Abstract
Among multiple genes aberrantly activated in cancers, invariably, there is a group related to the capacity of cell to self-renewal. Some of these genes are related to the normal process of development, including the establishment of a germline. This group, a part of growing family of Cancer/Testis (CT) genes, now includes the meiosis specific subunits of cohesin complex. The first reports characterizing the SMC1 and RAD21 genes, encoding subunits of cohesin, were published 20 years ago; however the exact molecular mechanics of cohesin molecular machine in vivo remains rather obscure notwithstanding ample elegant experiments. The matters are complicated by the fact that the evolution of cohesin function, which is served by just two basic types of protein complexes in budding yeast, took an explosive turn in Metazoa. The recent characterization of a new set of genes encoding cohesin subunits specific for meiosis in vertebrates adds several levels of complexity to the task of structure-function analysis of specific cohesin pathways, even more so in relation to their aberrant functionality in cancers. These three proteins, SMC1β, RAD21L and STAG3 are likely involved in a specific function in the first meiotic prophase, genetic recombination, and segregation of homologues. However, at present, it is rather challenging to pinpoint the molecular role of these proteins, particularly in synaptonemal complex or centromere function, due to the multiplicity of different cohesins in meiosis. The roles of these proteins in cancer cell physiology, upon their aberrant activation in tumors, also remain to be elucidated. Nevertheless, as the existence of Cancer/Testis cohesin complexes in tumor cells appears to be all but certain, this brings a promise of a new target for cancer therapy and/or diagnostics.
Similar content being viewed by others
Introduction
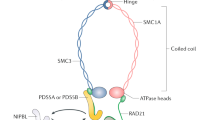
Cohesin is a protein complex that is essential for cell proliferation in all eukaryotic cells. Cohesin is the key activity that establishes sister chromatid cohesion (SCC) and then holds sister chromatids until the anaphase. The separation of sister chromatids in mitotic cell division requires the inactivation of SCC function by either proteolytic cleavage or stripping cohesin molecules from chromatin. The original (“mitotic” or “somatic”) cohesin complex is postulated to have the shape of a “ring” that is potentially able to physically embrace two chromatids [1]. All cohesin complexes are composed of four essential subunits. The chromatid-embracing core of the ring-like structure is formed by two SMC proteins, SMC1 and SMC3, which belong to the family of SMC (Structural Maintenance of Chromosomes) ATP-binding proteins [2]. SMC1 and SMC3 heterodimerize by joining via their hinge domains [3]. The “locking” of the ring is achieved via binding of two ATP molecules at the ATP-binding domains of SMC1 and SMC3 [4]. Such a locking of the ring is facilitated by the third subunit that is known as RAD21/SCC1/MCD1 in a variety of systems [5–7]. This very subunit is the target of proteolytic cleavage by the separase/separin coincidental with anaphase initiation [8–10]. The forth core cohesin subunit is a HEAT repeat protein known as SCC3 in yeast [11], and represented in vertebrates by two paralogs, SA1/STAG1 and SA2/STAG2 [12, 13]. The functionality of mitotic/somatic cohesin is well studied with respect to its involvement in SCC at the centromeres. The molecular role of cohesin at the chromosomal arms is more obscure, however it has been directly linked to DNA repair [14] and, albeit less conclusively, to many instances of gene expression regulation [15–18]. It is believed that cohesin, in yet to be uncovered fashion, facilitates the function of the multipurpose transcriptional regulator CTCF [19], at a subset of thousands of CTCF sites in the genomes of Metazoa where cohesin and CTCF colocalize [20, 21]. However, as CTCF itself is a multifunctional protein, delineating the molecular pathway linking cohesin to gene expression regulation has proven rather difficult. Furthermore, while the differential functions of SA1/STAG1 and SA2/STAG2 are not well defined, the most recent data indicate that SA1/STAG1, but not SA2/STAG2, is somehow linked to cohesin colocalization with CTCF [22].
With the exception of still unclear mechanistic links to non-SCC mediated processes, such as transcription, the biggest splash since the characterization of cohesin in the 1990s was made with the discovery of the meiotic function of cohesin. Indeed, upon entering meiosis I, in the preparation for the reductional division, a meiosis-specific subunit REC8 de facto replaces RAD21/SCC1/MCD1 and makes mei-cohesin to behave rather differently with respect to timing of SCC1 release. Namely, REC8 protects SCC throughout the process of homologous recombination and then maintains the association of homologues after recombination is complete, up to the point when chromosomes are ready to segregate. At this point, REC8-mediated cohesion is released along the arms, but persists at the centromeres to ensure proper sister chromatid orientation in meiosis II [23–25]. In yeast, REC8 is known to have some functions in addition to SCC per se, including chromosomal restructuring leading to recombination: assembly of axial elements, pairing and synapsis of homologues. Yeast REC8 can easily take the place of SCC1/MCD1 in the SMC1/SMC3 complex in vitro [26], however in vivo studies in mice show that there is little, if any, exchange of REC8 once it becomes chromatin-bound [27]. In general, in metazoan systems, the rebuilding of cohesin for meiosis is much more complex, evidently due to the evolutionary emergence of the sophisticated germline development process. In addition to REC8 [28], there is a meiosis-specific paralog of SMC1α, SMC1β [29], as well as the meiosis-specific SA3/STAG3 subunit [30].
