Abstract
Background
Congenital muscular dystrophy Type 1A (MDC1A) is a severe, recessive disease of childhood onset that is caused by mutations in the LAMA2 gene encoding laminin-α2. Studies with both mouse models and primary cultures of human MDC1A myogenic cells suggest that aberrant activation of cell death is a significant contributor to pathogenesis in laminin-α2-deficiency.
Methods
To overcome the limited population doublings of primary cultures, we generated immortalized, clonal lines of human MDC1A myogenic cells via overexpression of both CDK4 and the telomerase catalytic component (human telomerase reverse transcriptase (hTERT)).
Results
The immortalized MDC1A myogenic cells proliferated indefinitely when cultured at low density in high serum growth medium, but retained the capacity to form multinucleate myotubes and express muscle-specific proteins when switched to low serum medium. When cultured in the absence of laminin, myotubes formed from immortalized MDC1A myoblasts, but not those formed from immortalized healthy or disease control human myoblasts, showed significantly increased activation of caspase-3. This pattern of aberrant caspase-3 activation in the immortalized cultures was similar to that found previously in primary MDC1A cultures and laminin-α2-deficient mice.
Conclusions
Immortalized MDC1A myogenic cells provide a new resource for studies of pathogenetic mechanisms and for screening possible therapeutic approaches in laminin-α2-deficiency.
Similar content being viewed by others
Background
Congenital muscular dystrophy Type1A (MDC1A) is an autosomal recessive disease caused by mutations in the LAMA2 gene that encodes the extracellular protein laminin-α2 [1]. Mutations that result in complete loss of laminin-α2 function result in severe neuromuscular dysfunction, whereas mutations that result in partial loss of function are associated with less severe disease [2]. In skeletal muscles, laminin-α2 assembles with laminin-β1 and -γ1 to form laminin-211. Heterotrimeric laminins that include laminin-α2 have been termed merosins, and MDC1A has thus also been known as merosin-deficient congenital muscular dystrophy. Laminin-α2 has multiple binding partners in both the extracellular matrix and on the plasma membrane [3] so that loss of laminin-α2 is accompanied by both structural deficits and aberrant cell signaling.
Primary cultures of myogenic cells from human MDC1A patients have proven useful for analyzing molecular mechanisms of MDC1A pathogenesis in skeletal muscle. For example, myotubes formed in primary cultures of human MDC1A myoblasts in the absence of exogenous laminin show both a several-fold increase in caspase-3 activity and increased cell death compared to myotubes formed from healthy control myoblasts [4]. The increased caspase-3 activity in MDC1A myotubes in vitro appears to recapitulate the similarly increased caspase-3 activity seen in the skeletal muscles of laminin-α2-deficient mice and human MDC1A patients in vivo[5–9]. Thus, aberrant activation of caspase enzymatic activity is a cell autonomous property of laminin-α2-deficient myotubes. The aberrant caspase activation and cell death in muscle cells of MDC1A model systems is mediated by a BAX/KU70-dependent signaling pathway [4]. Importantly, inhibition of aberrant cell death in the skeletal muscles of laminin-α2-deficient mice leads to a significant amelioration of pathology, including a several-fold increase in lifespan and improved motor behavior [4, 10, 11], thereby demonstrating that aberrantly increased cell death is both a significant contributor to the overall pathology and a potential therapeutic target in human MDC1A.
The use of primary cultures of human MDC1A myogenic cells to analyze pathogenetic mechanisms has been constrained both by the small number of donors and by the limited replication capacity (typically approximately 50 to 60 population doublings) of human myogenic cells in primary culture. However, the replication limits of human myogenic cells can be overcome through forced expression of CDK4 and hTERT [12–14]. Using this technique, we now report the preparation and analysis of immortalized, clonal lines of human MDC1A myogenic cells. We found that the immortalized cells not only retained the capacity to differentiate into myotubes but also showed the aberrant activation of caspase activity as seen in primary cultures. This is the first report of immortalized human myogenic cells that recapitulate such a marked pathological change. Thus, these immortalized MDC1A myogenic cells can provide an essentially unlimited number of cells for study of MDC1A pathogenetic mechanisms, as well as for the identification and in vitro validation of therapeutic targets and strategies, including by high-throughput screening.
