Abstract
Background
Although it has been shown that acute beta-blocker administration may reduce the presence or severity of myocardial perfusion defects with dipyridamole stress, little information is available about the potential effect of chronic beta-blocker treatment on the sensitivity of dipyridamole myocardial perfusion imaging (DMPI).
Methods
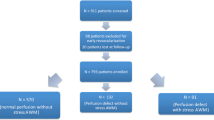
As a randomized clinical trial, one hundred twenty patients (103 male and 17 female) with angiographically confirmed CAD who were on long-term beta blocker therapy (≥3 months) enrolled in a randomized clinical trial study. The patients were allocated into two groups: Group A (n=60) in whom the beta-blocker agent was discontinued for 72h before DMPI and Group B (n=60) without discontinuation of beta-blockers prior to DMPI.
Results
No significant difference was noted between the groups concerning age, sex, type of the injected radiotracer and number of involved coronary vessels. The mean rank of total perfusion scores for whole myocardium (irrespective of reversibility or irreversibility) in group B was not significantly different from that of group A, (65.75 vs. 55.25, P=0.096). Regarding the only irreversible perfusion defects, the mean rank of perfusion score in group B was higher than that of group A for whole myocardium (72 vs. 49, P=0.0001); however, no difference was noted between two groups for only reversible perfusion defects (61.0 vs. 60.0, P=0.898). The overall sensitivity of DMPI for the diagnosis of CAD in group A (91.7%) was not statistically different from group B (90%).
Conclusion
Beta-blocker withholding before DMPI did not generally affect the sensitivity of the test for the diagnostic purposes in our study. Thus, beta-blocker withdrawal for just the purpose of diagnostic imaging is not mandatory particularly when medication discontinuation may cause the patients to face increased risk of heart events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scintigraphic myocardial perfusion imaging has been established as one of the most frequently used diagnostic tools in noninvasive assessment of the likelihood of coronary artery disease (CAD) [1–3]. Infusion of pharmacological vasodilators, including dipyridamole and adenosine or exercise treadmill test (ETT) are the main protocols of cardiac stressing in these imaging interventions, although less frequently other methods are also applied [4–6].
When ETT is selected as the stress method, discontinuation of agents affecting heart rate, most importantly beta-blockers and calcium channel blockers are usually advised, the underlying explanation of which is to allow heart rate to reach the age-predicted value [7]. However, there is some debate on the necessity of discontinuation of these agents for those patients undergoing pharmacological stress.
Some previous studies suggest that acute beta-blocker administration may reduce the presence and severity of myocardial perfusion defects with dipyridamole stress [8–17]. Nevertheless, most of such studies have been performed with short-term or acute beta-blocker treatment, some after intravenous administration, rather than after long-term oral beta-blocker treatment so that their methods differ from the usual clinical scenario encountered in many patients referring for dipyridamole myocardial perfusion imaging (DMPI) [8–17]. Few studies suggested that coronary flow reserve measured by means of positron emission tomography (PET) is improved in stenosis-dependent segments of the myocardium during long-term beta-blocker treatment, thereby b-blockers may decrease the contrast between ischemic and non-ischemic myocardium during hyperemia induced by dipyridamole [18]. However, they used metoprolol as a selective beta-1 receptor blocker and carvedilol as a non-selective beta blocker/alpha-1 blocker and thus this effect may not be generalized to other nonselective beta-blockers such as propranolol. To our knowledge, no clinical trial has been performed so far on the basis of DMPI with single-photon emission tomography (SPET) with random continuation or discontinuation of long-term beta-blocker medication, to study the effect of discontinuing chronic beta-blockade on the sensitivity of DMPI.
The current study was designed to evaluate the effect of discontinuing vs. continuing beta-blocker drugs on DMPI in patients who were on long-term treatment with these drugs.
Methods
Study population
The study was approved by the committee on ethics of Tehran University of Medical Sciences. All patients gave written informed consent before entering the study.
