Abstract
Background
The phylogeny of the Orthoptera was analyzed based on 6 datasets from 47 orthopteran mitochondrial genomes (mitogenomes). The phylogenetic signals in the mitogenomes were rigorously examined under analytical regimens of maximum likelihood (ML) and Bayesian inference (BI), along with how gene types and different partitioning schemes influenced the phylogenetic reconstruction within the Orthoptera. The monophyly of the Orthoptera and its two suborders (Caelifera and Ensifera) was consistently recovered in the analyses based on most of the datasets we selected, regardless of the optimality criteria.
Results
When the seven NADH dehydrogenase subunits were concatenated into a single alignment (NADH) and were analyzed; a near-identical topology to the traditional morphological analysis was recovered, especially for BI_NADH. In both the concatenated cytochrome oxidase (COX) subunits and COX + cytochrome b (Cyt b) datasets, the small extent of sequence divergence seemed to be helpful for resolving relationships among major Orthoptera lineages (between suborders or among superfamilies). The conserved and variable domains of ribosomal (r)RNAs performed poorly when respectively analyzed but provided signals at some taxonomic levels.
Conclusions
Our findings suggest that the best phylogenetic inferences can be made when moderately divergent nucleotide data from mitogenomes are analyzed, and that the NADH dataset was suited for studying orthopteran phylogenetic relationships at different taxonomic levels, which may have been due to the larger amount of DNA sequence data and the larger number of phylogenetically informative sites.
Similar content being viewed by others
Background
The Orthoptera is one of the oldest extant insect lineages, with fossils first appearing in the Upper Carboniferous (290 Mya) (Sharov 1968; Grimaldi and Engel 2005). The monophyly of this order was supported by both morphological and molecular data (Jost and Shaw 2006; Fenn et al. 2008 Ma et al. 2009). It is one of the largest and best researched of the hemimetabolous insect orders and consists of two suborders, the Caelifera (Acidoidea or Acrydoidea) and Ensifera (Tettigoniedea) (Handlirsh 1930; Ander 1939), which are widely accepted by most researchers. With regard to mid-level Caeliferan or Ensiferan relationships (among superfamilies, families, or subfamilies, respectively), there are a few hypotheses based on morphological and molecular data (Flook and Rowell 1997a 1997b 1998; Flook et al. 1999 2000; Fenn et al. 2008; Ma et al. 2009; Eades and Otte 2010; Sun et al. 2010; Zhao et al. 2010 2011). The lack of a consensus as to the phylogeny based only on morphologies makes it especially critical to use DNA data from highly polymorphic genetic markers such as mitogenomic sequences.
Mitogenomes of insects are typically small double-stranded circular molecules. They range in size from 14 to 19 kb and encode 37 genes (Wolstenholme 1992; Boore 1999). For the past 2 decades, mitogenomic data have been widely regarded as effective molecular markers of choice for both population and evolutionary studies of insects. The mitogenome is one of the most information-rich markers in phylogenetics and has extensively been used for studying phylogenetic relationships at different taxonomic levels (Ingman et al. 2000; Nardi et al. 2003). The utility of mitogenomic data may provide new insights into systematics within the Orthoptera.
Different DNA datasets from mitogenomes vary in the degree of phylogenetic usefulness. Protein-coding genes appear to be suited for studies of relationships among closely related species, because unconstrained sites (at the third codon position) in protein-coding genes and information from studies of amino acid substitutions in rapidly evolving genes may help decipher close relationships. In mitochondrial genes, phylogenetic trees based on ribosomal (r)RNA sequences can simultaneously reveal the evolutionary descent of nuclear and mitogenomes, because they are the only ones that are encoded by all organellar genomes and by nuclear and prokaryotic genomes (Gray 1989). The highly conserved regions of rRNA genes may be useful for deep levels of divergence (Simon et al. 1994). Formerly, the A + T-rich region in the mitogenome was rarely used in constructing phylogenies due to its high adenine and thymine contents and high variability (Zhang et al. 1995 Zhang and Hewitt 1997). However, Zhao et al. (2011) verified that the sequence of the conserved stem-loop secondary structure in this region discovered by Zhang et al. (1995) provides good resolution at the intra-subfamily level within the Caelifera.
Phylogenetic analyses have generally been performed with the maximum likelihood (ML) and Bayesian inference (BI) methods. There are yet no sufficient opinions to verify their superiority or inferiority in all cases. When the selected DNA sequence data are rather slowly evolving and large in amount, ML can lead to inferred phylogenetic relationships relatively close to those that would be obtained by analyzing the tree based on the entire genome (Nei and Kumar 2000). The recently proposed BI phylogeny appears to possess advantages in terms of its ability to use complex models of evolution, ease of interpretation of the results, and computational efficiency (Huelsenbeck et al. 2002).
Herein, we reconstructed the phylogeny of the Orthoptera as a vehicle to examine the phylogenetic utility of different datasets in the mitogenome to resolve deep relationships within the order. Also, we explored various methods of analyzing mitogenomic data in a phylogenetic framework by testing the effects of different optimality criteria and data-partitioning strategies.