Methods
Immortalization and cell cloning
Immortalization of myoblasts and isolation of myogenic clones was performed as previously described [12–14]. In brief, mouse CDK4 and hTERT cDNAs were inserted into pBabe vectors containing neomycin- and hygromycin-resistance genes, respectively. LoxP sites were included in the hTERT vector to allow optional excision of the hTERT expression cassette by Cre recombinase. To produce retroviral vectors, these plasmids were transfected into the Phoenix ecotropic packaging cell and the virus-containing supernatant was used to infect the amphotropic packaging cell line PA317 [15] to obtain stable virus-producing cell lines after selection with 0.5 mg/mL G418 or hygromycin (EMD Biosciences, San Diego, CA, USA). Infections were done with 2 μg/mL polybrene (Sigma-Aldrich). Clonal colonies were grown from the immortalized population by limiting dilution culture, and clonally-related cells were analyzed for CD56 expression by flow cytometry and for fusion potential in differentiation medium. Several independent clonal lines were isolated from each immortalized population and expanded for further assays. Telomere length and telomerase activity were assayed as before [13, 16].
Human myogenic cells
Table 1 summarizes the human myogenic cells used in this study. All human cells were obtained from German or USA biobanks (Table 1 and described below). All cells were anonymized prior to receipt and no personal identifications were available to us. The cells had been produced prior to our study from muscle biopsies collected under protocols approved by the appropriate institution that included informed donor consent and approval to publish results in accordance with standards of the Helsinki Declaration [17, 18]. Because our studies were of human cells that were obtained from cell banks and for which personal identification data were not obtainable by us, the studies were classified as exempt from Human Studies review by the Boston University Institutional Review Board in accordance with USA Department of Health and Human Services policy (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.101, accessed November, 4, 2013).
Primary MDC1A myoblasts from two different patients, designated as strains 38/03 and 96/04, were provided by the Muscle Tissue Culture Collection (MTCC) at the University of Munich (http://www.baur-institut.de/forschung/muskelbank/, accessed November 4, 2013) and were obtained from 4-month-old and 8-month-old male donors, respectively. Each donor had a clinical diagnosis of MDC1A with absence of laminin-α2 [4]. As controls, we analyzed primary myoblasts of a healthy 36-year-old man (unpublished strain 2/08, provided by the MTCC), as well as myoblasts derived from a biceps biopsy of a healthy 60-year-old woman, termed 15Vbic [17, 18]. As a disease control, we analyzed myoblasts derived from a biceps biopsy of a 67-year-old man with facioscapulohumeral dystrophy (FSHD), termed 15Abic [16–18]. Primary 15Abic and 15Vbic cells were prepared by and obtained from the Sen. Paul D. Wellstone Cooperative Research Center for FSHD (http://www.umassmed.edu/wellstone/materials.aspx, accessed November 4, 2013) and immortalization of these 15Abic and 15Vbic cells was reported previously [16]. Due to the young age of the MDC1A donor, it was not possible to obtain control myoblasts from age-matched donors. After immortalization, each clonal line was given a new identifier consisting of the original name followed by ‘-ct’ (for CDK4 + hTERT) and a clone number. Thus, 38/03-ct4 was the fourth clonal line derived from CDK4/hTERT immortalized 38/03 cells. Requests for immortalized 38/03-ct4, 96/04-ct8, and 2/08-ct7 myoblasts (Table 1) should be addressed to Dr. Peter Schneiderat (Peter.Schneiderat@med.uni-muenchen.de); and requests for immortalized 15Abic and 15Vbic myoblasts should be addressed to the Director of the Wellstone FSHD Center (charles.emersonjr@umassmed.edu).
Cell culture
Cells were cultured on Lab-Tek Permanox chamber slides (Nalge Nunc, Rochester, NY, USA) coated with 40 μg/mL poly-D-Lysine or 1% gelatin. In some cases as noted, slides were coated at 2 μg/cm2 with human placental laminin (Sigma-Aldrich cat. #L6274). Proliferating myoblasts were cultured at subconfluence in a high serum growth medium and differentiation was induced as cells neared confluence by switching the cultures to a low serum differentiation medium as described [17, 18]. Where noted, Laminin-111 (Sigma-Aldrich cat. #L2020 or BD Bioscience cat. #354239) was added to the culture medium at 5 μM. Cells were cultured under 5% CO2 at ambient oxygen concentration (normoxia), except, in some cases as noted, when cells were cultured under a low oxygen atmosphere of 2% O2, 5% CO2, 93% N2 (hypoxia) in gas-tight chambers as described [19].