One hundred twenty patients (103 male and 17 female) with angiographically confirmed CAD (i.e. more than 50% diameter stenosis in at least one coronary artery or major branches), who were on long-term treatment (≥3 months) with therapeutic dose of a beta blocker, enrolled in a randomized clinical trial. Patients with a past history of asthma, second degree type 2 or third degree atrio-ventricualr block, left ventricular ejection fraction less than 50%, previous angioplasty and/or coronary artery bypass graft were excluded from the study.
The patients were allocated into two groups, using permuted block randomization method [19]: Group A (n=60) in whom the beta-blocker agent was discontinued for 72 h before DMPI (i.e. for more than 5 drug half-lives for all beta-blockers used in the study) and Group B (n=60) without discontinuation of beta-blockers prior to DMPI. After randomization of patients, the average number of stenosed arteries in group A was 2.0 and in group B was 2.2 with no significant difference between groups (P=0.180). No patient with left main coronary artery disease was included in each group of the study. The similarity of the two groups related to the age, gender, dose of beta-blockers before randomization, severity, extent and significance of CAD is shown in Table 1.
SPET imaging
Patients were instructed to fast for at least 4h before stress phase imaging. Caffeine, theophylline or aminophylline were discontinued for 48 h before the pharmacological stress. For stress-phase imaging, a dose of 0.56 mg/kg dipyridamole was infused intravenously (i.v.) over a 4 min period. Radiotracer was injected i.v., 3-5 min after the completion of dipyridamole infusion. Post-stress imaging was performed following the injection of 925MBq technetium-99m methoxyisobutyl isonitrile (99mTc-MIBI) or 111MBq thallium-201 (201Tl) after dipyridamole infusion. Half an hour after injection of 99mTc-MIBI, the patients were encouraged to eat a fat-rich snack to accelerate hepatobiliary excretion of the radiotracer. Cardiac SPET acquisition was performed 0.5 h after the fatty meal or one hour after i.v. injection of the radiopharmaceutical. For 201Tl SPET, image acquisition was started 10-15 min after the radiotracer injection.
In the case of imaging with 99mTc-MIBI, the rest phase of the study was performed in the following day using injection of the same dose of 99mTc-MIBI at rest, while for 201Tl SPET myocardial perfusion imaging, it was done in the same day using re-injection of 37MBq 201Tl.
For 99mTc-MIBI SPET, image acquisitions were done using a rotating, dual head gamma camera (Solus, ADAC, Milpitas, CA) equipped with a low-energy high-resolution parallel-hole collimator. Patients were in a supine position during the SPET acquisition. Thirty-two azimuth images, 60s/projection, were obtained in a 180° circular orbit, beginning from 45° right anterior oblique to 135° left posterior oblique with step and shoot acquisition on a 128×128×16 matrix and 38.5 cm detector mask. For 201Tl SPET, all imaging parameters were similar except for using low-energy all-purpose collimator and 64×64×16 matrix. Filtered back-projected data was reconstructed into short-axis, vertical long-axis and horizontal long-axis slices.
Images were interpreted by three expert nuclear medicine physicians blinded to the patients’ clinical data and final decision was reached by consensus. For this purpose, the entire left ventricular myocardium was divided into 20 segments according to a predetermined 20-segment model (Figure 1). At first, the images were qualitatively interpreted as positive or negative for the presence of any perfusion defect in more than one segment throughout the myocardium. Thereafter, visual semiquantitative analysis was performed considering a variety of artefactual findings that can be identified only by visual assessment. The perfusion status in each segment was classified as normal, reversible or irreversible defect by visual comparison of the intensity of myocardial color-coded scintillation on the stress and rest images. The total reversible and irreversible perfusion scores for each patient were calculated separately based on the number of segments with any degree of reversible or irreversible perfusion defects, respectively.
Statistical analysis
Considering the angiographic results as the gold standard, the sensitivity of DMPI for the detection of CAD in each group was calculated based on 95% confidence interval (95%CI). The combined data from the perfusion scores of all patients in both groups were sorted and ranked from 1, assigned to the lowest score, to 120 intended to the highest score. The average rank in group A was compared to group B using Mann–Whitney-U-test. In this way, the number of times that a score from group A precedes a score from group B and the number of times that a score from group B precedes a score from group A were analyzed. SPSS for Windows (Release 11.5.0) was used for statistical analysis. The significance level was 0.05.