Making the sense of the multitude of cohesin subunits is becoming objectively more and more difficult. Recently, three groups characterized the RAD21L (RAD21-like) protein, which is yet another meiosis-specific cohesin subunit [31–33]. RAD21L is expressed strictly in germline, i.e. in spermatocytes and oocytes. The presence of putative RAD21L complexes in the repertoire of meiotic cohesins significantly increases the level of potential complexity in the task of defining separate types of cohesin complexes in meiosis. A recent review on the subject estimates that there are 18 potential cohesin complexes (Figure 1) that could be present in cells, based on purely combinatorial considerations [34]. However, RAD21L cohesin complexes appear to come in only two forms in vivo: with SMC3 and STAG3 complexed with either SMC1α or SMC1β [31, 35, 36].
Cohesin complexes based on all possible combinations of known cohesin subunits. Meiosis-specific complexes are boxed in blue, and mitotic, which are also found in meiosis, are boxed in red. The complexes that were not validated biochemically are shown shaded. (*) Interaction of REC8 with SA/STAG subunits has not been studied exhaustively in mammalian systems.
Rad21L appears at chromosomes in germline lineage in a fashion coordinated with REC8, at the pre-meiotic S phase. It resides on chromosomes from the establishment of SCC, through pairing of homologues and the establishment of the synapsis. More specifically, RAD21L appears to colocalize with axial elements upon meiosis initiation. Then, when homologues synapse, RAD21L stays associated with the synaptonemal complex all the way to the end of pachytene, when it disappears from the still persisting synaptonemal complex, according to [31]. However, there is some disagreement on the exact time of RAD21L disappearance [35], which is not possible to resolve based on purely cytological data. It is quite possible that REC8 is actually trailing RAD21L in chromosome loading in leptotene, making RAD21L the chief interface between cohesin and the initiation of synapsis. Upon cohabitation with REC8 the two proteins appear to enrich chromosomes in an alternating pattern [32], although high-resolution chromatin mapping data is required to confirm that that pattern is truly mutually exclusive.
The release of RAD21L from synaptonemal complex also coincides with the emergence of MLH1 foci at crossover sites. Thus, after the genetic recombination is finished, RAD21L leaves chromosomes, parting its ways with REC8, which stays bound at the axes of recombined chromosomes. As a result, RAD21L is apparently absent or has only marginal presence on chromosomes when they commit to segregation in meiosis I. This prompted a speculation that the RAD21L-containing cohesin represents the first case of cohesin complex which, at large, is actually uninvolved with SCC per se. Such a conclusion is indirectly supported by the observation that some of RAD21L loading onto chromosomes, apparently via the replacement of RAD21, in late pachytene may be replication independent [31, 32], i.e. happens after SCC establishment. However, if a direct exchange of RAD21 to RAD21L exists in situ, the situation becomes more complex to decipher. In any case, it would be premature to draw conclusions on any non-SCC function of RAD21L cohesin based solely on antibody staining data, without detailed molecular analysis and in the absence of mapping the distinct cohesin complexes in meiotic chromatin with high resolution.
RAD21L cohesin complexes are involved in homologue pairing
The peculiar dynamics of chromosomal associations of RAD21L indicates that it may have a direct molecular role in homologue pairing. Alternatively, RAD21L and REC8 cohesin complexes may correspond to chromosomal regions marked by other epigenetic marks facilitating pairing. Such marks may also themselves predestine theses regions to either undergo recombination or to be refractory to it. Indeed, the pattern of alternation of Rad21L and REC8 on meiotic chromosomes creates an appearance of a “barcode” [31, 32]. The functionality of this “barcode” is awaiting the investigation. While pre-mitotic bar-coding remains a hypothesis, the direct role of RAD21L cohesin complex in homologue pairing is supported by cytological data. Namely, RAD21L, unlike other known cohesin subunits, does interact physically with SYCP1, a component of the synaptonemal complex. SYCP1 forms axial elements of the synaptonemal complex at the beginning of the pathway resulting in recognition and pairing of homologues [37].
