Abstract
Exosomes are tiny membrane-bound vesicles that are over produced by most proliferating cell types during normal and pathological states. Their levels are up-regulated during pregnancy and disease states such as cancer. Exosomes contain a wide variety of proteins, lipids, RNAs, non-transcribed RNAs, microRNAs and small RNAs that are representative to their cellular origin and shuttle from a donor cell to a recipient cell. From intercellular communication to tumor proliferation, exosomes carry out a diverse range of functions, both helpful and harmful. Useful as biomarkers, exosomes may be applicable in diagnostic assessments as well as cell-free anti-tumor vaccines. Exosomes of ovarian cancer contain different set of proteins and miRNAs compared to exosomes of normal, cancer-free individuals. These molecules may be used as multiple “barcode” for the development of a diagnostic tool for early detection of ovarian cancer.
Similar content being viewed by others
Introduction
Exosomes, like the intraluminal vesicles range approximately from 30-100 nm in diameter secreted by live cells and were first observed in the early 1980s. Secretion of exosomes from cells was proposed to be a mechanism through which cells discard their garbage [1–4]. However, in recent years, exosomes have emerged as important molecules for inter-cellular communication that are involved both in normal and in pathophysiological conditions, such as lactation [5], immune response [6] and neuronal function [5], and also in the development and progression of diseases such as liver disease [7], neurodegenerative diseases [8] and cancer [9, 10]. They exhibit a round, saucer-like morphology and are encapsulated by a lipid bilayer [10, 11]. Exosomes are found in higher concentrations in the peripheral circulation during pregnancy and cancer [12]. A large number of biological functions of exosomes have been demonstrated however, in this review we will focus discussing on the functions of exosomes in ovarian cancer and immune tolerance related to cancer.
Exosome biogenesis and secretion
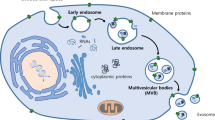
Exosomes in eukaryotic cells, throughout their life cycle periodically engulf small amounts of intracellular fluid, forming a small intracellular body called an endosome [13, 14]. As the early endosome matures and develops into the late endosome, it becomes characterized by the formation of intraluminal vesicles (ILV) inside the lumen of the endosome. Ranging from 30-100 nm in diameter, the ILVs are formed by inward budding of the endosomal membrane, randomly engulfing portions of the cytosolic contents and incorporating transmembrane and peripheral proteins into the invaginating membrane. The transformation into the late endosome can also be detected by observing a change in its shape and location: the early endosome is tube-like in shape and is usually located in the outer portion of the cytoplasm, whereas, the late endosome is spherical and found closer to the nucleus [14]. The late endosome containing ILVs is also called as multivesicular body (MVB) [15, 16].
The fate of the MVB may vary. Typically, it fuses with the lysosome and its contents are degraded by the hydrolysis inside the lysosome. It has been proposed that the contents of the MVB are sorted; proteins destined for degradation by the lysosome and are found in the ILVs, whereas proteins that may have another function or role are found outside the ILVs of the MVB. However, the mechanism by which this process occurs is not yet completely understood. Possible theories involving the sorting of proteins have been proposed [17, 18].
As an alternative to fusion with the lysosome, the MVB may fuse with the plasma membrane of the cell in a proposed ion-dependent manner instead, releasing its ILVs in an exocytotic fashion to the extracellular environment (Figure 1). These vesicles are then referred as exosomes [15]. Demonstrated both in vivo and in vitro, exosomes are secreted during both normal and pathological conditions and by a wide array of cell types [19]. First discovered in maturing mammalian reticulocyte (immature red blood cells) exosomes are secreted by wide range of mammalian cell types, including B and T cells [20], dendritic cells [16], mesenchymal stem cells [21], epithelial cells [22, 23], astrocytes [24], endothelial cells [25] and cancer cells [12, 26–37]. Exosomes have also been identified in most bodily fluids including urine and amniotic fluid [38], blood [39], serum [12, 40], saliva [41, 42], ascites [28, 43], breast milk [6], cerebrospinal fluid [44, 45] and nasal secretion [46]. Importantly, cancer cells have been shown to secrete exosomes in greater amounts than normal cells [12, 47].
Exosomes are mainly secreted by two different mechanisms, constitutive release via the Trans-Golgi network and inducible release [48, 49]. Studies from Thery and colleagues demonstrated that a number of Rab family proteins, including Rab27a and Rab27b, act as key regulators of exosomes secretion [50]. Apart from Rab 27a and 27b, another Rab family member, Rab 35 and Rab 11 have also been shown to regulate the secretion of exosomes by interacting with GTPase-activating protein TBC1 domain family member 10A-C (TBC1D10A-C) [51, 52]. The Rab family of proteins are often mutated (constitutively active) or over-expressed in cancer cells.