Results
No significant difference was noted between the groups concerning age, sex, type of the radiotracer injected, average dose of beta-blockers being administered orally before randomization, number of involved coronary vessels and the frequency of left anterior descending artery (LAD) stenosis (Table 1).
The mean rank of total perfusion scores for whole myocardium, irrespective of reversibility or irreversibility, in group B (65.75) was not significantly different from that of group A (55.25, P=0.096) (Figure 2). Regarding the only irreversible perfusion defects, the mean rank of perfusion score in group B was substantially higher than that of group A for whole myocardium (72 vs. 49, P=0.0001), (Figure 2); however, no difference was noted between group A and B for only reversible perfusion defects (60.0 vs. 61.0, respectively, P=0.898).
Comparison of the mean rank of perfusion score for total, reversible and irreversible segments between patients discontinued beta-blocker (Group A) and those on beta-blockade (Group B) during dipyridamole myocardial perfusion imaging. (The mean rank of total perfusion scores for whole myocardium, irrespective of reversibility or irreversibility, in group B (65.75) was not significantly different from that of group A (55.25, P=0.096). Regarding the only irreversible perfusion defects, the mean rank of perfusion score in group B was substantially higher than that of group A for whole myocardium (72 vs. 49, P=0.0001); however, no difference was noted between group A and B for only reversible perfusion defects (60.0 vs. 61.0, respectively, P=0.898)).
The overall sensitivity of DMPI for the diagnosis of CAD in group A was 91.7% (95%CI: 83.1%-98.7%) and in group B was 90% (95%CI: 82.2%-97.8%). The difference was not statistically significant (P=0.945).
Additional analysis was performed based on the type of beta-blockers. For this purpose, the patients in groups A and B were divided into two subgroups. The first subgroup included the patients who were taking selective β1 receptor antagonists (i.e. metoprolol and atenolol) and the second subgroup was composed of those who were on nonselective β1/β2 receptor antagonists (i.e. propranolol and carvedilol) before entering the study. The sensitivity of DMPI for the diagnosis of CAD did not differ between groups, A and B, even across separate subgroups of patients based on the type of beta-blocker, gender and age (Table 2).
Discussion
Based on our study findings, the diagnostic sensitivity of DMPI is not significantly affected by the chronic consumption of beta-blockers. There is a common and overlapping sub-cellular and molecular pathways of action between dipyridamole and beta-blockers. Dipyridamole increases the endogenous concentration of adenosine, the underlying mechanism of which is inhibition of adenosine deaminase (ADA) enzyme. Adenosine in turn binds to A2 receptors and subsequently enhances the activity of its corresponding G-proteins. The cascade will eventually lead to increase in cellular concentration of cAMP, an event which leads to vasodilatation [20–24]. In atherosclerotic coronaries, vasodilatory response is restricted leading to the shift of blood to normal coronaries (steal phenomenon). This event is the rationale for using dipyridamole infusion to unveil possible myocardial ischemia [25–30]. On the other hand, beta adrenergic receptors bind to the same stimulatory G-proteins [31, 32]. Therefore, theoretically it can be judged that inhibitors of such receptors may decrease activation of the corresponding G-proteins, which eventually lead to decreased cellular concentration of cAMP and reduced response to dipyridamole infusion [17, 26, 33]. Based on these facts, others concluded that “much of the coronary vasodilation associated with hypoxia is dependent on adrenergic activation and that adenosine may only play a role in sustained hypoxic vasodilation when adrenergic receptors are intact” [33]. Such a probable effect practically could result in reduced sensitivity of DMPI. This unpleasant outcome was supposed to be easily avoided with discontinuation of beta-blockers before stress protocol of dipyridamole infusion.
On the other hand, dipyridamole infusion causes an increase in rate-pressure product, which can be considered as one of the main explanations for dipyridamole-induced steal phenomenon. The masking effect of beta-blockade on such an index has been considered as another reason for decreased sensitivity of DMPI [34].