While pairing of homologues is a feature of all eukaryotes with a meiotic cycle, RAD21L is present in vertebrates only. Unfortunately, however, little data on this protein is available in non-mammalian systems. In mammals, it is clear that at least some RAD21L cohesin complexes do colocalize as well as show signs of physical interaction with the proteins of axial elements, SYCP3 and SYCP2 [38, 39]. Mouse genetic data allow one to make the functional connection between cohesin subunits, axial elements’ structure, and genetic recombination itself. Single-gene knock-outs of Smc1 β, Rec8 and Rad21L show that spermatocytes arrest at the stage reminiscent of zygotene to pachytene transition, despite assembling axial elements and achieving a partial synapse between homologues [29, 33, 36, 40]. When both Rec8 and Rad21L are deleted in mice, spermatogenic cells get arrested at a stage corresponding to leptotene, with chromosomes showing no pairing, apparently as a result of axial elements dysfunction. This stage approximately corresponds to the stage IV among the twelve morphological phases of seminiferous epithelium progression towards spermiogenesis. Spermatocytes with such a double knockout (dKO) stay viable up to that stage but later disappear, apparently as a result of arrest-induced apoptosis [36]. Such a dramatic meiotic defect of dKO has an inevitable consequence of infertility, i.e. all dKO males are sterile, visibly lacking any post meiotic cells in their seminiferous tubules, and with three-fold reduction in total testis size (at the age 6–8 week). While this phenotype is quite severe, it appears to be largely additive of similar defects in Rec8-deleted and Rad21L-deleted mice [33, 36, 40], indicating that REC8 cohesin complexes and RAD21L-containing complexes are specialized rather strictly.
In Rec8-Rad21L dKO spermatocytes, SYCP3 and SYCP2 fail to localize to chromosomes and the formation of axial elements is impaired. A punctate SYCP3 and/or SYCP2 staining reveals the accumulation of aggregates, which are devoid of SYCP1 [36]. However, chromosomes in mouse Sycp1-Sycp3 dKO are still loaded with meiotic cohesin complexes, and the synapsis as well as recombination initiation both occur in such meiotic cells [41]. The sum of phenotypic analyses unequivocally indicates that either meiotic cohesin complexes are essential for the initiation of axial elements and the assembly of lateral elements or themselves are bona fide components of axial elements [36].
For understanding the underlying physiological abnormalities in dKO Rad21L and Rec8 males mice it is important to consider that spermatocytes are not only defective in axial elements assembly, but are also unable to initiate a specific recombination pathway mediated by RAD51. This defect is signified by up to ten-fold reduction in RAD51 foci numbers observed in spermatocytes [36]. RAD51 normally assembles on bivalents within axial elements and lateral elements in leptotene and disappears later from all chromatin that was synapsed [42]. However, the number of DMC foci was unaffected in leptonema of the dKO spermatocytes, as well as the DMC1-dependent repair of DSB, which dKO generated (likely by SPO11) with normal frequency, was largely unaffected. Despite that, some repair detected by gamma-H2AX staining appeared to persist in chromosomes of arrested dKO spermatocytes [36]. This indicates either that the normal meiotic DMC pathway is partially perturbed, or that DSB were generated by a non-physiological mechanism. Taken together, this dKO spermatocyte phenotype indicates that while physiological DSBs priming recombination events are induced with normal frequency and kinetics, RAD21L and REC8 complexes are evidently involved in cooperative non-redundant pathways leading to axial elements formation followed by the assembly of the synaptonemal complex [36]. A direct involvement of meiotic cohesin complexes in genetic recombination may have substantial implications for cancer cells that express these proteins.
Why another cohesin? Spermatogenesis vs oogenesis
KO of both Rad21L and Rec8 in mice should have left the somatic cohesin fully intact in meiocyte lineage. Early binding of RAD21 to chromatin is indicative of its involvement in the establishment of SCC in the S phase preceding the meiosis [36]. Indeed, the proper establishment of SCC in the premeiotic S-phase seems unperturbed, by cytological criteria, while RAD21 remains chromosome-bound from leptonema to diplonema (or until pachytene in [32]) in mouse spermatocytes [36]. These observations seemingly suggest that meiotic cohesin complexes are not required for SCC, at least not as much as for their other functions, i.e. synaptonemal complex formation. On the other hand, in a specialized meiotic system in marsupials, STAG3 (most likely as a part of the incorporating cohesin complex) is involved in segregating sex chromosomes that undergo no recombination. This is achieved via assembly of a unique structure called dense plate [43]. These two polar considerations, i.e. the apparent un-involvement in SCC in mice and the participation in meiotic segregation that does not involve any recombination, both indicate that meiotic cohesins occupy a rather specialized niche in meiosis.