Caspase enzyme assays
Caspase enzymatic activity was measured in cell homogenates using either the CaspACE Colorimetric Caspase-3 Activity Assay (50 to 100 μg protein per assay; Promega, Madison, WI, USA) or the more sensitive luminescence-based Caspase-Glo 3/7 Assay System (5 μg protein per assay; Promega) according to the manufacturer’s instructions and with signal detection on a Safire2 or Infinite M1000 microplate reader (Tecan, Durham, NC, USA).
Antibodies and immunocytochemistry
Myosin heavy chain isoforms were detected with one of three antibodies: (1) mouse mAb F59 [20] used at 1:10 dilution of hybridoma supernatant; (2) mouse mAb F20 [21] (used at 1:10; Developmental Studies Hybridoma Bank, Iowa City, IA, USA), or (3) rabbit anti-MYH3 (Sigma-Aldrich, St. Louis, MO, USA). Desmin was detected with mouse mAb D1033 (Sigma-Aldrich) used at 1:100 for 1 h. Activated caspase-3 antibody was from Cell Signaling Technologies (Beverly, MA, USA; cat. #9661, used at 1:400); and KU70 antibody was from Novus Biologicals (Littleton, CO, USA; cat #NB100-1915, used at 1:300). Dr. Lydia Sorokin (University of Münster) generously provided the rat anti-laminin-α2 mAb 4H8-2 which reacts with an epitope within the L4b globular domain [22]. Cultures were fixed with 4% formaldehyde or 100% methanol. Primary antibody binding was visualized with appropriate species-specific secondary antibodies conjugated to either Alexa Fluor 488 or Alexa Fluor 594 (Life Technologies, Grand Island, NY, USA). Slides were imaged using a Nikon E800 microscope (Melville, NY, USA) with SPOT Software (version 4.1) and SPOT Insight camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Results and discussion
Using forced expression of CDK4 and hTERT followed by cell cloning, we first produced immortalized myogenic cell lines from primary human myoblasts obtained from MDC1A (38/03, 96/04), normal control (2/08, 15Vbic), and FSHD (15Abic) donors (Table 1 and Figure 1). The FSHD cells served as a disease control to determine if pathological changes were disease-specific or shared. Though primary myoblast cultures reached a replicative limit at approximately 50 to 60 cumulative population doublings, the immortalized cells proliferated indefinitely (not shown, compare to [13]). Cells that were CDK4 plus hTERT immortalized had higher telomerase enzymatic activity and maintained longer telomeres at higher population doublings than either primary cells or cells with CDK4 alone (Figure 1A). Culture under low oxygen conditions (2% O2, 5% CO2, 93% N2) did not significantly alter proliferation or differentiation of the immortalized normal, MDC1A, or FSHD lines compared to culture under normoxic conditions (not shown).
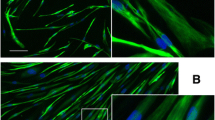
Characterization of immortalized compared to primary myogenic cells. (A) CDK4 + hTERT immortalized cells had higher telomerase enzymatic activity (left) and maintained longer telomeres (right) than primary cells or cells with CDK4 only. Results from 38/03 MDC1A cells (telomerase activity by telomeric repeat amplification protocol assay) and healthy control 2/08 cells (telomere length by hybridization assay) are shown as examples. The cervical carcinoma cell line HeLa served as a positive control. Lanes re-arranged for presentation. PD = population doublings. (B) 100% of CDK4 + hTERT immortalized cells expressed desmin (green), with MDC1A 96/04-ct8 cells shown as an example. (C) CDK4 + hTERT immortalized MDC1A myogenic cells showed normal differentiation by fusing into multinucleate cells that expressed myosin heavy chain (MyHC, red) isoforms. (D) Similar percentages of nuclei were incorporated into multinucleate (≥2 nuclei) myotubes formed from immortalized healthy control, MDC1A, and FSHD myoblasts. Error bars = SD, n = 5.