These hypotheses were supported with findings of some reports, which showed decreased sensitivity of DMPI in those patients with history of calcium channel blocker or beta-blocker treatment [34–39], although most of them were retrospective and suffered from limitations such as low sample size, lack of gold standard, lack of individual and separate evaluation of beta-blocker effect rather than combined effect of multiple anti-anginal drugs [36] and acute versus chronic beta blockade [9]. Furthermore, controversies exist so far, as our study and also some of the other reports demonstrate opposite findings [14, 40–42].
According to the previous assumptions, beta-blocker withdrawal in patients with long-term beta-blockade is expected to result in restoring adrenergic activation and consequently better dipyridamole effect [33], rising the heterogeneity of regional perfusion between stress and rest phases, leading to better detection of reversible defects that might have been overlooked in case of ongoing beta-blocker treatment. Conversely, in our study, enduring beta-blocker treatment in the time of DMPI was associated with the detection of more irreversible perfusion defects, but its effect on the extent of reversible perfusion abnormalities were not established. This means that beta-blocker treatment may induce a reduction in dipyridamole-phase perfusion similar to the reduction in resting perfusion in the areas of stenotic coronary arteries. The effect of beta-blocker treatment with metoprolol on the coronary flow rate of patients with severe CAD was assessed in another study in which the patients underwent dipyridamole NH3-PET imaging while randomly on and off beta-blocker medications [10]. In these patients, both resting and hyperemic blood flow in either stenotic or normal myocardial segments were decreased by beta-blocker treatment, in accordance with the finding of the present study [10]. There are two possible explanations for these results. Adrenergic blockade may blunt the adenosine effect and coronary vasodilatation during ischemia due to a direct interference with the adenosine receptor or adenosine concentration [10], resulting in less contrast between resting and post-dipyridamole images of the ischemic areas, possibly causing overestimation of the fixed defects. Another possible mechanism is that the systolic pressure tends to be decreased by beta-blockade during dipyridamole stress; the driving pressure might be reduced and thereby may reduce the myocardial perfusion in stenotic areas during dipyridamole- and rest-phase imaging [10]. Thus, beta-blocker withdrawal may represent more accurate estimation of the extent of fixed versus reversible defects.
On the other hand, the effect of continuing beta-blocker treatment on the heterogeneity of regional myocardial perfusion had no significant impact on the sensitivity of the DMPI in our study. As a result, regarding the acknowledged risks of discontinuation of anti-anginal medications before any diagnostic testing, on-medication imaging protocol may be considered adequate to reveal the presence of underlying CAD. Nevertheless, there are some inaccuracies in the estimation of myocardial defect size caused by beta-blocker treatment, e.g. overestimation in the size of fixed defects in our study as well as underestimation of dipyridamole-induced defect size in a previously reported study [43]. Therefore, for the accurate analysis of the size and extent of fixed versus reversible myocardial defects especially in a known case of previous insult or candidates for long-term risk stratification, withholding beta-blocker treatment may be considered on a case-by-case basis.
Study limitations
Because of ethical considerations (unnecessary exposure to radiation, inherent risks to the stress phase of MPI-such as myocardial infarction, arrhythmia, etc.), we were unable to conduct a within-subject study to evaluate the effect of beta-blocker withdrawal on the sensitivity of DMPI in a single population of patients; however, the two groups in our study were comparable as far as the gender, age, type of radiotracer used for imaging and the extent of CAD were concerned. For future studies and to come into the conclusion that beta-blockers did not affect the sensitivity of the test using the same population with double measurement (one for continuation and the other for discontinuation) could be more justified. In this approach the potential confounder effects of baseline clinical characteristics of patients would be omitted.
Also the study was conducted in patients treated with four different beta-blocking agents. It would have been ideal to assess each beta-blocker disjointedly, but since the consumption of some kinds of beta-blockers such as carvedilol was very uncommon in our patients, a well-powered analysis of the data by considering each type of drug, individually, was unfeasible.
Conclusion
No difference was noted between the sensitivity of DMPI with or without discontinuing of either selective or nonselective beta-blocking agents in our study. Thus, in view of the fact that medication discontinuation may cause the patients to face increased risk of heart events, discontinuing long-term beta-blocker treatment 3 days before DMPI is not suggested especially when only the diagnosis of CAD is the main concern. Beta-blocker withholding before MPI may be indicated on a case-by-case basis just in those patients referred for the accurate estimation of the size of fixed versus reversible defects.