Methods
Data partitioning
In total, 47 available orthopteran mitogenomes were included in our analyses (Table 1). Ramulus hainanense from the Phasmatodea and Sclerophasma paresisense from the Mantophasmatodea were selected as outgroups. We created six datasets to study the effect of different partitioning schemes on the topology of mitogenomic phylogenies: (1) ATP, (2) cytochrome oxidase (COX), (3) COX + cytochrome (Cyt) b, (4) NADH, (5) the concatenated conserved domain of ribosomal RNA (rRNA(C)), and (6) the concatenated variable domain of rRNA (rRNA(V)).
DNA alignment was inferred from the amino acid alignment of each of the 13 protein-coding genes using MEGA vers. 5.0 (Tamura 2011). rRNA genes were individually aligned with ClustalX using default settings (Thompson et al. 1997). The 15 separate nucleic acid sequence alignments were manually refined.
Phylogenetic analyses
MrModeltest 2.3 (Nylander 2004) and ModelTest 3.7 (Posada and Crandall 1998) were respectively used to select the model for the BI and ML analyses. According to the Akaike information criterion, the GTR + I + G model was selected as the most appropriate for these datasets. The BI analysis was performed using MrBayes, vers. 3.1.2 (Ronquist and Huelsenbeck 2003) under this model. Two simultaneous runs of 106 generations were conducted for the matrix. Each set was sampled every 100 generations with a burn-in of 25%. Bayesian posterior probabilities were estimated on a 50% majority rule consensus tree of the remaining trees. The ML analysis was performed using the program RAxML, vers. 7.0.3 (Stamatakis 2006) with the same model. A bootstrap analysis was performed with 100 replicates.
Results and discussion
Results
Phylogenetic relationships within the Orthoptera
Different optimality criteria and dataset compilation techniques have been applied to find the best method of analyzing complex mitogenomic data (Stewart and Beckenbach 2009 Cameron et al. 2004 Castro and Dowton 2005 Kim et al. 2005). In this paper, we compared the effect of partitioning according to different protein-coding genes (NADH, COX, COX + Cyt b, and ATP) and different regions in rRNA (rRNA(C) (small subunit ribosomal RNA (rrnS) (III) + large subunit ribosomal RNA (rrnL) (III + IV + V)) and rRNA(V) (rrnS (I + II) + rrnL (I + II + VI))).
Different partitioning schemes had greater or lesser influences on the phylogenetic reconstruction in terms of both the topology and nodal support. When all available data were analyzed, the monophyly of two Orthoptera suborders, the Caelifera and Ensifera, was consistently recovered in the context of our taxon sampling based on most analyses (Figures 1, 2, 3, and 4A,B).
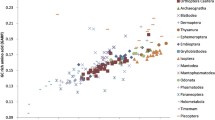
Phylogenetic tree built by the Bayesian method based on the NADH dataset. The posterior probabilities are shown close to the nodes. Ac., Acridinae; Br., Bradyporinae; Cal., Calliptaminae; Cat., Catantopinae; Co., Conocephalinae; Cy., Cyrtacanthacridinae; Ep., Episactinae; Go., Gomphocerinae; Grylli., Gryllinae; Gryllo., Gryllotalpinae; Mec., Meconematinae; Mel., Melanoplinae; My., Myrmecophilinae; Oe., Oedipodinae; Ox., Oxyinae; Ph., Phaneropterinae; Py., Pyrgomorphinae; Ro., Romaleinae; Tetr., Tetriginae; Tett., Tettigoniinae; Th., Thrinchinae; Tri., Tridactylinae; Tro., Troglophilinae.
Phylogenetic relationships within the Ensifera
Based on most analyses, within the Ensifera, the Rhaphidophoridae clustered into one group with the Tettigoniidae and together supported the monophyly of the Tettigonioidea (Figures 1, 2, 3, 4, and 5A,B). This was consistent with results presented by Flook and Rowell (1999), Fenn et al. (2008), Ma et al. (2009), Sun et al. (2010), Zhao et al. (2010), and Zhou et al. (2010), but conflicted with results of Jost and Shaw (2006). Jost and Shaw (2006) found that the Rhaphidophoridae was more closely related to the Grylloidea than to the Tettigoniidae. Relationships among the five subfamilies within the Tettigoniidae were only recovered in the analyses (BI_NADH, BI_(COX + Cyt b), ML_NADH, and ML/BI_rRNA(C)) (Figures 1, 2B, 4A,B, and 6), (Meconematinae + ((Phaneropterinae + Conocephalinae) + (Bradyporinae + Tettigoniinae))). These results were similar to those by Storozhenko (1997), Gwynne and Morris (2002), and Zhou et al. (2010).