It has also been shown that activation of the tumor suppressor protein, p53, stimulates and increases the rate of exosome secretion by regulating transcription of the various genes such as TSAP6 and CHMP4C, which activate exosome production [53]. If the cell experiences stresses such as hypoxia or toxic stress, DNA may become damaged. The p53 protein responds to this process by regulating the transcription of number of genes (Figure 2). This results in what is known as “bystander effect”, during which the cell secretes certain proteins that communicate with nearby cells to help in recruiting them for a response to compensate the stress [54]. Irradiation of human prostate cancer cells strongly indicated DNA damage that could indeed induce a p53-dependent increase in exosome secretion by activating the cells [54]. In addition, secretion of exosomes appears to be increased by K+-induced depolarization in neuronal cells, and the crosslinking of CD3 with T cells, and intracellular Ca2+ levels [14, 15]. Various other stimuli and change in membrane pH have also been shown to trigger the secretion of exosomes from various cell types [55, 56].
Once exosomes are released from the parent cells, they carry out a variety of functions and may eventually be taken up by another cell [57] (Figure 2). It has been reported that certain exosomes, such as those that have been secreted by tumor cells, carry phosphatidylserine (PS) on their membranes. PS acts as a signal and has a large role in exosome uptake by certain cells [58]. When an exosome is taken up by another cell, the entire exosome may be endocytosed and re-legated to clathrin-coated pits, or may simply release its inner contents into the cell and remain united with the cell membrane [57].
Composition of exosomes
A large amount of exosomal proteins have been identified, and the consistency among exosomes stemming from like-cell types suggests that a sorting mechanism is acting during exosomal development. TSG101 and Alix have been identified in exosomal analysis and are known components of the endosomal sorting complex required for transport (ESCRT) machinery. ESCRT functions to sort the cargo proteins of ILVs. It works at the endosomal/MVB membrane and recognizes ubiquitinylated proteins that are associated with tetraspanins and possibly those which are GPI-anchored, arranges them in such a way on the membrane that they are fated to become contents of exosomes [15, 17].
The function of the original cell from which an exosome originate can be deduced by the makeup of proteins, lipids, miRNAs, mRNA, non-coding RNAs and other molecules found in a particular exosome. Exosomes contain many different cell surface molecules and are able to engage many different cell receptors simultaneously. This allows them to participate in the exchange of materials between cells, for example proteins, lipids, carbohydrates, and pathogens [13]. Recent information from different cell type reveals that exosomes contains 4,563 proteins, 194 lipids, 1639 mRNA and 764 microRNA [59, 60] demonstrating their complexity. Exosomes are rich in molecules that are involved in antigen presentation, such as CD1 and the major histocompatibility molecules (MHC) class I and II, which play a key role in immune-regulation by possessing antigenic peptides [61]. MHC I also may be involved in the presentation of antigen fragments to T cells [62, 63]. Also exosomes contain tetraspanins CD9, CD63, CD81, and CD82, as well as co-stimulatory molecules (CD86) and adhesion molecules CD11b and CD54 [33, 61, 62]. Other common exosomal proteins include heat shock proteins HSP70 and HSP90, which expedite peptide loading onto MHC I and II, as well as play a role in the cellular response to environmental stresses. Inside the cell, HSPs assist in protein folding and trafficking, acting as chaperones [62, 63]. Exosomes in general also contain cytoplasmic proteins such as annexins and rab proteins, both of which may promote the fusion of MVB with the cell membrane and expulsion of exosomes. In the last few years, exosomes have been reported to contain nucleic acid such as DNA, RNA, miRNA and none coding RNA [12, 58, 59, 64–69] in addition to proteins. RNA containing exosomes may represent alternate pathway of cellular communication with significant implications in the modification of cell phenotypes. The mRNAs found in exosomes are found to be functional and could be transferred to target cells and translated into proteins [70, 71]. Specific miRNA such as let-7, miR-1, miR-15, miR-16, miR-151 and miR 375 which play role in angiogenesis, hematopoiesis, exocytosis and tumorigenesis have been reported in exosomes [65]. Along with DNA, RNA, miRNA, cytoplasmic tubulin, actin, and actin-binding proteins are also commonly found in exosomes [12, 58, 59]. Membrane proteins CD55 (decay accelerating factor) and CD59 have been reported in exosomes which provide protection against the complement system and thus are an instrumental part in the stability of the exosome outside the cell [15]. Exosomes also commonly contain signal transduction proteins such as G-proteins and protein kinases [58, 59]. In addition, exosomes tend to have a rich content of proteolysis enzymes, suggesting that exosomes may increase cell migration [10, 13]. It is to note that exosomes do not contain mitochondrial, nuclear, and endoplasmic proteins [13].