References
Zaman MU, Hashmi I, Fatima N: Recent developments and future prospects of SPECT myocardial perfusion imaging. Ann Nucl Med. 2010, 24 (8): 565-569. 10.1007/s12149-010-0400-z.
Cuocolo A, Petretta M, Acampa W, De Falco T: Gated SPECT myocardial perfusion imaging: the further improvements of an excellent tool. Q J Nucl Med Mol Imaging. 2010, 54 (2): 129-144.
Gholamrezanezhad A, Mirpour S, Esfehani AF, Saghari M, Mirpour K, Beiki D, Soheilifar M: A correlative study comparing current different methods of calculating left ventricular ejection fraction. Nucl Med Commun. 2007, 28 (1): 41-48. 10.1097/01.mnm.0000237990.37325.74.
Fallahi B, Beiki D, Gholamrezanezhad A, Mahmoudian B, Ansari Gilani K, Eftekhari M, Fard-Esfahani A, Mohseni Z, Saghari M: Single 99mTc Sestamibi injection, double acquisition gated SPECT after stress and during low-dose dobutamine infusion: a new suggested protocol for evaluation of myocardial perfusion. Int J Cardiovasc Imaging. 2008, 24 (8): 825-835. 10.1007/s10554-008-9328-y.
Baskot B, Obradović S, Gligić B, Orozović V, Ristić-Angelkov A, Romanović R, Jung R, Ivanović V, Bikicki M, Pavlović M: Adenosine stress protocols for myocardial perfusion imaging. Vojnosanit Pregl. 2008, 65 (1): 47-50. 10.2298/VSP0801047B.
Bokhari S, Ficaro EP, McCallister BD: Adenosine stress protocols for myocardial perfusion imaging. J Nucl Cardiol. 2007, 14 (3): 415-416. 10.1016/j.nuclcard.2007.04.005.
Gholamrezanezhad A, Mirpour S, Hajimohammadi H, Pourmoslemi A: Submaximal target heart rate and the detection of myocardial ischemia by stress myocardial perfusion imaging using the treadmill exercise Bruce protocol. Anadolu Kardiyol Derg. 2008, 8 (3): 192-196.
Patel RN, Arteaga RB, Mandawat MK, Thornton JW, Robinson VJ B: Pharmacologic stress myocardial perfusion Imaging. South Med J. 2007, 100 (10): 1006-1014. 10.1097/SMJ.0b013e318153f9c6.
Taillefer R, Ahlberg AW, Masood Y, White CM, Lamargese I, Mather JF, McGill CC, Heller GV: Acute beta-blockade reduces the extent and severity of myocardial perfusion defects with dipyridamole 99mTc-sestamibi SPECT imaging. J Am Coll Cardiol. 2003, 42 (8): 1475-1483. 10.1016/S0735-1097(03)01046-5.
Bøttcher M, Refsgaard J, Madsen MM, Randsbaek F, Kaltoft A, Bøtker HE, Nielsen TT: Effect of antianginal medication on resting myocardial perfusion and pharmacologically induced hyperemia. J Nucl Cardiol. 2003, 10 (4): 345-352. 10.1016/S1071-3581(03)00454-9.
Shehata AR, Gillam LD, Mascitelli VA, Herman SD, Ahlberg AW, White MP, Chen C, Waters DD, Heller GV: Impact of acute propranolol administration on dobutamine-induced myocardial ischemia as evaluated by myocardial perfusion imaging and echocardiography. Am J Cardiol. 1997, 80 (3): 268-272. 10.1016/S0002-9149(97)00344-5.
Martin GJ, Henkin RE, Scanlon PJ: Beta blockers and the sensitivity of the thallium treadmill test. Chest. 1987, 92 (3): 486-487. 10.1378/chest.92.3.486.
Burns RJ, Kruzyk GC, Armitage DL, Druck MN: Effect of antianginal medications on the prognostic value of exercise thallium scintigraphy. Can J Cardiol. 1989, 5 (1): 29-32.