The exact degree of meiotic specialization of the three types of cohesin complexes built around RAD21, RAD21L or REC8 remains to be fully uncovered, however such a task may involve more than just analyzing the meiotic prophase. Indeed, their chromatin binding properties and turnover not just vary in the first prophase; they also behave differently through metaphases of both the first and the second meiotic divisions. The latter includes a substantial enrichment of RAD21L at centromeres at the metaphase II [33]. RAD21L is also the first cohesin subunit with a strong sex-dependent phenotype resulting from its inactivation. This somewhat challenges the hypothesis that the corresponding cohesin has a universal, essential and direct role in homologue pairing. In the context of such a hypothesis, it is difficult to explain why RAD21L appears to be distinctly distributed in male and female germlines. Namely, in the metaphase of meiosis I in spermatocytes, RAD21L is apparently left at the centromeres, while in oocytes it is not detectable at metaphase chromosomes [32]. This difference is not likely to be purely technical. Instead, it may either reflect a deep biological difference or stem from the distinctly differential dynamics of meioses in male and female germlines [44].
Genetic data also strongly indicate that RAD21L function is distinct in male and female germlines. RAD21L cohesin complex may be sex-restricted in its function, even though other meiotic cohesin subunits that were mutated did not show such a severe dichotomy. For example, SMC1β depletion leads to sterility in both males and females. However, the SMC1β-devoid mouse spermatocytes undergo an arrest in pachytene, while oocytes only suffer precocious dissolution of SCC during meiotic metaphase II [45]. Upon REC8 inactivation, both sexes in mice show sterility correlating with the impaired synapsis of homologues and the defective formation of chiasmata [40, 46]. At the same time, germ cells of male Rad21L−/− mice, as was mentioned, cannot achieve the complete synapsis of homologous chromosomes during the first meiotic prophase. As a result of this defect, and possibly others yet to be uncovered, spermatocytes run into zygotene arrest leading to the complete absence of spermatozoa. In contrast, female mice lacking RAD21L are fertile, but develop sterility at the age of 6 months. The molecular basis of this aging process is yet to be understood, as the original report seemingly excluded a probable defect in SCC as the leading cause, and the synapsed bivalents in KO females apparently have wild type amount of STAG3 loaded [33]. Furthermore, it appears that this difference between the sexes is not dependent on the differential regulation of meiotic checkpoints in males and females, particularly in relation to dictyate. Rather, this difference could be a reflection of the fact that RAD21L is not involved in homologue segregation in mouse oogenesis [33]. Thus, for RAD21L, we have evidence of both the sex-limited specialization and the redundancy with REC8. The latter is possibly reflected in evolutionary data that strongly suggest that only mammals need both proteins simultaneously, as RAD21L is not present in amphibians, while REC8 is absent in birds [35].
In the absence of data from non-mammal vertebrates it is not possible to judge whether such differences really represent the sex-specific functional properties of meiotic cohesins themselves, or result from a differential chromatin organization in sexes of placental animals. The latter, could be due to potentially differential roles of the global transcriptional regulator and chromatin-structuring factor CTCFL/BORIS in mammalian spermatocytes and oocytes [47, 48]. Indeed, in adult mouse testis, the CTCF paralog BORIS/CTCFL is expressed, likely transiently, in late spermatogonia and in the pre-leptotene germ cells [49]. An answer to the question whether BORIS/CTCFL facilitates, even if partially, RAD21L localization in male meiosis, considering that RAD21 notably co-localizes with CTCF in soma, will likely linger until one generates high resolution chromatin maps for meiotic cohesin complexes and compare them to the recent chromatin immunoprecipitation maps of CTCF and CTCFL in the germline [49].