The monophyly of the Grylloidea was supported by the analyses (ML_NADH, ML/BI_(COX + Cyt b), and ML/BI_COX) (Figures 2, 3A,B, and 6). However, the Myrmecophilidae clustered into one clade with the Gryllotalpidae, and the Gryllidae formed an independent monophyletic group in the BI_NADH (Figure 1). These results were consistent with those based on a mitochondrial (mt)DNA sequence by Zhou et al. (2010) but conflicted with results based on three rRNA gene sequences by Flook et al. (1999). The two datasets of COX + Cyt b and NADH may be good choices for resolving deep relationships within the suborder Ensifera.
Phylogenetic relationships within the Caelifera
The monophyly of the Caelifera is widely accepted and is supported by morphological and molecular data (Xia 1994 Fenn et al. 2008 Eades and Otte 2010 Sheffield et al. 2010 Sun et al. 2010 Zhao et al. 2010 2011). In the present study, five superfamilies of Caelifera lineages were included, and they clustered as a monophyletic clade. Our results may provide evidence for resolving phylogenetic relationships among those superfamilies within the Caelifera. The monophyly of five superfamilies within the Caelifera was well supported by our analyses (BI_NADH, ML/BI_(COX + Cyt b), and ML/BI_COX) (Figures 1, 2, and 3A,B). Relationships among the five superfamilies were (Tridactyloidea + (Tetrigoidea + (Eumastacoidea + (Pneumoroidea + (Acridoidea))))). The Tridactyloidea occupied the basal position.
Within the Acridoidea, the BI_NADH analysis produced an identical topology to the OSF system (Eades and Otte 2010) (Figure 1). The respective monophylies of the Acrididae, Romaleidae, and Pamphagidae were well recovered only by this analysis.
Phylogenetic relationships within the Acrididae
With regard to relationships among those subfamilies within the Acrididae, divergent tree topologies were resolved from the different datasets we selected in this study. Our initial analyses using the six datasets led to quite different tree topologies, and neither the ML nor BI trees based on these datasets well resolved deep phylogenetic relationships with the exception of the BI_NADH analysis (Figure 1). The relationships among the eight acridid subfamilies were (Cyrtacanthacridinae + (Calliptaminae + (Catantopinae + (Oxyinae + (Melanopline + (Acridinae + (Oedipodinae + Gomphocerinae))))))).
The five subfamilies, the Cyrtacanthacridinae, Catantopinae, Calliptaminae, Oxyinae, and Melanopline, were placed in one family, the Catantopidae, in Xia's (1958) system. In the BI_NADH analysis, the five subfamilies were split into three clades, Cyrtacanthacridinae, (Calliptaminae + Catantopinae), and (Oxyinae + Melanopline). The Acridinae species were split into two clades. Phlaeoba albonema formed one monophyletic clade, while the other two species were grouped together into one clade with species of the Oedipodinae, which was in conflict with the morphological taxonomy and previously reported topologies (Fenn et al. 2008 Sheffield et al. 2010 Sun et al. 2010 Zhao et al. 2010 2011). For the Gomphocerinae, both subfamilies Arcypterinae and Gomphocerinae in Xia's (1958) system were consolidated into one group. This result supports the monophyletic group of the Gomphocerinae in the OSF system (Eades and Otte 2010).
Few of the analyses recovered a topology completely congruent with other studies. Most datasets scattered members of the Acrididae throughout the tree and failed to resolve most of the clades. The topology within the Acridoidea based on rRNA(V) was almost consistent with that of BI_NADH with the sole exception of the Pamphagidae which was located away from the Acridoidea (Figure 5A,B). In the analyses with this dataset, eight subfamilies within the Acrididae adopted in this work were grouped into three clades: (1) clade 1 containing (the Catantopinae + Cyrtacanthacridinae + Calliptaminae + Oxyinae + Melanopline); (2) clade 2 containing (the Oedipodinae + Acridinae), and (3) clade 3 containing the Gomphocerinae.
Discussion
Phylogenetic analyses in this study
We performed 12 separate phylogenetic analyses to test the effect of the optimality criteria and data-partitioning strategies on mitogenomic phylogenies of the Orthoptera. The results indicated that the differing datasets had much larger effects than the optimality criteria on both the topologies and levels of support.
In terms of the ability to resolve deeper-level relationships in the Orthoptera, conserved gene data (COX + Cyt b and COX) resolved the relationships among major Orthoptera lineages (between suborders or among superfamilies), but were unable to unambiguously resolve intra-subfamily relationships within the Acrididae (Figures 2 and 3A,B). This suggests that the two datasets might not have sufficient phylogenetic signals to resolve relationships among closely related species.
The ATP topologies were the worst among the analyses performed herein (Figure 7A,B). The ATP dataset gave tree topologies that were wildly incongruent with the other datasets and with previously accepted orthopteran phylogenies (Fenn et al. 2008 Ma et al. 2009 Sun et al. 2010 Zhao et al. 2010 2011).