Bonaduce D, Muto P, Morgano G, Pace L, Ferrara N, Salvatore M, Condorelli M: Effect of beta-blockade on thallium-201 dipyridamole myocardial scintigraphy. Acta Cardiol. 1984, 39 (6): 399-408.
Zoghbi GJ, Dorfman TA, Iskandrian AE: The effects of medications on myocardial perfusion. J Am Coll Cardiol. 2008, 52 (6): 401-416. 10.1016/j.jacc.2008.04.035.
Hurst RT, Askew JW, Lee R: Resolution of myocardial bridge-related wall motion abnormality and associated myocardial perfusion defect with beta-blocker therapy. J Invasive Cardiol. 2005, 17 (12): E40-E42.
Brandts B, Dirkmann D, Borchard R, Wickenbrock I, Van Bracht M, Prull MW, Trappe HJ: Propranolol inhibits IK(Ado) by competitive A1-receptor interaction. Z Kardiol. 2004, 93 (7): 533-539.
Koepfli P, Wyss CA, Namdar M, Klainguti M, von Schulthess GK, Lüscher TF, Kaufmann PA: Beta-adrenergic blockade and myocardial perfusion in coronary artery disease: differential effects in stenotic versus remote myocardial segments. J Nucl Med. 2004, 45 (10): 1626-1631.
Efird J: Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health. 2011, 8 (1): 15-20.
Dobson JG: Mechanism of adenosine inhibition of catecholamine-induced responses in heart. Circ Res. 1983, 52 (2): 151-160. 10.1161/01.RES.52.2.151.
Wang T, Mentzer RM, Van Wylen DG: Interstitial adenosine with dipyridamole: effect of adenosine receptor blockade and adenosine deaminase. Am J Physiol. 1992, 263 (2 Pt 2): 552-558.
Sopena M, Viña J, Calderón J, Pallardó F, Rodrigo F, Canós J, Cabo J: In vitro study of inhibition of adenosine-deaminase by dipyridamole. Rev Esp Fisiol. 1970, 26 (4): 303-306.
Gresele P, Arnout J, Deckmyn H, Vermylen J: Mechanism of the antiplatelet action of dipyridamole in whole blood: modulation of adenosine concentration and activity. Thromb Haemost. 1986, 55 (1): 12-18.
Meisel P, Meisel M, Grisk A: Some properties of adenosine deaminase from vascular smooth muscle and its inhibition by various vasodilators. Cor Vasa. 1976, 18 (1): 56-65.
Gould KL, Lipscomb K, Calvert C: Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation. 1975, 51 (6): 1085-1094. 10.1161/01.CIR.51.6.1085.
Gould KL, Lipscomb K: Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974, 34 (1): 48-55. 10.1016/0002-9149(74)90092-7.
Flameng W, Wüsten B, Schaper W: On the distribution of myocardial flow. Part II: Effects of arterial stenosis and vasodilation. Basic Res Cardiol. 1974, 69 (4): 435-446.
Wüsten B, Flameng W, Schaper W: The distribution of myocardial flow. Part I: Effects of experimental coronary occlusion. Basic Res Cardiol. 1974, 69 (4): 422-434.
Flameng W, Schaper W, Wüsten B: Myocardial blood flow distribution in experimental coronary occlusion without myocardial infarct. Verh Dtsch Ges Kreislaufforsch. 1973, 39: 199-202. 10.1007/978-3-642-85288-6_37.
Becker LC: Conditions for vasodilator-induced coronary steal in experimental myocardial ischemia. Circulation. 1978, 57 (6): 1103-1110. 10.1161/01.CIR.57.6.1103.
Johnson JA, Liggett SB: Cardiovascular pharmacogenomics of adrenergic receptor signaling: clinical implications and future directions. Clin Pharmacol Ther. 2011, 89 (3): 366-378. 10.1038/clpt.2010.315.
Wallukat G: The beta-adrenergic receptors. Herz. 2002, 27 (7): 683-690. 10.1007/s00059-002-2434-z.
Herrmann SC, Feigl EO: Adrenergic blockade blunts adenosine concentration and coronary vasodilation during hypoxia. Circ Res. 1992, 70 (6): 1203-1216. 10.1161/01.RES.70.6.1203.