Cancer connection
The reference to CTCFL/BORIS brings about an important connection of some meiotic proteins to cancers. Indeed, germline-specific CTCFL/BORIS is aberrantly activated in many tumors and cancer cell lines [48, 50, 51], and its presence there was largely overlooked by the literature devoted to CTCF-cohesin colocalization in cancer cells of somatic origin. On the other hand, in the past several years it became evident that chromosome destabilization, as a result of mutation or epimutation, is likely one of the earliest steps in transforming a normal somatic cell into a cancer cell [52, 53]. Cohesin deregulation, in particular, may provide a rich potential source of such destabilizing changes, as the failure to resolve sister chromatid cohesion in mitosis universally results in chromosomal damage [54]. Indeed, both mutations of separase [55] and somatic cohesin subunits SMC1L1 and SMC3/CSPG6 themselves were correlated with tumors [56]. Tumor-borne mutations were also identified in the putative cohesin loader SCC2/NIPBL as well as in STAG3 [56]. COSMIC database of cancer genomic data contains 15 examples of mutations in RAD21L, some of them reoccurring, 83 mutations in SMC1β, and 88 in STAG3. While REC8 activation in somatic cells has not been reported, its differential methylation was linked to poor cancer prognoses in gastrointestinal stromal tumor [57]. Similarly to RAD21L and REC8, the expression of SMC1β and STAG3 are known to be transcriptionally silenced in somatic cells, and this repression involves a set of diverse mechanisms, such as methylation of histone H3 on both lysine 9 and lysine 27 [58]. Despite such safeguards preventing the transcription of meiotic cohesin subunits in soma, RAD21L and other meiotic cohesin proteins are known to be aberrantly activated in cancers and thus fall into a special group of factors named Cancer/Testis genes, and Antigens [59–61]. RAD21L, SMC1β and STAG3 subunits have drastically distinct patterns of activation/overexpression in cancers, however. STAG3 activation is found practically in every dataset in Oncomine (September 2012 release), including all types of cancers, with some of datasets showing over 50% of samples overexpressing STAG3 over 2-fold. On the contrary, SMC1β is only expressed (over 2 fold increase over baseline) in 3.4% of samples in the same Oncomine release (public datasets), mostly in invasive breast carcinomas, and RAD21L is only found in 5 samples using the same cutoff. This indicates that the potential misregulation of somatic cohesin by STAG3 is a common event in cancers, while the expression of full meiotic cohesin, to include SMC1β and RAD21L, appears to be rare. It must be noted, however, that the baseline in gene expression experiments in somatic cancers may have been too high to detect a biologically meaningful activation of SMC1b and RAD21L. Furthermore, when the experimental approach is specifically targeted at CT genes, the outcome could be quite different. For example in a recently published dataset of only 33 cancer cell lines, six were shown to express RAD21L, with two co-expressing SMC1b with RAD21L [62]. Thus, as cohesin functions are intimately intertwined with chromosome segregation mechanisms, meiotic subunits could potentially serve as the source of epimutations leading to chromosome instability in cancers, especially considering how many “unnatural” complexes could be formed (Figure 1).
Among the biological and mechanistic questions about the nature of meiotic cohesins’ involvement in chromosome recombination and segregation in the norm, three especially stand out with respect to cancer biology. First, it is important to study whether somatic cohesin’s properties in vivo are altered upon STAG3 binding, as STAG3 activation and overexpression is a common event in multiple cancers. Second, it would be useful to determine whether the SMC1β-SMC3-RAD21L-STAG3 complex is actually able to participate in SCC. Third, it would be important to know whether RAD21L is cleavable by separase. If meiotic cohesin is able to establish SCC in principle, as the experimental results seemingly suggest, then a cancer cell where these proteins are activated may have a problem in resolving SCC in anaphase, recapitulating a well-characterized defect in SCC resolution [54]. This could only happen, if the meiosis-specific regulation of cohesin removal or a hypothetical RAD21L cleavage is not in place in mitosis in such cancer cells. It is plausible however, that mitotic prophase cohesin removal pathway mediated by PLK1 could remove RAD21L complexes in cancer cells similarly to the corresponding pathway engaged in meiosis I [32]. Nevertheless, the absence of cleavage of residual RAD21L complexes, particularly at centromeres where they can be targeted based on meiotic data [33], may pose a significant hurdle for chromosome segregation. Experimental investigation of these questions is feasible. While the ectopic expression of individual components of meiotic cohesin in cell lines does not appear to cause dramatic defects, neither correct stoichiometry was modeled nor detailed phenotypic analysis was performed in these studies with respect to chromosomes [35]. There were also no reports on the ectopic co-expression of all three meiotic subunits, SMC1β, STAG3 and RAD21L, in somatic cells.
Cancer cells may, however, lack the regulatory machinery to fully engage subunits of meiotic cohesin complexes, either ectopically expressed or aberrantly activated. It was recently reported that SMC3, STAG3, and REC8, as well as interacting factors SYCP2, SYCP3, HORMAD1, and HORMAD2, are specifically phosphorylated in the prophase I [63]. In case of SMC3, for example, the form phosphorylated at an ATM/ATR consensus mostly localizes to disjoined homologues’ regions, prior to synapsis. Furthermore, this regulation is probably tied into the DSB formation pathway, as SPO11 is required for it to occur [63]. Thus the experimental testing of the functionality of aberrantly activated meiotic cohesin is essential for understanding its molecular role in cancer cells.
Notwithstanding only limited information on the functionality of meiotic cohesin subunits in tumor cells, it is unlikely that their activation has an adaptive benefit for cell proliferation. On the contrary, such events are probably detrimental for chromosome integrity and segregation fidelity. Therefore, it is possible that the activation of meiotic cohesin genes in soma is involved in the earlier stages of tumorigenesis, thus providing a set of more universal targets for potential therapy compared to adaptive late-onset tumor mutations. Cancer/Testis genes were indeed reported as promising targets for the immunotherapy of cancer in model systems and in humans [64–66]. This strategy benefits from the tight repression of corresponding genes in soma, which enables one to diminish or eliminate potential side effects of treatments targeting these proteins. The activation of meiotic cohesin subunits may also provide an attractive target to find synthetic treatments targeting their combinations with other common mutations in cancers, especially ones in DNA damage response pathways.