Exosomes derived from B cells, T cells, dendritic cells, intestinal epithelial cells and many other cell types contain specific-proteins pertaining to their particular functions in addition to commonly found proteins in exosomes. Exosomes secreted by intestinal epithelial cells display syntaxin 3, C26, and the A33 antigen, depending upon which side of the epithelial membrane it is released such as apical or basolateral [15]. Exosomes from T lymphocytes contain many T cell receptors, indicating exosomes role in intercellular communication. Exosomes secreted by dendritic cells contain CD80 and CD86 proteins, which are involved in the activation of naïve CD4+ T cells [10]. Microglial cells appear to release exosomes, which display CD13, the active aminopeptidase that cleaves neuropeptides [15]. Some of the major proteins reported in the exosomes are shown in Table 1.
Functions of exosomes
Exosomes are involved in many physiological processes, both beneficial and pathological. Initially, exosomes were characterized as a means for the maturing reticulocyte to get rid of superfluous proteins. Studies have now shown that exosomes are involved in elimination of unnecessary proteins or unwanted molecules from the cell, exchange of materials between cells, intercellular communication, propagation of pathogens, functions of the immune system (both stimulatory and inhibitory), antigen presentation, and many more. By observing the varying protein contents, it is quite easy to understand how exosomes that come from different cell types can have differential functions [13, 62] and prove to be highly useful molecules in various biological functions.
Exosomes in ovarian tumor
There is a paradox about exosomes that some may have beneficial effects, such as those released from B cells and dendritic cells. These have powerful anti-tumor and immune-stimulatory effects and the latter also provides co-stimulatory proteins that are used to activate T cells when presented with tumor antigens. On the other hand, exosomes secreted from tumor cells have been observed to enhance tumor invasiveness and angiogenesis, while suppressing the immune response [72]. Breast cancer tumor-derived exosomes and prostasomes (exosome-like vesicles released by the prostate) can also promote angiogenesis in individuals with cancer, whereas prostasomes in a healthy individual inhibit angiogenesis, enhance sperm motility, and delay and stabilize the acrosome reaction, as well as act immune suppressive in its local environment [35, 57]. It is not only cancer cell-derived exosomes that have pervasive qualities; exosomes secreted by platelets have demonstrated to promote tumor growth and metastasis of lung cancer cells [55].
Tumor cell-secreted exosomes include many of the common exosomal proteins; they also contain tumor antigens that are reflective of the tumors they are derived from [13]. The heat shock proteins (HSP) found in tumor exosomes are taken up by dendritic cells and macrophages, which process and present the HSP to the lymph nodes. The HSP then release the tumor-specific antigens they hold, which are presented to cytotoxic T cells leading to their activation. Studies have demonstrated the elimination of various cancers using HSP-stimulated immune cells [62].
Exosomes released by tumor cells are quite unique and have recently come into the spotlight, with many groundbreaking discoveries of their unique proteins and their potential to be diagnostic biomarkers. Tumor exosomes are known to have a significant role in the communication and interaction with tumor cells, immune cells and its surrounding environment. In cancer cells, exosomes entail the transfer of cancer-promoting cellular contents to surrounding cells within the tumor microenvironment or to the circulation to act at distant sites, thereby enabling cancer progression. Several studies have demonstrated the presence of exosomes in ovarian cancer cell cultures and ovarian cancer patients’ plasma/serum or ascites [12, 38, 39, 43, 58, 62, 63, 73–76] in addition to many other cancers. Three to 4-fold higher amount of exosomes has been reported in circulation of patients with ovarian cancer compared to normal individuals [77]. Ovarian cancer is one of the most lethal forms of cancer in women, and until recently, lacked consistent biomarkers useful for its detection. Approximately 70% of cases of ovarian cancer are diagnosed in advanced stage, which has only a 20% survival rate within 5 years of diagnosis. However, if diagnosed at early stage (at Stage I), survival rate is over 90% [12]. Therefore, early diagnosis using exosomal contents may be highly important and may save large number of patients dying from ovarian cancer due to late diagnosis. Exosomes provide stable, disease-specific markers for detection, disease characterization, and disease prognosis [73]. Even though change in exosome content in circulation can be used as a diagnostic tool to detect cancer, but measuring the content of circulating exosomes is not very specific and authentic technique. However, proteomic and genetic profiling of circulating exosomes can provide better diagnosis and monitoring of therapeutic response and diagnostic tool [73].