Among the total evidence analyzed, there were no apparent effects of different optimality criteria on the tree topologies with rRNA(C) and rRNA(V) (Figures 4 and 5A,B). However, different optimality criteria did result in different topologies among the other datasets. In both the MP and BI analyses based on COX + Cyt b (Figure 2A,B), Acrididae species were split into four clades: (1) clade 1 containing the (Gomphocerinae + Melanopline); (2) clade 2 containing the (Romaleinae + Pamphaginae + Cyrtacanthacridinae + Calliptaminae + Catantopinae + P. albonema); (3) clade 3 containing the (Acridinae (Acrida) + Arcyptera coreana); and (4) clade 4 containing the (Oedipodinae). However, the positions of clades 1 and 2 were reversed in the two analyses. Among the remaining datasets (NADH, COX, and ATP), different optimality criteria greatly influenced reconstruction of the ingroup topology (Figures 1, 3, 6, and 7A,B). Nodal support values also appeared to be affected by the optimality criteria in that bootstrap values for the ML analyses were generally lower than those for the BI analyses, which was consistent with analyses by Fenn et al. (2008).
Here, genes with intermediate rates of evolution might have had better phylogenetic utility for the questions at hand.
Choice of genes and their contribution to a total evidence tree
Correction or weighting of DNA-sequence data based on the level of variability can improve phylogenetic reconstructions in some cases. So gene choice is of critical importance.
Evolution rates of rRNA genes considerably vary along the length of molecules (Hillis and Dixon 1991 Simon et al. 1991). Short-range stems and loops tend to be less conserved compared to long-range stems (Hixson and Brown 1986 Simon et al. 1990). Unpaired regions joining domains tend to be highly conserved. rRNA domains evolve at different average rates dictated by their functional constraints. For example, in rrnS, the 5′ half (domains I and II) has many fewer conserved nucleotide strings than the 3′ half (domain III) (Clary and Wolstenholme 1985 De Rijk et al. 1993 Van de Peer et al. 1993). So, domain III has routinely been used in insect systematic studies as a molecular marker (Simon et al. 1994). Similarly, in rrnL, domains I, II, and VI, on average, are less conserved than domains III, IV, and V (Uhlenbusch et al. 1987; Gutell et al. 1992). So the majority of structural and phylogenetic studies mainly focused on the 3′ half of the rrnL molecule (Kambhampati et al. 1996 Flook and Rowell 1997a b; Buckley et al. 2000). The 3′ halves of rrnS and rrnL are not very useful for phylogenetic studies of recently diverged species, because they contain few sites that vary (Simon et al. 1994). Milinkovitch et al. (1993) successfully analyzed relationships among 16 whale taxa using only the most conserved domains of these two ribosomal genes. rRNA genes are most likely to be useful at the population level and at deep levels of divergence. However, if a researcher is choosing a study of relationships among closely related species, a protein-coding gene might be a better first choice.
Protein-coding genes may be more appropriate for phylogenetic analyses at intermediate levels of divergence. The phylogenetic performance of different genes is related to their particular rates of evolution. The three protein-coding genes, atp6, atp8, and nad4L, are the fastest evolving genes, while COX subunits and Cyt b show much-slower overall rates of evolution (Russo et al. 1996; Zardoya and Meyer 1996; Cameron et al. 2004). Cox1 is the most conserved gene in terms of amino acid evolution. In the past, coxl, cox2, cytb, and nad2 were extensively used for phylogenetic analyses (Liu and Beckenbach 1992; Simon et al. 1994; Caterino et al. 2000; Chapco et al. 2001; Litzenberger and Chapco 2001; Chapco and Litzenberger 2002; Amédégnato et al. 2003). Cox2 is the most widely used mitochondrial protein-coding gene in insects (Simon et al. 1994). Both nad4 and nad5 are large genes, and their protein sequence divergences seem to be helpful in constructing trees for distantly related species (Russo et al. 1996). The nad6 gene was often omitted because it is coded on the light strand, and its properties differ from those of the other 12 protein-coding genes (Springer et al. 2001). Zardoya and Meyer (1996) classified mitochondrial protein-coding genes into three groups, good (nad4, nad5, nad2, cytb, and cox1), medium (cox2, cox3, nadl, and nad6), and poor (atp6, nad3, atp8, and nad4L) phylogenetic performers. Some genes seem to be consistently more-reliable tracers of evolutionary history than others.
Conclusions
Our findings suggest that the best phylogenetic inferences can be made when moderately divergent nucleotide data from mitogenomes are analyzed, and that the NADH dataset was suited for studying orthopteran phylogenetic relationships at different taxonomic levels, which may have been due to the larger amount of DNA sequence data and the larger number of phylogenetically informative sites.
Abbreviations
- Cox1 - 3:
-
Cytochrome oxidase subunits I - III
- COX:
-
Concatenated cox1 - 3
- nad1-6 and 4 L:
-
NADH dehydrogenase subunits 1 to 6 and 4 L
- NADH:
-
The concatenated nad1-6 and 4 L
- atp6 and 8:
-
ATP synthase subunits 6 and 8
- ATP:
-
Concatenated atp6 and 8
- cytb:
-
Cytochrome b
- rRNA:
-
Ribosomal RNA
- rrnL and rrnS:
-
Large and small subunit ribosomal RNAs
- rRNA(C):
-
Concatenated conserved domains of two rRNA genes
- rRNA(V):
-
Concatenated variable domains of two rRNA genes
- OSF:
-
Orthoptera species file.
