pin techniques [11]. A 3.3% recurrence rate was reported for laparoscopic-guided abomasopexy [20].
Seventy-six percent of cows were milking satisfactorily six months later, while 79% of cows that were bred were back in calf. This compares favourably with the left paralumbar omentopexy study [24] but less so with the smaller study for a ventral laparoscopic abomasopexy method in a group of 17 cows [16], with rates of 100% and 88%, respectively.
A decision-tree analysis study of treatment alternatives for LDA carried out in the USA [19], suggests that surgical treatment had the highest expected monetary value (EMV) of $1,661. Closed surgical techniques, e.g., 'roll and tack', had an EMV of $1,636, whilst rolling had the lowest EMV at $764. This, however, was greater than the EMV of $480 obtained by selling the animal.
This present study highlights the potential benefits of surgical correction of LDA in a practice setting.
References
Baird AN, Harrison S: Surgical treatment of left displaced abomasums. Compend Contin Educ Pract Vet. 2001, 23: S102-S108.
Cameron REB, Dijk PB, Herdt TH, Kaneene JB, Miller R, Bucholtz F, Liesman JS, Vandehaar MJ, Emery RS: Dry cow diet, management, and energy balance as risk factors for displaced abomasum in high producing dairy herds. Journal of Dairy Science. 1998, 81 (1): 132-139. 10.3168/jds.S0022-0302(98)75560-2.
Constable PD, Miller GY, Hoffsis GF, Hull BF, Rings DF: Risk factors for abomasal volvulus and left abomasal displacement in cattle. American Journal of Veterinary Research. 1992, 53: 1184-1192.
Correa MT, Erb H, Scarlett JM: Path analysis for seven postpartum disorders of Holstein cows. Journal of Dairy Science. 1993, 76: 1305-1312. 10.3168/jds.S0022-0302(93)77461-5.
Cottrell DF: Vagal reflex inhibition of motility in the abomasal body of sheep by antral and duodenal tension receptors. Veterinary Research Communications. 1994, 18: 319-330. 10.1007/BF01839200.
Dawson LJ, Aalseth EP, Rice LE, Adams GD: Influence of fiber form in a complete mixed ration on incidence of left displaced abomasum in postpartum dairy cows. Journal of American Medical Association. 1992, 200: 1989-1992.
Detilleux JC, Gröhn YT, Eicker SW, Quaas RL: Effects of left displaced abomasum on test day milk yields of Holstein cows. Journal of Dairy Science. 1997, 80 (1): 121-126. 10.3168/jds.S0022-0302(97)75919-8.
Dirksen G: Dilation, displacement and torsion of the abomasum in cattle. Deutsche Tierarztl Wochenschr. 1962, 68: 8-12.
Fubini SL, Ducharme NG: Farm animal surgery. 2004, Philadelphia: Saunders
Fubini SL, Ducharme NG, Erb HN, Shiels RL, Hollis NE: A comparison in 101 dairy cows of right paralumbar fossa omentopexy and right paramedian abomasopexy for treatment of left displacement of the abomasum. Canadian Veterinary Journal. 1992, 33: 318-324.
Kelton DF, García J, Guard CL, Dinsmore RP, Powers PM, Smith MC, Stelman S, Ralston N, White ME: Bar suture (toggle pin) vs open surgical abomasopexy for treatment of left displaced abomasum in dairy cattle. Journal of the American Veterinary Medical Association. 1988, 193 (5): 557-559.
Lotthammer KH: Epidemiological studies of the occurrence of abomasal displacement in dairy cows. Tierarztliche Umschau. 1992, 47: 320-328.
Martin W: Left abomasal displacement: an epidemiological study. Canadian Veterinary Journal. 1972, 13: 61-68.
Massey CD, Wang C, Donovan A, Beeda DK: Hypocalcaemia at parturition as a risk factor for left displacement of the abomasum in dairy cows. Journal of the American Veterinary Medical Association. 1993, 203: 852-853.
Moore GR, Riley WF, Westcott RW: Displacement of the bovine abomasum. Veterinary Medicine. 1954, 49: 49-51.
Mulon YP, Babkine M, Desrochers A: Ventral laparascopic abomasopexy in 18 cattle with displaced abomasum. Veterinary Surgery. 2006, 35: 347-355. 10.1111/j.1532-950X.2006.00156.x.
Petty RD: Surgical correction of left displaced abomasum in cattle: a retrospective study of 143 cases. Journal of the American Veterinary Medical Association. 1981, 178: 1274-1276.
Robertson JM: Left displacement of the bovine abomasum: epizootiologic factors. American Journal of Veterinary Research. 1968, 29: 421-434.
Ruegg PL, Carpenter TE: Decision-tree analysis of treatment alternatives for left displaced abomasum. Journal of the American Veterinary Medical Association. 1989, 195 (4): 464-467.
Seeger T, Kumper H, Failing K, Doll K: Comparison of laparascopic-guided abomasopexy versus omentopexy via right flank laparotomy for the treatment of left abomasal displacement in dairy cows. American Journal of Veterinary Research. 2006, 67 (3): 472-478. 10.2460/ajvr.67.3.472.
van Winden CL, Kuiper R: Left displacement of the abomasum in dairy cattle: recent developments in epidemiological and etiological aspects. Veterinary Research. 2003, 34: 47-56. 10.1051/vetres:2002060.
Verhoef M: Techniques for treating the left displaced abomasums (LDA), with emphasis on laparascopic repositioning and fixation in the standing cow. Proceedings of the first joint AVSPNI and CAVI conference. 2005, 90-92.
Vlaminck L, Martens A, Steenhaut F, Gasthuys F, Desmet P, De Moor A: Surgical correction of abomasal displacement in cows: a review of 163 cases. Vlaams Dier Tijd. 1984, 201-206.
Vlaminck L, Steenhaut M, Gasthuys F, Martens A, Desmets P, Vanden Abeele K, De Moor A: Omentopexy for correction of left displaced abomasum: outcome results in 53 cattle. Vlaams Dier Tijd. 1998, 67: 118-122.
Whitlock RH: Pathophysiology of lower gastrointestinal tract problems in the bovine. Proceedings of the Ninth Annual Convention of the American Association of Bovine Practitioners. 1976, 43-48.
Acknowledgements
The authors would like to thank: colleagues and staff at Riverview Veterinary Clinic for all their help and assistance; students Elena Nani, Torino, Lisa Ebner, Ingolstadt and Christiane Freytag, Leipzig; and the kind staff at the veterinary library, UCD.
Author information
Authors and Affiliations
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sexton, M., Buckley, W. & Ryan, E. A study of 54 cases of left displacement of the abomasum: February to July 2005. Ir Vet J 60, 605 (2007). https://doi.org/10.1186/2046-0481-60-10-605
Published:
DOI: https://doi.org/10.1186/2046-0481-60-10-605