Recent discoveries of the presence of microRNAs, epithelial cell adhesion molecule (EpCAM) and CD24 in ovarian tumor-derived exosomes have been highly promising alternatives for early detection of ovarian cancer. EpCAM is a glycoprotein that facilitates homo-typical adhesion of cells. It has been found in pseudo-stratified, transitional and simple epithelia on the basolateral surfaces and has been identified as a cargo protein in exosomes. EpCAM is expressed to a certain degree in the normal epithelia of many organs and is highly overexpressed in multiple types of carcinomas. The overexpression is correlated with an escalation of epithelial cell proliferation, as occurs in tumor development [66]. Studies by some investigators have used the magnetic activated cell sorting procedure (MACS) using magnetic micro-beads coated with EpCAM antibodies to isolate exosomes that specifically express EpCAM [12, 77]. Results from these studies showed very low levels of EpCAM (0.039+0.030 mg/ml) in exosomes of normal individuals and an increase in levels related to stage and severity of ovarian carcinoma, reaching a significantly higher level of 1.42 + 0.228 mg/ml for Stage IV ovarian carcinomas. This indicates that EpCAM can be useful as a biomarker for the diagnosis of ovarian cancer as well as the detection of tumor-derived exosomes [12, 66]. High EpCAM concentration is usually associated with a high concentration of CD24 [43]. CD24 is recognized as a tumor marker and is associated with poor prognosis of ovarian carcinomas. It is also expressed by many other types of malignancies as well as by some normal cell types. CD24 is a glycoprotein linked to the cell membrane via a GPI-anchor. CD24 can also be found in the cytoplasm inside MVBs and released into the extracellular environment via exosomes, in which case it is correlated with more aggressive forms of ovarian carcinoma, worsening the prognoses and therefore, shortening patients’ survival times [43].
Presently, ~2,000 microRNAs have been described in human [78] microRNAs have been reported to play important role in in cellular and developmental processes, including developmental timing, organ development, differentiation, proliferation, apoptosis and immune regulation [67]. Recently, Taylor and his colleagues reported the presence of diagnostic miRNAs from EpCAM-positive exosomes from ovarian cancer patients’ serum [12]. The discoveries for the presence of microRNAs, EpCAM, and CD24 in ovarian tumor-derived exosomes have provided promising alternatives to early detection. MicroRNAs are small non-coding RNAs ([22–25] nucleotides) that are important in the regulation of cellular processes such as differentiation, proliferation, cell death, and maturation. MicroRNAs from normal cells and tumor cells of the same tissue type have remarkably different makeup. These findings, along with the previous knowledge of the secretion of exosomes by tumor cells, have recently led to research that microRNAs may be useful as biomarkers. Ideal microRNAs used for diagnosis purpose would be absent or present at non-detectable level in the circulating exosomes of cancer-free individuals, but are aberrantly expressed in ovarian cancer and it is postulated that they may be useful in the diagnosis and prognosis of ovarian cancer. There are 8 microRNAs (miR-21, miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-205 and miR-214) that are confirmed to be diagnostic [12]. The levels of these microRNAs in serum differ slightly between the early and late stages of ovarian cancer, but differ significantly between exosomes from individuals with benign disease and those with advance stages of malignancy. Specific microRNAs have been profiled from a direct tumor tissue samples and compared to microRNAs from exosomes from the peripheral circulation. Results showed that the microRNA from tumor-derived exosomes are a correct reflection of the microRNA from the tumor, indicating that profiling the exosomal microRNA may serve as a useful tool for early diagnosis of this deadly disease instead of sampling of ovarian cancer tissues [12].
Exosomes in immune tolerance
While some exosomes have demonstrated immune-stimulatory effects, whereas, others, such as exosomes released by intestinal epithelial cells have demonstrated the ability to induce antigen-specific tolerance. In a key study by Karlsson, these so-called “tolerosomes” were shown to carry MHC II molecules and thus assist the body in building a tolerance to food antigens [36]. Exosomes have also been reported to stimulate an immune tolerance during pregnancy [79–82].
Interestingly, in contrast to intestinal epithelial cell-derived exosomes helping to create antigen-specific tolerance, exosomes released from dendritic cells that are mounted with tumor peptides produce a specific anti-tumor response in vitro in human [10]. Findings by Andre and colleagues used exosomes from dendritic cells that had been pulsed with tumor peptides showed a tumor-rejection effect in patients with melanoma, prostate cancer, lymphoma, and renal cancer [33], indicating that exosomes secreted by tumor cells have the ability to stimulate an anti-tumor response by transferring tumor-specific antigens to dendritic or other antigen-presenting cells, initiating a specific immune response and thus have much potential in cancer immunotherapy [10, 11]. In order for a T cell-mediated immune response to a tumor to occur, the antigen-presenting dendritic cells must be presented and then process a tumor antigen, which then may be cross-presented on MHC-I molecules to activate a T cell response, which has been demonstrated in an in vitro model [37].