References
Amédégnato C, Chapco W, Litzenberger G: Out of South America? Additional evidence for a southern origin of melanopline grasshoppers. Mol Phylogenet Evol 2003, 29: 115–119. 10.1016/S1055-7903(03)00074-5
Ander K: Vergleichend-anatomische und phylogenetische Studien über die Ensifera (Saltatoria). Opuscula Ent Suppl 1939, 2: 1–306.
Boore JL: Animal mitochondrial genomes. Nucleic Acids Res 1999, 27: 1767–1780. 10.1093/nar/27.8.1767
Buckley TR, Simon C, Flook PK, Misof B: Secondary structure and conserved motifs of the frequently sequenced domains IV and V of the insect mitochondrial large subunit rRNA gene. Insect Mol Biol 2000, 9: 565–580. 10.1046/j.1365-2583.2000.00220.x
Cameron SL, Miller KB, D'Haese CA, Whiting MF, Barker SC: Mitochondrial genome data alone are not enough to unambiguously resolve the relationships of Entognatha, Insecta and Crustacea sensu lato (Arthropoda). Cladistics 2004, 20: 534–557. 10.1111/j.1096-0031.2004.00040.x
Cameron SL, Barker SC, Whiting MF: Mitochondrial genomics and the new insect order Mantophasmatodea. Mol Phylogenet Evol 2006, 38: 274–279. 10.1016/j.ympev.2005.09.020
Castro LR, Dowton M: The position of the Hymenoptera within the Holometabola as inferred from the mitochondrial genome of Perga condei (Hymenoptera: Symphyta: Pergidae). Mol Phylogenet Evol 2005, 34: 469–479. 10.1016/j.ympev.2004.11.005
Caterino MS, Cho S, Sperling FAH: The current state of insect molecular systematics: a thriving tower of Babel. Annu Rev Entomol 2000, 45: 1–54. 10.1146/annurev.ento.45.1.1
Chapco W, Litzenberger G: A molecular phylogenetic study of two relict species of melanopline grasshoppers. Genome 2002, 45: 313–318. 10.1139/g01-156
Chapco W, Litzenberger G, Kuperus WR: A molecular biogeographic analysis of the relationship between North American melanoploid grasshoppers and their Eurasian and South American relatives. Mol Phylogenet Evol 2001, 18: 460–466. 10.1006/mpev.2000.0902
Clary DO, Wolstenholme DR: The mitochondrial DNA molecule of Drosophila yakuba : nucleotide sequence, gene organization, and genetic code. J Mol Evol 1985, 22: 252–271. 10.1007/BF02099755
De Rijk P, Neefs JM, Van Y, de Peer R, Wachter D: Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res 1993,20(Suppl):2075–2089.
Ding FM, Shi HW, Huang Y: Complete mitochondrial genome and secondary structures of lrRNA and srRNA of Atractomorpha sinensis (Orthoptera, Pyrgomorphidae). Zool Res 2007, 28: 580–588.
Eades DC, Otte D: Orthoptera species file 2.0/3.5. 2010.
Erler S, Ferenz HJ, Moritz RFA, Kaatz HH: Analysis of the mitochondrial genome of Schistocerca gregaria gregaria (Orthoptera: Acrididae). Biol J Linn Soc 2010, 99: 296–305. 10.1111/j.1095-8312.2009.01365.x
Fenn JD, Cameron SL, Whiting MF: The complete mitochondrial genome sequence of the Mormon cricket ( Anabrus simplex : Tettigoniidae: Orthoptera) and an analysis of control region variability. Insect Mol Biol 2007, 16: 239–252. 10.1111/j.1365-2583.2006.00721.x
Fenn JD, Song H, Cameron SL, Whiting MF: A preliminary mitochondrial genome phylogeny of Orthoptera (Insecta) and approaches to maximizing phylogenetic signal found within mitochondrial genome data. Mol Phylogenet Evol 2008, 49: 59–68. 10.1016/j.ympev.2008.07.004
Flook PK, Rowell CHF, Gellissen G: Homoplastic rearrangements of insect mitochondrial tRNA genes. Naturwissenschaften 1995, 82: 336–337. 10.1007/BF01131531
Flook PK, Rowell CHF: The phylogeny of the Caelifera (Insecta, Orthoptera) as deduced from mtrRNA gene sequences. Mol Phylogenet Evol 1997, 8: 89–103. 10.1006/mpev.1997.0412
Flook PK, Rowell CHF: The effectiveness of mitochondrial rRNA gene sequences for the reconstruction of the phylogeny of an insect order (Orthoptera). Mol Phylogenet Evol 1997, 8: 177–192. 10.1006/mpev.1997.0425
Flook PK, Rowell CHF: Inferences about orthopteroid phylogeny and molecular evolution from small subunit nuclear ribosomal DNA sequences. Insect Mol Biol 1998, 7: 163–178. 10.1046/j.1365-2583.1998.72060.x
Flook PK, Klee S, Rowell CHF: Combined molecular phylogenetic analysis of the Orthoptera (Arthropoda, Insecta) and implications for their higher systematics. Syst Biol 1999, 48: 233–253. 10.1080/106351599260274
Flook PK, Klee S, Rowell CHF: Molecular phylogenetic analysis of the basal Acridomorpha (Orthoptera, Caelifera): resolving morphological character conflicts with molecular data. Mol Phylogenet Evol 2000, 15: 345–354. 10.1006/mpev.1999.0759
Gao J, Cheng CH, Huang Y: Analysis of complete mitochondrial genome sequence of Aeropus licenti Chang. Zool Res 2009, 30: 603–612.