References
Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K: The cohesin ring concatenates sister DNA molecules. Nature 2008, 454: 297–301. 10.1038/nature07098
Strunnikov AV, Larionov VL, Koshland D: SMC1 : An essential yeast gene encoding a putative head-rod-tail protein is required for nuclear division and defines a new ubiquitous protein family. JCB 1993, 123: 1635–1648. 10.1083/jcb.123.6.1635
Hirano M, Hirano T: Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J 2002, 21: 5733–5744. 10.1093/emboj/cdf575
Hirano M, Hirano T: Opening closed arms: long-distance activation of SMC ATPase by hinge-DNA interactions. Mol Cell 2006, 21: 175–186. 10.1016/j.molcel.2005.11.026
Birkenbihl RP, Subramani S: Cloning and characterization of rad21 an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. NAR 1992, 20: 6605–6611. 10.1093/nar/20.24.6605
Guacci V, Koshland D, Strunnikov A: A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae . Cell 1997, 91: 47–57. 10.1016/S0092-8674(01)80008-8
Michaelis C, Ciosk R, Nasmyth K: Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 1997, 91: 35–45. 10.1016/S0092-8674(01)80007-6
Gruber S, Haering CH, Nasmyth K: Chromosomal cohesin forms a ring. Cell 2003, 112: 765–777. 10.1016/S0092-8674(03)00162-4
Nakamura T, Nagao K, Nakaseko Y, Yanagida M: Cut1/separase C-terminus affects spindle pole body positioning in interphase of fission yeast: pointed nuclear formation. Genes Cells 2002, 7: 1113–1124. 10.1046/j.1365-2443.2002.00586.x
Uhlmann F: Separase regulation during mitosis. Biochem Soc Symp 2003, 70: 243–251.
Toth A, Ciosk R, Uhlmann F, Galova M, Schleiffer A, Nasmyth K: Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev 1999, 13: 320–333. 10.1101/gad.13.3.320
Losada A, Yokochi T, Kobayashi R, Hirano T: Identification and characterization of SA/Scc3p subunits in the Xenopus and human cohesin complexes. J Cell Biol 2000, 150: 405–416. 10.1083/jcb.150.3.405
Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM: Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol 2000, 151: 749–762. 10.1083/jcb.151.4.749
Unal E, Heidinger-Pauli JM, Koshland D: DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7). Science 2007, 317: 245–248. 10.1126/science.1140637
Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, Itoh T, Watanabe Y, Shirahige K, Uhlmann F: Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 2004, 430: 573–578. 10.1038/nature02742
Lin S, Ferguson-Smith AC, Schultz RM, Bartolomei MS: Nonallelic transcriptional roles of CTCF and cohesins at imprinted loci. Mol Cell Biol 2011, 31: 3094–3104. 10.1128/MCB.01449-10
Nitzsche A, Paszkowski-Rogacz M, Matarese F, Janssen-Megens EM, Hubner NC, Schulz H, de Vries I, Ding L, Huebner N, Mann M, et al.: RAD21 cooperates with pluripotency transcription factors in the maintenance of embryonic stem cell identity. PLoS One 2011, 6: e19470. 10.1371/journal.pone.0019470
Renault NK, Renault MP, Copeland E, Howell RE, Greer WL: Familial skewed X-chromosome inactivation linked to a component of the cohesin complex, SA2. J Hum Genet 2011, 56: 390–397. 10.1038/jhg.2011.25
Lobanenkov VV, Nicolas RH, Adler VV, Paterson H, Klenova EM, Polotskaja AV, Goodwin GH: A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene 1990, 5: 1743–1753.
Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, et al.: Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 2008, 451: 796–801. 10.1038/nature06634
Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM: Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J 2008, 27: 654–666. 10.1038/emboj.2008.1
Remeseiro S, Cuadrado A, Gomez-Lopez G, Pisano DG, Losada A: A unique role of cohesin-SA1 in gene regulation and development. EMBO J 2012, 31: 2090–2102. 10.1038/emboj.2012.60
Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, Nasmyth K: A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 1999, 98: 91–103. 10.1016/S0092-8674(00)80609-1
Severson AF, Ling L, van Zuylen V, Meyer BJ: The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev 2009, 23: 1763–1778. 10.1101/gad.1808809
Kudo NR, Anger M, Peters AH, Stemmann O, Theussl HC, Helmhart W, Kudo H, Heyting C, Nasmyth K: Role of cleavage by separase of the Rec8 kleisin subunit of cohesin during mammalian meiosis I. J Cell Sci 2009, 122: 2686–2698. 10.1242/jcs.035287
Kaganskii AM, Strunnikov AV: A new protein complex (meicohesin) regulating cohesion of sister chromatids in meiosis in Saccharomyces cerevisiae. Dokl Biol Sci 2004, 394: 82–86.
Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K: Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 2010, 24: 2505–2516. 10.1101/gad.605910
Parisi S, McKay MJ, Molnar M, Thompson MA, van der Spek PJ, van Drunen-Schoenmaker E, Kanaar R, Lehmann E, Hoeijmakers JH, Kohli J: Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol Cell Biol 1999, 19: 3515–3528.
Novak I, Wang H, Revenkova E, Jessberger R, Scherthan H, Hoog C: Cohesin Smc1beta determines meiotic chromatin axis loop organization. J Cell Biol 2008, 180: 83–90. 10.1083/jcb.200706136
Prieto I, Suja JA, Pezzi N, Kremer L, Martinez AC, Rufas JS, Barbero JL: Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 2001, 3: 761–766. 10.1038/35087082
Lee J, Hirano T: RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol 2011, 192: 263–276. 10.1083/jcb.201008005
Ishiguro K, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y: A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep 2011, 12: 267–275. 10.1038/embor.2011.2
Herran Y, Gutierrez-Caballero C, Sanchez-Martin M, Hernandez T, Viera A, Barbero JL, de Alava E, de Rooij DG, Suja JA, Llano E, Pendas AM: The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 2011, 30: 3091–3105. 10.1038/emboj.2011.222
Jessberger R: Cohesin complexes get more complex: the novel kleisin RAD21L. Cell Cycle 2011, 10: 2053–2054. 10.4161/cc.10.13.15802
Gutierrez-Caballero C, Herran Y, Sanchez-Martin M, Suja JA, Barbero JL, Llano E, Pendas AM: Identification and molecular characterization of the mammalian alpha-kleisin RAD21L. Cell Cycle 2011, 10: 1477–1487. 10.4161/cc.10.9.15515
Llano E, Herran Y, Garcia-Tunon I, Gutierrez-Caballero C, de Alava E, Barbero JL, Schimenti J, de Rooij DG, Sanchez-Martin M, Pendas AM: Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol 2012, 197: 877–885. 10.1083/jcb.201201100
Qiao H, Chen JK, Reynolds A, Hoog C, Paddy M, Hunter N: Interplay between synaptonemal complex, homologous recombination, and centromeres during mammalian meiosis. PLoS Genet 2012, 8: e1002790. 10.1371/journal.pgen.1002790
Suja JA, Barbero JL: Cohesin complexes and sister chromatid cohesion in mammalian meiosis. Genome Dyn 2009, 5: 94–116.
Calvente A, Viera A, Parra MT, de la Fuente R, Suja JA, Page J, Santos JL, de la Vega CG, Barbero JL, Rufas JS: Dynamics of cohesin subunits in grasshopper meiotic divisions. Chromosoma 2013, 122: 77–79. 10.1007/s00412-012-0393-6
Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC: Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 2004, 40: 184–194. 10.1002/gene.20085
Kouznetsova A, Benavente R, Pastink A, Hoog C: Meiosis in mice without a synaptonemal complex. PLoS One 2011, 6: e28255. 10.1371/journal.pone.0028255
Suwaki N, Klare K, Tarsounas M: RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol 2011, 22: 898–905. 10.1016/j.semcdb.2011.07.019
Page J, Viera A, Parra MT, de la Fuente R, Suja JA, Prieto I, Barbero JL, Rufas JS, Berrios S, Fernandez-Donoso R: Involvement of synaptonemal complex proteins in sex chromosome segregation during marsupial male meiosis. PLoS Genet 2006, 2: e136. 10.1371/journal.pgen.0020136
Houmard B, Small C, Yang L, Naluai-Cecchini T, Cheng E, Hassold T, Griswold M: Global gene expression in the human fetal testis and ovary. Biol Reprod 2009, 81: 438–443. 10.1095/biolreprod.108.075747
Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R: Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 2004, 6: 555–562. 10.1038/ncb1135
Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ: Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 2005, 8: 949–961. 10.1016/j.devcel.2005.03.018
Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, Mannan P, Larsson E, Kanduri C, Vostrov AA, et al.: BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 2002, 99: 6806–6811. 10.1073/pnas.092123699
Pugacheva EM, Suzuki T, Pack SD, Kosaka-Suzuki N, Yoon J, Vostrov AA, Barsov E, Strunnikov AV, Morse HC 3rd, Loukinov D, Lobanenkov V: The structural complexity of the human BORIS gene in gametogenesis and cancer. PLoS One 2010, 5: e13872. 10.1371/journal.pone.0013872