In addition to exosomes acting as immune-stimulants, they have also demonstrated the ability to encourage tolerance in the immune system. Exosomes introduced to a patient from a donor prior to transplant surgery allow the patient longer transplant acceptance time by the immune system [71]. Studies using a rat model have shown that rat treated with bone marrow-derived exosomes prior to a heart allograft procedure showed an increase in alloantibody production, but also experienced a decreased anti-donor T cell response, and longer graft survival [83].
Another means of tumor promotion by exosomes is their capability to suppress the immune response. Experiments performed by Liu et al. [84] indicated an exosome-mediated reduction of natural killer (NK) T cells, unrelated to actions of T-regulatory cells. The same group found tumor exosomes derived from myeloid-derived suppressor cells (MDSCs) [85] and Clayton and colleagues found tumor exosomes derived from mesothelioma [85] to express (membrane-associated) Transforming Growth Factor-β (TGFβ1). Instead of acting as a cell cycle regulator in normal cells to induce apoptosis or cease cell proliferation, TGFβ1 from tumor exosomes assists in immune response evasion by tumor cells and also induces an antiproliferative effect on tumor growth and lymphocytes. The exosomal, membrane-associated form of TGFβ1 was more potent in its inhibitory effect than the soluble form at significantly lower doses [86].
Another proposed method of immune evasion by tumor exosomes is induction of T cell apoptosis. It is known that tumor exosomes are able to inhibit T cell proliferation. Many cancers coincide with notable amounts of Fas Ligand (FasL), which is correlated with poor prognosis [87]. FasL is a transmembrane protein in the tumor necrosis factor family. FasL is found in the membrane of exosomes that are released from tumors and it has been concluded that FasL does induce apoptosis of T cells, suppressing the immune response [16, 85, 88, 89].
Another mechanism of immune defense involves antigen-presenting cells. Dendritic cell-derived exosomes express MHC I and II and T cell co-stimulatory molecules and deliver these complexes to dendritic cells, which are capable of activating CD4+ and CD8+ T cells and suppressing tumor growth [16, 90, 91] (Table 1). However, if antigen-presenting cells such as dendritic cells are not involved, tumor-derived exosomes may possess a self-promoting function. Studies have recently stressed the importance of microvesicles (exosomes) secreted by tumor cells an important means of communication between tumor cells and their environment.
Exosomes and microvesicles
In this review we focused on exosomoes that are secreted from the inner cell membrane compartment. We did not discuss involvement of larger vesicles (100 nm-1μm) that are secreted from the cell surface membranes. Augmenting evidence indicates that both types of vesicles - smaller (exosomes) and larger (microvesicles) are secreted from the activated cells. Since ovarian cancer cells express highly tissue factor (TF) [92], they can easily activate blood platelets that are rich source of both exosomes and microvesicles. It has been demonstrated that TF-directed activation of platelets enhances in exosome/microvesicle-dependent manner metastasis of several types of cancer cells [86, 93, 94]. Moreover, a concept has been presented that tumor growth and expansion is directed by a network of extracellular vesicles secreted by various types of normal and malignant cells present in tumor microenvironment [95]. Its involvement in progression of ovarian cancer needs further studies.
Exosomes and therapies
Research and clinical studies have already proven exosomes to be an accessible and reliable source of biomarkers and early diagnosis of disease, with many more avenues yet to be explored. Exosomes bode well in their role as a means of intercellular communication; they contain many different sets of molecules that have roles in various cellular processes and possess the ability to carry antigenic information. They have the advantage of being nonliving and they are easily recovered from biological fluids [15]. Perhaps the most advantageous of exosomal possibilities is their potential to serve as a key factor in finding a cure for cancer. Tumor-derived exosomes have been used to carry tumor antigens and present them to T cells, priming them to induce the anti-tumor response, resulting in tumor cell death [10, 15, 62]. Their ability to do this suggests that tumor-derived exosomes may be useful in developing a cell-free anti-cancer vaccine [96, 97].
With the growing knowledge of exosomes functions and the inner workings of the immune response, immunotherapeutic treatments are continuously being investigated and developed. Vaccines are being created on the basis of the knowledge of antigen presenting dendritic cells that have the ability to activate CD4+ and CD8+ T cells, as well as natural killer cells. In a phase I clinical trial, dendritic cell exosomes demonstrated the ability to activate NK cell proliferation and activation, shown by an increased quantity of circulating NK cells in patients. By observing such role of exosomes, it is plausible that in near future, a cell-free anticancer vaccine will be available.