The clonal, immortalized myogenic cells were 100% positive for expression of the muscle-specific intermediate filament protein desmin (Figure 1B), whereas primary cultures were 70% to 95% desmin-positive (not shown), as was consistent with a small proportion of non-myogenic cells in the non-clonal primary cultures. Immortalized MDC1A, FSHD, and normal control myogenic cells all formed multinucleate myotubes when switched to low serum differentiation medium, and, as in primary cell cultures, the percentage of nuclei that were within multinucleate cells was similar for disease and control cultures (Figure 1C, D, and not shown). We also confirmed that myotubes formed from immortalized MDC1A myoblasts failed to express laminin-α2, whereas myotubes formed from immortalized control myoblasts did express laminin-α2 (Figure 2A, B), thus demonstrating that the laminin-α2-deficient phenotype was maintained in the immortalized MDC1A cultures.
Immortalized MDC1A myogenic cells did not express laminin-α2 and showed an altered distribution of KU70. (A) Laminin-α2 (red) appeared in a punctate pattern in multinucleate myotubes (arrows) formed from immortalized healthy control myoblasts. (B) As expected, no laminin-α2 was found in myotubes (arrows) formed from immortalized MDC1A myoblasts. (C) Immortalized myogenic cells from healthy control donors showed KU70 (green) both in the cytoplasm (arrows) and in nuclei. (D) KU70 (green) was restricted to nuclei of immortalized MDC1A myogenic cells.
We next examined whether immortalized MDC1A myogenic cells also showed the pathological changes that we had previously found in primary MDC1A cultures [4]. We first compared KU70 immunostaining patterns in cultures of immortalized control and MDC1A myogenic cells. We found that KU70 immunostaining was restricted to the nuclei of immortalized MDC1A myogenic cells, whereas both the cytoplasm and nuclei of immortalized normal control cells showed KU70 staining (Figure 2A, B). Because primary cultures of MDC1A myogenic cells also show decreased KU70 expression in the cytoplasm [4], it is clear that immortalization did not affect this aberrant property of MDC1A myogenic cells. KU70 is a multifunctional protein with roles in the nucleus, cytoplasm, and perhaps at the cell surface [23]. In the cytoplasm, KU70 normally binds to BAX, thereby inhibiting BAX activation and subsequent cell death [4, 24–26]. Loss of KU70 from the cytoplasm would promote BAX activation and cell death, which is consistent with the increased cell death phenotype in laminin-α2-deficient mouse muscles and MDC1A human muscles [5–9].
Our next step was to examine caspase-3 activation in cultures of immortalized MDC1A vs. immortalized normal and FSHD myogenic cells. Caspase-3 activation is associated with activation of the BAX-mediated pathway of cell death in MDC1A cell cultures [4]. Using immunohistochemistry with an antibody specific for the cleaved, enzymatically active form of caspase-3, we found positive immunostaining in approximately 1% to 3% of the differentiated, myosin heavy chain-positive (MyHC) cells in MDC1A cultures (Figure 3A to D). For example, in one survey of an MDC1A 38/03-ct4 culture after 4 days of differentiation on gelatin, we found caspase-3 immunostaining in 15 out of 1,084 (1.4%) of the MyHC-positive cells in the culture. The caspase-3 immunostaining in MDC1A cells appeared to often fill the cytoplasm and was sometimes also in nuclei as expected for ongoing cell death (Figure 3A to D). In some cases, the caspase-3-positive cells appeared to be undergoing degeneration as evidenced by fragmented and/or aggregated MyHC staining (Figure 3C, D). Furthermore, nuclei in caspase-3-positive cells were often irregularly shaped, condensed, or fragmented (Figure 3E), which are signs of incipient cell death. We did not see such caspase-3-positive cells with aberrant nuclei in differentiated cultures of immortalized normal or FSHD myogenic cells (not shown).