Gray MW: Origin and evolution of mitochondrial DNA. Annu Rev Cell Biol 1989, 5: 25–50. 10.1146/annurev.cb.05.110189.000325
Grimaldi D, Engel MS: Evolution of the insects. New York: Cambridge University Press; 2005.
Gutell RR, Schnare MN, Gray MW: A compilation of large subunit (23S- and 23S-like) ribosomal RNA structures. Nucleic Acids Res 1992,20(Suppl):2095–2109. 10.1093/nar/20.suppl.2095
Gwynne DT, Morris GK: Tettigoniidae. Katydids, long-horned grasshoppers and bushcrickets. 2002.
Handlirsh A: Mantodea order Fangheuschrecken. In Handbuch der Zoologie. Edited by: Kükenthal W, Krumbach T. Berlin, Leipzig, Germany: de Gruyter; 1930:803–819. 4(i)
Hillis DM, Dixon MT: Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 1991, 66: 411–453. 10.1086/417338
Hixson JE, Brown WM: A comparison of the small ribosomal RNA genes from the mitochondrial DNA of the great apes and humans: sequence, structure, evolution, and phylogenetic implications. Mol Biol Evol 1986, 3: 1–18.
Huelsenbeck JP, Larget B, Miller RE: Potential applications and pitfalls of Bayesian inference of phylogeny. Syst Biol 2002, 51: 673–688. 10.1080/10635150290102366
Ingman M, Kaessmann H, Pääbo S, Gyllensten U: Mitochondrial genome variation and the origin of modern humans. Nat 2000, 408: 708–713. 10.1038/35047064
Jost MC, Shaw KL: Phylogeny of Ensifera (Hexapoda: Orthoptera) using three ribosomal loci, with implications for the evolution of acoustic communication. Mol Phylogenet Evol 2006, 38: 510–530. 10.1016/j.ympev.2005.10.004
Kambhampati S, Kjer KM, Thorne BL: Phylogenetic relationship among termite families based on DNA sequence of mitochondrial 16S ribosomal RNA gene. Insect Mol Biol 1996, 5: 229–238. 10.1111/j.1365-2583.1996.tb00097.x
Kim I, Cha SY, Yoon MH, Hwang JS, Lee SM, Sohn HD, Jin BR: The complete nucleotide sequence and gene organization of the mitochondrial genome of the oriental mole cricket, Gryllotalpa orientalis (Orthoptera: Gryllotalpidae). Gene 2005, 353: 155–168. 10.1016/j.gene.2005.04.019
Litzenberger G, Chapco W: A molecular phylogeographic perspective on a fifty-year-old taxonomic issue in grasshopper systematics. Heredity 2001, 86: 54–59. 10.1046/j.1365-2540.2001.00806.x
Liu H, Beckenbach AT: Evolution of the mitochondrial cytochrome oxidase II gene among 10 orders of insects. Mol Phylogenet Evol 1992, 1: 41–52. 10.1016/1055-7903(92)90034-E
Liu Y, Huang Y: Sequencing and analysis of complete mitochondrial genome of Chorthippus chinensis Tarb. Chin J Biochem 2008, 24: 329–335.
Liu N, Huang Y: Complete mitochondrial genome sequence of Acrida cinerea (Acrididae: Orthoptera) and comparative analysis of mitochondrial genomes in Orthoptera. Comp Funct Genomics, ID 2010., 319486: doi:10.1155/2010/319486
Ma C, Liu C, Yang P, Kang L: The complete mitochondrial genomes of two band-winged grasshoppers, Gastrimargus marmoratus and Oedaleus asiaticus. BMC Genomics 2009, 10: 1–12. 10.1186/1471-2164-10-1
Milinkovitch MC, Orti G, Meyer A: Revised phylogeny of whales suggested by mitochondrial ribosomal DNA sequences. Nat (Lond) 1993, 361: 346–348. 10.1038/361346a0
Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F: Hexapod origins: monophyletic or paraphyletic? Sci 2003, 299: 1887–1889. 10.1126/science.1078607
Nei M, Kumar S: Molecular evolution and phylogenetics. New York: Oxford University Press; 2000.