Ferrara N, Coltorti F, Leosco D, Sederino S, Abete P, Caccese P, Landing P, Longobardi G, Verde R, Rengo F: Protective effect of beta-blockade on dipyridamole-induced myocardial ischaemia. Role of heart rate. Eur Heart J. 1995, 16 (7): 903-908.
Hockings B, Saltissi S, Croft DN, Webb-Peploe MM: Effect of beta adrenergic blockade on thallium-201 myocardial perfusion imaging. Br Heart J. 1983, 49 (1): 83-89. 10.1136/hrt.49.1.83.
Sharir T, Rabinowitz B, Livschitz S, Moalem I, Baron J, Kaplinsky E, Chouraqui P: Underestimation of extent and severity of coronary artery disease by dipyridamole stress thallium-201 single-photon emission computed tomographic myocardial perfusion imaging in patients taking antianginal drugs. J Am Coll Cardiol. 1998, 31 (7): 1540-1546. 10.1016/S0735-1097(98)00142-9.
Kubota I, Ikeda K, Igarashi H, Kawashima S, Yamaki M, Yasumura S, Tsuiki K, Yasui S: Inhibition of dipyridamole-induced myocardial ischemia by diltiazem in patients with coronary artery disease. J Cardiovasc Pharmacol. 1987, 9 (3): 363-367. 10.1097/00005344-198703000-00013.
Lattanzi F, Picano E, Bolognese L, Piccinino C, Sarasso G, Orlandini A, L’Abbate A: Inhibition of dipyridamole-induced ischemia by antianginal therapy in humans. Correlation with exercise electrocardiography. Circulation. 1991, 83 (4): 1256-1262. 10.1161/01.CIR.83.4.1256.
Reyes E, Stirrup J, Roughton M, D’Souza S, Underwood SR, Anagnostopoulos C: Attenuation of adenosine-induced myocardial perfusion heterogeneity by atenolol and other cardioselective beta-adrenoceptor blockers: a crossover myocardial perfusion imaging study. J Nucl Med. 2010, 51 (7): 1036-1043. 10.2967/jnumed.109.073411.
Bridges AB, Kennedy N, McNeill GP, Cook B, Pringle TH: The effect of atenolol on dipyridamole 201Tl myocardial perfusion tomography in patients with coronary artery disease. Nucl Med Commun. 1992, 13 (1): 41-46. 10.1097/00006231-199201000-00007.
Lakkireddy D, Aronow WS, Bateman T, McGhee I, Nair C, Khan IA: Does beta blocker therapy affect the diagnostic accuracy of adenosine single-photon-emission computed tomographic myocardial perfusion imaging?. Am J Ther. 2008, 15 (1): 19-23. 10.1097/MJT.0b013e31804c71a7.
Yoon AJ, Melduni RM, Duncan SA, Ostfeld RJ, Travin MI: The effect of beta-blockers on the diagnostic accuracy of vasodilator pharmacologic SPECT myocardial perfusion imaging. J Nucl Cardiol. 2009, 16 (3): 358-367. 10.1007/s12350-009-9066-0.
Gerson MC: Reduction in dipyridamole-induced single-photon emission computed tomography myocardial defect size by beta-blockers: time to re-examine the patient preparation protocol for pharmacologic stress testing. J Am Coll Cardiol. 2003, 42 (8): 1484-1486. 10.1016/S0735-1097(03)01047-7.
Acknowledgment
This research has been supported by Tehran University of Medical Sciences, under grant 85-02-58-3934, Tehran, Iran. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
DB was involved in all aspects of the study and the corresponding author. BF and DB designed and planed the study, deduced the data and revised the manuscript. AG did bibliography and drafted the article. AF and SA acquired data. FA, SI, JE interpreted data and revised the manuscript. AG and BF conducted the data analysis, interpreted data. MS and ME edited/revised the article. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Fallahi, B., Beiki, D., Akbarpour, S. et al. Withholding or continuing beta-blocker treatment before dipyridamole myocardial perfusion imaging for the diagnosis of coronary artery disease? A randomized clinical trial. DARU J Pharm Sci 21, 8 (2013). https://doi.org/10.1186/2008-2231-21-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-21-8