Conclusions
Because of multiple biological and therapeutic functions of exosomes, studies on application of exosomes are moving forward with new applications of exosomes. Identification of new proteins and biomolecules in exosomes and novel mechanisms by which they communicate and deliver their functions are of great interest for diagnosis, drug delivery and therapeutic purposes.
References
Trams EG, Lauter CJ, Salem N Jr, Heine U: Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981, 645: 63–70. 10.1016/0005-2736(81)90512-5
Pan BT, Teng K, Wu C, Adam M, Johnstone RM: Electron microscopic evidence for xternalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985, 101: 942–948. 10.1083/jcb.101.3.942
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C: Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987, 262: 9412–9420.
Johnstone RM, Bianchini A, Teng K: Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 1989, 74: 1844–1851.
Harding C, Heuser J, Stahl P: Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 1983, 97: 329–39. 10.1083/jcb.97.2.329
Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S: Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007, 179: 1969–1978.
Masyuk AI, Masyuk TV, Larusso NF: Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol 2013, 59: 621–625. 10.1016/j.jhep.2013.03.028
Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF: The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J 2008, 37: 323–332. 10.1007/s00249-007-0246-z
Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN: Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol 2004, 31: 114–21. 10.1165/rcmb.2003-0238OC
Schorey JS, Bhatnagar S: Exosome function: from tumor immunology to pathogen biology. Traffic 2008, 9: 871–881. 10.1111/j.1600-0854.2008.00734.x
Koga K, Matsumoto K, Akiyoshi T, Kubo M, Yamanaka N, Tasaki A, Nakashima H, Nakamura M, Kuroki S, Tanaka M, Katano M: Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Res 2005, 25: 3703–3707.
Taylor DD, Gercel-Taylor C: MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008, 110: 13–21. 10.1016/j.ygyno.2008.04.033
Théry C, Zitvogel L, Amigorena S: Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002, 2: 569–579.
Keller S, Sanderson MP, Stoeck A, Altevogt P: Exosomes: from biogenesis and secretion to biological function. Immunol Lett 2006, 107: 102–108. 10.1016/j.imlet.2006.09.005
van Niel G, Porto-Carreiro I, Simoes S, Raposo G: Exosomes: A Common Pathway for a Specialized Function. J. Biochem 2006, 140: 13–21. 10.1093/jb/mvj128
Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S: Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999, 147: 599–610. 10.1083/jcb.147.3.599
Simons M, Raposo G: Exosomes - vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009, 21: 575–81. 10.1016/j.ceb.2009.03.007
Johnstone RM, Ahn J: A common mechanism may be involved in the selective loss of plasma membrane functions during reticulocyte maturation. Biomed Biochim Acta 1990, 49: S70-S75.
Henderson MC, Azorsa DO: The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol 2012, 2: 38.
Zech D, Rana S, Büchler MW, Zöller M: Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal 2012, 10: 37. 10.1186/1478-811X-10-37
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK: Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010, 4: 214–222. 10.1016/j.scr.2009.12.003
Van Niel G, Mallegol J, Bevilacqua C, Candalh C, Brugière S, Tomaskovic-Crook E, Heath JK, Cerf-Bensussan N, Heyman M: Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 2003, 52: 1690–1697. 10.1136/gut.52.12.1690
Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN: Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum 2005, 52: 1517–1521. 10.1002/art.21005
Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E: Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem 2012, 287: 21384–1395. 10.1074/jbc.M112.340513
Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Wang X, Qian LJ: Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway nvolving exosomes. Biochem Biophys Res Commun 2009, 387: 229–233. 10.1016/j.bbrc.2009.06.095
Keryer-Bibens C, Pioche-Durieu C, Villemant C, Souquère S, Nishi N, Hirashima M, Middeldorp J, Busson P: Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer 2006, 6: 283. 10.1186/1471-2407-6-283
Savina A, Furlán M, Vidal M, Colombo MI: Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 2003, 278: 20083–20090. 10.1074/jbc.M301642200
Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B, Bailey-Wood R, Wilson K, Tabi Z, Mason MD, Adams M: Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis 2005, 35: 149–152. 10.1016/j.bcmd.2005.06.008
Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH: Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 2009,10(1):42–46. 10.3816/CLC.2009.n.006
Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A: Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta 1819, 2012: 1154–1163.
Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T, Hamdy FC: Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer 2012, 106: 768–774. 10.1038/bjc.2011.595
Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y, Ikeda N, Isozaki Y, Maruyama T, Akanuma N, Komatsu A, Jitsukawa M, Matsubara H: Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer 2013, 108: 644–652. 10.1038/bjc.2013.8
AAndre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L: Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360: 295–305. 10.1016/S0140-6736(02)09552-1
Février B, Vilette D, Laude H, Raposo G: Exosomes: a bubble ride for prions? Traffic 2005, 6: 10–17. 10.1111/j.1600-0854.2004.00247.x
Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE: Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4: 4019–4031. 10.1002/pmic.200400876
Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E: "Tolerosomes" are produced by intestinal epithelial cells. Eur J Immunol 2001, 31: 2892–2900. 10.1002/1521-4141(2001010)31:10<2892::AID-IMMU2892>3.0.CO;2-I
Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L: Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001, 7: 297–303. 10.1038/85438
Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager HD, Abdel-Bakky MS, Gutwein P, Altevogt P: CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int 2007, 72: 1095–1102. 10.1038/sj.ki.5002486
Li QL, Bu N, Yu YC, Hua W, Xin XY: Exvivo experiments of human ovarian cancer ascites-derived exosomes presented by dendritic cells derived from umbilical cord blood for immunotherapy treatment. Clin Med Oncol 2008, 2: 461–467.
Almqvist N, Lönnqvist A, Hultkrantz S, Rask C, Telemo E: Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology 2008, 125: 21–27. 10.1111/j.1365-2567.2008.02812.x
Gallo A, Tandon M, Alevizos I, Illei GG: The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 2012, 7: e30679. 10.1371/journal.pone.0030679
Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R: Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 2008,31(6):1059–1062. 10.1248/bpb.31.1059
Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P: Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol 2007, 107: 563–571. 10.1016/j.ygyno.2007.08.064
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF: Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 2012, 287: 3842–3849. 10.1074/jbc.M111.277061
Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW: Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 2012, 10: 5. 10.1186/1479-5876-10-5
Qiu S, Duan X, Geng X, Xie J, Gao H: Antigen-specific activities of CD8+ T cells in the nasal mucosa of patients with nasal allergy. Asian Pac J Allergy Immunol 2012, 30: 107–13.
Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi E, Brambilla D, Venturi G, Lozupone F, Santinami M, Huber V, Maio M, Rivoltini L, Fais S: High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One 2009, 4: e5219. 10.1371/journal.pone.0005219
Record M, Subra C, Silvente-Poirot S, Poirot M: Exosomes as ntercellular signalosomes and pharmacological effectors. Biochem Pharmacol 2011, 81: 1171–1182. 10.1016/j.bcp.2011.02.011
Record M: Exosomal Lipids in Cell-Cell Communication. In Emerging Concepts of Tumor Exosome-Mediated Cell-Cell Communication. Edited by: Zhang H-G. New York, NY USA: Springer; 2013:47–68.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C: Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010,12(1–13):19–30.
Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, Barr FA, Simons M: Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010, 189: 223–32. 10.1083/jcb.200911018
Savina A, Vidal M, Colombo MI: The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 2002, 115: 2505–2515.
Yu X, Harris SL, Levine AJ: The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006, 66: 4795–4801. 10.1158/0008-5472.CAN-05-4579
Yu XT, Riley T, Levine AJ: The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J 2009, 276: 2201–2212. 10.1111/j.1742-4658.2009.06949.x
Azmi AS, Bao B, Sarkar FH: Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 2013. Epub ahead of print
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S: Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 2009, 284: 34211–34222. 10.1074/jbc.M109.041152
Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G: The biogenesis and functions of exosomes. Traffic 2002, 3: 321–330. 10.1034/j.1600-0854.2002.30502.x
Keller S, König AK, Marmé F, Runz S, Wolterink S, Koensgen D, Mustea A, Sehouli J, Altevogt P: Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett 2009, 278: 73–81. 10.1016/j.canlet.2008.12.028
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ: ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 2012, 40: D1241-D1244. 10.1093/nar/gkr828
Mathivanan S, Simpson RJ: ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9: 4997–5000. 10.1002/pmic.200900351
Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB: Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002, 270: 211–226. 10.1016/S0022-1759(02)00330-7
Cho JA, Park H, Lim EH, Lee KW: Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer 2005, 114: 613–622. 10.1002/ijc.20757
Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN: Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol 2004, 164: 1807–1815. 10.1016/S0002-9440(10)63739-X
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO: Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007, 9: 654–659. 10.1038/ncb1596
Taylor DD, Zacharias W, Gercel-Taylor C: Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol 2011, 728: 235–246. 10.1007/978-1-61779-068-3_15
Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM: Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433: 769–773. 10.1038/nature03315
Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS: Non-coding RNAs: regulators of disease. J Pathol 2010, 220: 126–139. 10.1002/path.2638
Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, Kim JH, Choi DS, Kim YK, Hwang D, Gho YS: Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics 2009, 10: 556. 10.1186/1471-2164-10-556
Hannafon BN, Ding WQ: Intercellular Communication by Exosome-Derived microRNAs in Cancer. Int J Mol Sci 2013, 14: 14240–14269. 10.3390/ijms140714240
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ: Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20: 847–56. 10.1038/sj.leu.2404132
Fevrier B, Raposo G: Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004, 16: 415–421. 10.1016/j.ceb.2004.06.003
Taylor DD, Gercel-Taylor C: Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 2011, 33: 441–54. 10.1007/s00281-010-0234-8
Liang B, Peng P, Chen S, Li L, Zhang M, Cao D, Yang J, Li H, Gui T, Li X, Shen K: Characterization and proteomic analysis of ovarian cancer-derived exosomes. Proteomics 2013, 80C: 171–182.