Caspase-3 activity was aberrantly activated in myotubes formed from immortalized MDC1A myoblasts. (A, B, C, D) Activated caspase-3 (green) was found in approximately 1% to 3% of the MyHC-expressing (red) differentiated cells in cultures of immortalized MDC1A cells. Two examples of 38/03-ct4 cultures are shown after 4 days in differentiation medium. (E) Arrows indicate nuclei with aberrant morphology that were in caspase-3-positive cells (caspase staining not shown). Nearby nuclei in caspase-3-negative cells showed normal morphology. Four fields are shown. (F) Quantification of caspase-3 enzymatic activity by two different assays showed that, after 4 days in differentiation medium, cultures of immortalized MDC1A cells (96/04-ct8 and 38/03-ct4) had significantly elevated levels of caspase-3 compared to cultures of immortalized healthy control cells (2/08-ct and 15Vbic-ct16) or immortalized FSHD (15Abic-ct24) cells. **P <001 (t-test, ‘n’ as shown).
The finding that only a small fraction of the differentiated, myosin-expressing cells were positive for caspase-3 at any one time suggests that onset of cell death was asynchronous in the differentiating MDC1A cultures. Caspase-3-positive cells typically have a short half-life (<5 h); eventually detach from the culture dish [4]; and could possibly be replaced by remaining undifferentiated myoblasts in the cultures. The mechanism(s) that underlie the progression of cells from a state in which there are limited signs of pathology (for example, KU70 reduced in the cytoplasm) to a state with high level activation of caspase-3 followed by cell death remain to be determined in future work.
Finally, to confirm the immunocytochemistry results, we measured caspase-3 enzymatic activity in differentiated cultures of MDC1A vs. normal and FSHD myogenic cells. After 4 days of differentiation, cultures of MDC1A cells had significantly more caspase-3 enzymatic activity than did cultures of normal control or FSHD cells (Figure 3F). This approximate four- to six-fold increase in caspase-3 activity in immortalized MDC1A lines was similar to the increase we saw previously in primary MDC1A vs. primary normal cultures [4]. We found similar results with two different immortalized MDC1A lines (38/03-ct4 and 96/04-ct8) and with two different caspase-3 enzymatic activity assays. Culture under low oxygen did not alter the extent of caspase-3 activation (not shown). The increased caspase-3 activation in the MDC1A cultures was at least partially laminin-dependent, as growth on human placental laminin (which includes laminin-211) or in the presence of mouse laminin-111 was sufficient to prevent approximately 30% to 50% of the aberrant increase in caspase-3 (not shown), a result similar to that we found previously in primary MDC1A cultures [4].
In summary, we have immortalized laminin-α2-deficient MDC1A myogenic cells and shown that the immortalized cells not only retain the capacity for differentiation, but also recapitulate cell autonomous pathological changes that have been reported in primary MDC1A myogenic cell cultures, in laminin-α2-deficient mouse muscles, and in human MDC1A muscles [4–9]. Among these changes were a reduction in the amount of KU70 in the cytoplasm and aberrant activation of caspase-3 with associated abnormalities of nuclear morphology. The immortalized MDC1A myogenic cells should provide an essentially unlimited source of laminin-α2-deficient cells for future studies. In particular, these cells will be valuable for studies of myogenic cell-autonomous mechanisms in MDC1A pathology, including, for example, aberrant induction of cell death and increased autophagy [4, 27]. Combining results from studies of the human MDC1A myogenic cells with results from studies of laminin-α2-deficient mice should be particularly useful for further analyses of disease mechanisms. Pathological changes that arise due to interactions of human MDC1A myogenic cells with motor nerve, vascular, inflammatory, or connective tissue cells could possibly be studied in co-cultures. Xenotransplant models might also be useful if the immortalized MDC1A myogenic cells can form a significant number of innervated myofibers after transplant into immunodeficient mice [14]. Finally, the immortalized MDC1A cells and the pathological changes in these cells that we have identified should be useful for developing cell-based screening assays, including high-throughput screening, as part of pre-clinical studies to identify therapeutic interventions that reverse MDC1A pathology.
Conclusions
Immortalized myogenic cells from laminin-α2-deficient MDC1A patients recapitulate aspects of MDC1A pathology including aberrant induction of caspase-3 and KU70 relocalization. The immortalized MDC1A cells provide a new resource for studies of pathogenetic mechanisms and for screening possible therapeutic approaches in laminin-α2-deficiency.