Nylander JAA: MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre: Uppsala University, Uppsala, Sweden; 2004.
Posada D, Crandall KA: Modeltest: testing the model of DNA substitution. Bioinform 1998, 14: 817–818. 10.1093/bioinformatics/14.9.817
Ronquist F, Huelsenbeck JP: MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinform 2003, 19: 1572–1574. 10.1093/bioinformatics/btg180
Russo CAM, Takezaki M, Nei M: Efficiencies of different genes and different tree-building methods in recovering a known vertebrate phylogeny. Mol Biol Evol 1996, 13: 525–536. 10.1093/oxfordjournals.molbev.a025613
Sharov AG: Phylogeny of the Orthopteroidea. Akad Nauk SSSR Trudy Paleontologicheskogo Inst 1968, 118: 1–216.
Sheffield NC, Hiatt KD, Valentine MC, Song H, Whiting MF: Mitochondrial genomics in Orthoptera using MOSAS. Mitochondr DNA 2010, 21: 87–104. 10.3109/19401736.2010.500812
Shi HW, Ding FM, Huang Y: Complete sequencing and analysis of mtDNA in Phlaeoba albonema Zheng. Chin J Biochem 2008, 24: 604–611.
Simon CS, Pääbo TK, Wilson AC: Evolution of the mitochondrial ribosomal RNA in insects as shown by the polymerase chain reaction. In Molecular evolution. Volume 122. Edited by: Clegg M, O'Brien S. New York: UCLA Symposia on Molecular and Cellular Biology; 1990:235–244.
Simon C, Franke A, Martin A: The polymerase chain reaction: DNA extraction and amplification. In Molecular techniques in taxonomy. Edited by: Hewitt GM, Johnson AWB, Young JPW. Berlin: Springer-Verlag; 1991:329–355.
Simon C, Rati FF, Beckenbach A, Crespi B, Liu H, Flook P: Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 1994, 87: 651–701.
Springer MS, DeBry RW, Douady C, Amrine HM, Madsen O, de Jong WW, Stanhope MJ: Mitochondrial versus nuclear gene sequences in deep-level mammalian phylogeny reconstruction. Mol Biol Evol 2001, 18: 132–143. 10.1093/oxfordjournals.molbev.a003787
Stamatakis A: RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinform 2006, 22: 2688–2690. 10.1093/bioinformatics/btl446
Stewart JB, Beckenbach AT: Characterization of mature mitochondrial transcripts in Drosophila , and the implications for the tRNA punctuation model in arthropods. Gene 2009, 445: 49–57. 10.1016/j.gene.2009.06.006
Storozhenko SY: Fossil history and phylogeny of orthopteroid insects. In The bionomics of grasshoppers, katydids and their kin. Edited by: Gangwere SK, Muralirangan MC, Muralirangan M. Oxford&New York: CAB International, Oxon; 1997:59–82.
Sun HM, Zheng ZM, Huang Y: Sequence and phylogenetic analysis of complete mitochondrial DNA of two grasshopper species Gomphocerus rufus (Linnaeus, 1758) and Primnoa arctica (Zhang and Jin, 1985) (Orthoptera: Acridoidea). Mitochondr DNA 2010, 21: 115–131. 10.3109/19401736.2010.482585
Tamura K: MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011, 28: 2731–2739. 10.1093/molbev/msr121
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG: The CLUSTAL X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997, 25: 4876–4882. 10.1093/nar/25.24.4876
Uhlenbusch I, McCracken A, Gellissen G: The gene for the large (16S) ribosomal RNA from the Locusta migratoria mitochondrial genome. Curr Genet 1987, 11: 631–638. 10.1007/BF00393927
Van de Peer Y, Neefs JM, De Rijk P, De Wachter R: Reconstructing evolution from eukaryotic small-ribosomal-subunit RNA sequences: calibration of the molecular clock. J Mol Evol 1993, 37: 221–232. 10.1007/BF02407359
Wolstenholme DR: Animal mitochondrial DNA: structure and evolution. Int Rev Cytol 1992, 141: 173–216.
Xia KL: Classification summary of grasshoppers in China. Beijing, China: Science Press; 1958. (in Chinese)
Xia KL: Fauna Sinica Insecta. Volume 4. Beijing, China: Science Press; 1994. in Chinese
Xiao B, Chen W, Hu CC, Jiang GF: Complete mitochondrial genome of the groundhopper Alulatettix yunnanensis (Insecta: Orthoptera: Tetrigoidea). Mitochondr DNA 2012, 23: 286–287. 10.3109/19401736.2012.674122
Xiao B, Feng X, Miao WJ, Jiang GF: The complete mitochondrial genome of grouse locust Tetrix japonica (Insecta: Orthoptera: Tetrigoidea). Mitochondr DNA 2012, 23: 288–289. 10.3109/19401736.2012.674123
Yang H, Huang Y: Analysis of the complete mitochondrial genome sequence of Pielomastax zhengi . Zool Res 2011, 3: 353–362.