Sleutels F, Soochit W, Bartkuhn M, Heath H, Dienstbach S, Bergmaier P, Franke V, Rosa-Garrido M, van de Nobelen S, Caesar L, et al.: The male germ cell gene regulator CTCFL is functionally different from CTCF and binds CTCF-like consensus sites in a nucleosome composition-dependent manner. Epigenetics Chromatin 2012, 5: 8. 10.1186/1756-8935-5-8
D’Arcy V, Pore N, Docquier F, Abdullaev ZK, Chernukhin I, Kita GX, Rai S, Smart M, Farrar D, Pack S, et al.: BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br J Cancer 2008, 98: 571–579. 10.1038/sj.bjc.6604181
Martin-Kleiner I: BORIS in human cancers – a review. Eur J Cancer 2012, 48: 929–935. 10.1016/j.ejca.2011.09.009
Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, et al.: Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011, 144: 27–40. 10.1016/j.cell.2010.11.055
Inaki K, Liu ET: Structural mutations in cancer: mechanistic and functional insights. Trends Genet 2012, 28: 550–559. 10.1016/j.tig.2012.07.002
Strunnikov AV: One-hit wonders of genomic instability. Cell Div 2010, 5: 15. 10.1186/1747-1028-5-15
Mukherjee M, Ge G, Zhang N, Huang E, Nakamura LV, Minor M, Fofanov V, Rao PH, Herron A, Pati D: Separase loss of function cooperates with the loss of p53 in the initiation and progression of T- and B-cell lymphoma, leukemia and aneuploidy in mice. PLoS One 2011, 6: e22167. 10.1371/journal.pone.0022167
Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, et al.: Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A 2008, 105: 3443–3448. 10.1073/pnas.0712384105
Okamoto Y, Sawaki A, Ito S, Nishida T, Takahashi T, Toyota M, Suzuki H, Shinomura Y, Takeuchi I, Shinjo K, et al.: Aberrant DNA methylation associated with aggressiveness of gastrointestinal stromal tumour. Gut 2012, 61: 392–401. 10.1136/gut.2011.241034
Storre J, Schafer A, Reichert N, Barbero JL, Hauser S, Eilers M, Gaubatz S: Silencing of the meiotic genes SMC1beta and STAG3 in somatic cells by E2F6. J Biol Chem 2005, 280: 41380–41386. 10.1074/jbc.M506797200
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ: Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005, 5: 615–625. 10.1038/nrc1669
Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, et al.: Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A 2008, 105: 20422–20427. 10.1073/pnas.0810777105
Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, Hoda SA, Simpson AJ, Old LJ, Caballero O, Neville AM: Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One 2011, 6: e17876. 10.1371/journal.pone.0017876
Feichtinger J, Aldeailej I, Anderson R, Almutairi M, Almatrafi A, Alsiwiehri N, Griffiths K, Stuart N, Wakeman JA, Larcombe L, McFarlane RJ: Meta-analysis of clinical data using human meiotic genes identifies a novel cohort of highly restricted cancer-specific marker genes. Oncotarget 2012, 3: 843–853.
Fukuda T, Pratto F, Schimenti JC, Turner JM, Camerini-Otero RD, Hoog C: Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet 2012, 8: e1002485. 10.1371/journal.pgen.1002485
Mkrtichyan M, Ghochikyan A, Davtyan H, Movsesyan N, Loukinov D, Lobanenkov V, Cribbs DH, Laust AK, Nelson EL, Agadjanyan MG: Cancer-testis antigen, BORIS based vaccine delivered by dendritic cells is extremely effective against a very aggressive and highly metastatic mouse mammary carcinoma. Cell Immunol 2011, 270: 188–197. 10.1016/j.cellimm.2011.05.007
Nishikawa H, Maeda Y, Ishida T, Gnjatic S, Sato E, Mori F, Sugiyama D, Ito A, Fukumori Y, Utsunomiya A, et al.: Cancer/testis antigens are novel targets of immunotherapy for adult T-cell leukemia/lymphoma. Blood 2012, 119: 3097–3104. 10.1182/blood-2011-09-379982
Tsuji K, Hamada T, Uenaka A, Wada H, Sato E, Isobe M, Asagoe K, Yamasaki O, Shiku H, Ritter G, et al.: Induction of immune response against NY-ESO-1 by CHP-NY-ESO-1 vaccination and immune regulation in a melanoma patient. Cancer immunology, immunotherapy: CII 2008, 57: 1429–1437. 10.1007/s00262-008-0478-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that he has no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Strunnikov, A. Cohesin complexes with a potential to link mammalian meiosis to cancer. Cell Regen 2, 4 (2013). https://doi.org/10.1186/2045-9769-2-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2045-9769-2-4