References
Gawlik KI, Durbeej M: Skeletal muscle laminin and MDC1A: pathogenesis and treatment strategies. Skelet Muscle 2011, 1: 9. 10.1186/2044-5040-1-9
Geranmayeh F, Clement E, Feng LH, Sewry C, Pagan J, Mein R, Abbs S, Brueton L, Childs AM, Jungbluth H, De Goede CG, Lynch B, Lin JP, Chow G, Sousa C, O’Mahony O, Majumdar A, Straub V, Bushby K, Muntoni F: Genotype-phenotype correlation in a large population of muscular dystrophy patients with LAMA2 mutations. Neuromuscul Disord 2010, 20: 241-250. 10.1016/j.nmd.2010.02.001
Yurchenco PD, Patton BL: Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 2009, 15: 1277-1294. 10.2174/138161209787846766
Vishnudas V, Miller JB: Interaction of Ku70 with Bax regulates pathogenesis in laminin-alpha2-deficient models of congenital muscular dystrophy. Hum Mol Genet 2009, 18: 4467-4477. 10.1093/hmg/ddp399
Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S: Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett 1997, 415: 33-39. 10.1016/S0014-5793(97)01007-7
Mukasa T, Momoi T, Momoi MY: Activation of caspase-3 apoptotic pathways in skeletal muscle fibers in laminin alpha2-deficient mice. Biochem Biophys Res Commun 1999, 260: 139-142. 10.1006/bbrc.1999.0829
Hayashi YK, Tezak Z, Momoi T, Nonaka I, Garcia CA, Hoffman EP, Arahata K: Massive muscle cell degeneration in the early stage of merosin-deficient congenital muscular dystrophy. Neuromuscul Disord 2001, 11: 350-359. 10.1016/S0960-8966(00)00203-0
Girgenrath M, Dominov JA, Kostek CA, Miller JB: Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J Clin Invest 2004, 114: 1635-1639.
Girgenrath M, Beermann ML, Vishnudas VK, Homma S, Miller JB: Pathology is alleviated by doxycycline in a laminin-alpha2-deficient mouse model of congenital muscular dystrophy. Ann Neurol 2008, 65: 47-56. 10.1002/ana.21523
Dominov JA, Kravetz AJ, Ardelt M, Kostek CA, Beermann ML, Miller JB: Muscle-specific BCL2 expression ameliorates muscle disease in laminin-alpha2-deficient, but not dystrophin-deficient, mice. Hum Mol Gen 2005, 14: 1029-1040. 10.1093/hmg/ddi095
Meinen S, Lin S, Thurnherr R, Erb M, Meier T, Rüegg MA: Apoptosis inhibitors and mini-agrin have additive benefits in congenital muscular dystrophy mice. EMBO Mol Med 2011, 3: 465-479. 10.1002/emmm.201100151
Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, Di Santo JP, Butler-Browne GS, Wright WE: Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 2007, 6: 515-523. 10.1111/j.1474-9726.2007.00306.x
Stadler G, Chen JC, Wagner K, Robin JD, Shay JW, Emerson CP, Wright WE: Establishment of clonal myogenic cell lines from severely affected dystrophic muscles - CDK4 maintains the myogenic population. Skelet Muscle 2011, 1: 12. 10.1186/2044-5040-1-12
Mamchaoui K, Trollet C, Bigot A, Negroni E, Chaouch S, Wolff A, Kandalla PK, Marie S, Di Santo J, St Guily JL, Muntoni F, Kim J, Philippi S, Spuler S, Levy N, Blumen SC, Voit T, Wright WE, Aamiri A, Butler-Browne G, Mouly V: Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skelet Muscle 2011, 1: 34. 10.1186/2044-5040-1-34
Miller AD, Buttimore C: Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol 1986, 6: 2895-2902.
Stadler G, Rahimov F, King OD, Chen JC, Robin JD, Wagner KR, Shay JW, Emerson CP, Wright WE: Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat Struct Mol Biol 2013, 20: 671-678. 10.1038/nsmb.2571
Homma S, Chen JCJ, Rahimov F, Beermann ML, Hanger K, Bibat GM, Wagner KR, Kunkel LM, Emerson CP, Miller JB: A unique library of myogenic cells from facioscapulohumeral muscular dystrophy subjects and unaffected relatives: Family, disease, & cell function. Eur J Hum Genet 2011, 20: 404-410.