Ye W, Dang JP, Xie LD, Huang Y: Complete mitochondrial genome of Teleogryllus emma (Orthoptera: Gryllidae) with a new gene order in Orthoptera. Zool Res 2008, 29: 236–244. 10.3724/SP.J.1141.2008.00236
Yin H, Zhi YC, Jiang HD, Wang PX, Yin XC, Zhang DC: The complete mitochondrial genome of Gomphocerus tibetanus Uvarov, 1935 (Orthoptera: Acrididae: Gomphocerinae). Gene 2012, 494: 214–218. 10.1016/j.gene.2011.12.020
Zardoya R, Meyer A: Phylogenetic performance of mitochondrial protein-coding genes in resolving relationships among vertebrates. Mol Biol Evol 1996, 13: 933–942. 10.1093/oxfordjournals.molbev.a025661
Zhang DX, Hewitt FM: Insect mitochondrial control region: a review of its structure, evolution and usefulness in evolutionary studies. Biochem Syst Ecol 1997, 25: 99–120. 10.1016/S0305-1978(96)00042-7
Zhang CY, Huang Y: Complete mitochondrial genome of Oxya chinensis (Orthoptera, Acridoidea). Acta Biochim Biophys Sin 2008, 40: 7–18. 10.1111/j.1745-7270.2008.00375.x
Zhang DX, Szymura JM, Hewitt GM: Evolution and structural conservation of the control region of insect mitochondrial DNA. J Mol Evol 1995, 40: 382–391. 10.1007/BF00164024
Zhang DC, Zhi YC, Yin H, Li XJ, Yin XC: The complete mitochondrial genome of Thrinchus schrenkii (Orthoptera: Caelifera, Acridoidea, Pamphagidae). Mol Biol Rep 2011, 38: 611–619. 10.1007/s11033-010-0147-6
Zhang HL, Zeng HH, Huang Y, Zheng ZM: The complete mitochondrial genomes of three grasshoppers, Asiotmethis zacharjini , Filchnerella helanshanensis and Pseudotmethis rubimarginis (Orthoptera: Pamphagidae). Gene 2013, 517: 89–98. 10.1016/j.gene.2012.12.080
Zhang HL, Zhao L, Huang Y, Zheng ZM: The complete mitochondrial genome of the Gomphocerus sibiricus (Orthoptera: Acrididae) and comparative analysis in four Gomphocerinae mitogenomes. Zool Sci 2013, 30: 192–204. 10.2108/zsj.30.192
Zhao L, Zheng ZM, Huang Y, Sun HM: A comparative analysis of mitochondrial genomes in Orthoptera (Arthropoda: Insecta) and genome descriptions of three grasshopper species. Zool Sci 2010, 27: 662–672. 10.2108/zsj.27.662
Zhao L, Zheng ZM, Huang Y, Zhou ZJ, Wang L: Comparative analysis of the mitochondrial control region in Orthoptera. Zool Stud 2011, 50: 385–393.
Zhou ZJ, Huang Y, Shi FM: The mitochondrial genome of Ruspolia dubia (Orthoptera: Conocephalidae) contains a short A + T-rich region of 70 bp in length. Genome 2007, 50: 855–866. 10.1139/G07-057
Zhou ZJ, Shi FM, Huang Y: The complete mitogenome of the Chinese bush cricket, Gampsocleis gratiosa (Orthoptera: Tettigonioidea). J Genet Genom 2008, 35: 341–348. 10.1016/S1673-8527(08)60050-8
Zhou ZJ, Huang Y, Shi FM, Ye HY: The complete mitochondrial genome of Dercantha onos (Orthoptera: Bradyporidae). Mol Biol Rep 2009, 36: 7–12. 10.1007/s11033-007-9145-8
Zhou ZJ, Ye HY, Huang Y, Shi FM: The phylogeny of Orthoptera inferred from mtDNA and description of Elimaea cheni (Tettigoniidae: Phaneropterinae) mitogenome. J Genet Genom 2010, 37: 315–324. 10.1016/S1673-8527(09)60049-7
Acknowledgments
The authors are grateful to Z.J. Zhou and L. Zhao for their valuable suggestions for the manuscript and help with experiments. This study was supported by the National Natural Science Foundation of China (30970346 and 31172076) and the Fundamental Research Funds for the Central Universities (GK201001004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HZ carried out the molecular genetic studies and drafted the manuscript, YH gave some important advices in the frame of this manuscript, LL and XW participated in the sequence alignment and data analyses, and ZZ modified and checked the language of this manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhang, HL., Huang, Y., Lin, LL. et al. The phylogeny of the Orthoptera (Insecta) as deduced from mitogenomic gene sequences. Zool. Stud. 52, 37 (2013). https://doi.org/10.1186/1810-522X-52-37
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1810-522X-52-37