Taylor DD, Gerçel-Taylor C: Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 2005, 92: 305–311.
Peng P, Yan Y, Keng S: Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep 2011, 25: 749–762.
Taylor DD, Gercel-Taylor C: The origin, function, and diagnostic potential of RNA within extracellular vesicles present in human biological fluids. Front Genet 2013, 4: 142.
Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S: Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004, 35: 122–128. 10.1016/j.humpath.2003.08.026
Oliveira LJ, Barreto RS, Perecin F, Mansouri-Attia N, Pereira FT, Meirelles FV: Modulation of maternal immune system during pregnancy in the cow. Reprod Domest Anim 2012,47(Suppl 4):384–393.
Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB: Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA 2013, 110: 12048–12053. 10.1073/pnas.1304718110
Williams JL, Gatson NN, Smith KM, Almad A, McTigue DM, Whitacre CC: Serum exosomes in pregnancy-associated immune modulation and neuroprotection during CNS autoimmunity. Clin Immunol 2013, 149: 236–243. 10.1016/j.clim.2013.04.005
Taylor DD, Akyol S, Gercel-Taylor C: Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 2006, 176: 1534–1542.
Pêche H, Heslan M, Usal C, Amigorena S, Cuturi MC: Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 2003, 76: 1503–1510. 10.1097/01.TP.0000092494.75313.38
Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang HG: Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 2006, 176: 1375–1385.
Xiang X, Liu Y, Zhuang X, Zhang S, Michalek S, Taylor DD, Grizzle W, Zhang HG: TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol 2010, 177: 1606–1610. 10.2353/ajpath.2010.100245
Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z: Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res 2007, 67: 7458–7466. 10.1158/0008-5472.CAN-06-3456
Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ: Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006, 46: 1199–209. 10.1111/j.1537-2995.2006.00871.x
Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP: Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 2005, 35: 169–173. 10.1016/j.bcmd.2005.07.001
Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N: Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 2009, 4: e4942. 10.1371/journal.pone.0004942
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK: A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005, 3: 9. 10.1186/1479-5876-3-9
Admyre C, Grunewald J, Thyberg J, Gripenbäck S, Tornling G, Eklund A, Scheynius A, Gabrielsson S: Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J 2003, 22: 578–583. 10.1183/09031936.03.00041703
Abu Saadeh F, Norris L, O'Toole S, Mohamed BM, Langhe R, O'Leary J, Gleeson N: Tumour expresion of tissue factor and tissue factor pathway inhibitor in ovarian cancer- relationship with venous thrombosis risk. Thromb Res 2013, 132: 627–634. 10.1016/j.thromres.2013.09.016
Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ: Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 2005, 113: 752–60. 10.1002/ijc.20657
Wysoczynski M, Liu R, Kucia M, Drukala J, Ratajczak MZ: Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: distinct role of PAR1 and PAR3 signaling. Mol Cancer Res 2010, 8: 677–90. 10.1158/1541-7786.MCR-10-0019
Wysoczynski M, Ratajczak MZ: Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer 2009, 125: 1595–603. 10.1002/ijc.24479
Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, Xiang X, Guo H, Zhang L, Dryden G, Yan J, Miller D, Zhang HG: Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther 2013.
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, Roth M, Welti R, Mobley J, Jun Y, Miller D, Zhang HG: Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther 2013, 21: 1345–1357. 10.1038/mt.2013.64
Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Zhang L, Xiang X, Wang B, Yan J, Miller D, Zhang HG: Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun 1867, 2013: 4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AB prepared the first draft of the manuscript; SSK and MZR participated in its design and coordination, and wrote and revised the manuscript; HGZ provided in depth input and edited the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Beach, A., Zhang, HG., Ratajczak, M.Z. et al. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res 7, 14 (2014). https://doi.org/10.1186/1757-2215-7-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1757-2215-7-14