Jones TI, Chen JC, Rahimov F, Homma S, Arashiro P, Beermann ML, King OD, Miller JB, Kunkel LM, Emerson CP, Wagner KR, Jones PL: Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: evidence for disease modifiers and a quantitative model of pathogenesis. Hum Mol Genet 2012, 21: 4419-4430. 10.1093/hmg/dds284
Wright WE, Shay JW: Inexpensive low-oxygen incubators. Nat Protoc 2006, 1: 2088-2090. 10.1038/nprot.2006.374
Miller JB, Crow MT, Stockdale FE: Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J Cell Biol 1985, 101: 1643-1650. 10.1083/jcb.101.5.1643
Bader D, Masaki T, Fischman DA: Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol 1982, 1982: 763-770.
Schuler F, Sorokin LM: Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci 1995, 108: 3795-3805.
Muller C, Paupert J, Monferran S, Salles B: The double life of the Ku protein: facing the DNA breaks and the extracellular environment. Cell Cycle 2005, 4: 438-441. 10.4161/cc.4.3.1565
Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP: SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells fromstress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 2008, 28: 6384-6401. 10.1128/MCB.00426-08
Amsel AD, Rathaus M, Kronman N, Cohen HY: Regulation of the proapoptotic factor Bax by Ku70-dependent deubiquitylation. Proc Natl Acad Sci U S A 2008, 105: 5117-51122. 10.1073/pnas.0706700105
Li Y, Yokota T, Gama V, Yoshida T, Gomez JA, Ishikawa K, Sasaguri H, Cohen HY, Sinclair DA, Mizusawa H, Matsuyama S: Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ 2007, 14: 2058-2067. 10.1038/sj.cdd.4402219
Carmignac V, Svensson M, Körner Z, Elowsson L, Matsumura C, Gawlik KI, Allamand V, Durbeej M: Autophagy is increased in laminin α2 chain-deficient muscle and its inhibition improves muscle morphology in a mouse model of MDC1A. Hum Mol Genet 2011, 20: 4891-4902. 10.1093/hmg/ddr427
Acknowledgements
We thank biopsy donors for their generosity; Dr. Sachiko Homma (Boston University School of Medicine) for much helpful advice; Ms. Katherine Bankert for technical assistance with initial experiments; and Dr. Lydia Sorokin (University of Münster) for the laminin-α2 mAb. We also thank Dr. Kathryn Wagner and Dr. Genila Bibat (Kennedy-Krieger Institute and Johns Hopkins School of Medicine) who obtained 15Abic and 15Vbic biopsies as described [17]; and Dr. Jennifer CJ Chen and Ms. Kendal Hanger (University of Massachusetts Medical School) who prepared the primary myogenic cell cultures from these biopsies as described [17].
Funding
Supported by grants from the NIH (R01AR060328 to JBM; R01AR062587 to Peter L. Jones, subcontract to JBM); the Muscular Dystrophy Association (#216422 to JBM); and the Boston University Undergraduate Research Opportunity Program to EVS. Work with FSHD cells was performed within the framework of the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center for FSHD, supported by NIH grant 5U54HD060848. The Muscle Tissue Culture Collection (MTCC) at the University of Munich is part of the German network on muscular dystrophies (MD-NET, service structure S1, 01GM0601) funded by the German Ministry of Education and Research (BMBF, Bonn, Germany). MTCC is a partner of Eurobiobank (http://www.eurobiobank.org) and TREAT-NMD (http://www.treat-nmd.eu). Funders had no role in design or implementation of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GS and WEW developed the immortalization method, immortalized and cloned the normal, MDC1A, and FSHD lines, and assayed telomerase activity and telomere length (Figure 3A). SY, MLB, EVS, and JAW carried out the remaining experimental studies (Figures 1B-D, 2, and 3, and data in text) which were planned with JBM. PS provided primary MDC1A and control myogenic cells and biobanking services. JBM wrote the manuscript. All authors participated in editing the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yoon, S., Stadler, G., Beermann, M.L. et al. Immortalized myogenic cells from congenital muscular dystrophy type1A patients recapitulate aberrant caspase activation in pathogenesis: a new tool for MDC1A research. Skeletal Muscle 3, 28 (2013). https://doi.org/10.1186/2044-5040-3-28
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2044-5040-3-28