Abstract
Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO) is a proapoptogenic mitochondrial protein that is released to the cytosol in response to diverse apoptotic stimuli, including commonly used chemotherapeutic drugs. In the cytosol, Smac/DIABLO interacts and antagonizes inhibitors of apoptosis proteins (IAPs), thus allowing the activation of caspases and apoptosis. This activity has prompted the synthesis of peptidomimetics that could potentially be used in cancer therapy. For these reasons, several authors have analyzed the expression levels of Smac/DIABLO in samples of patients from different tumors. Although dissimilar results have been found, a tissue-specific role of this protein emerges from the data. The objective of this review is to present the current knowledge of the Smac/DIABLO role in cancer and its possible use as a marker or therapeutic target for drug design.
Similar content being viewed by others
Background
Cancer cells share six features that distinguish them from normal cells: Autocrine production of growth signals, inability to respond to anti-growth signals, sustained angiogenesis, limitless replicative potential, tissue invasion and metastasis, and apoptosis avoidance [1]. This type of cell death is fundamental for the maintenance of tissue homeostasis and immune system development [2]. Tumor cells are subjected to stressful internal and external environments, but nevertheless are resistant to apoptosis.
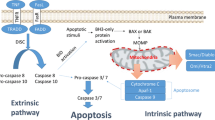
Apoptosis can be activated through two pathways: The extrinsic pathway (mediated by death receptors) or the intrinsic pathway (mediated by mitochondria). The former is activated in response to the engagement of ligands such as CD95 or TNF-α with their receptors. This in turn induces the recruitment of adapter proteins (FADD, TRADD o RAIDD) to form the so-called death-inducing signal complex (DISC), which activates caspase-8. In turn, caspase 8 activates effector caspases by catalytic cleavage. The intrinsic pathway is induced by several different stimuli like antineoplastic drugs, hypoxia, irradiation, growth factor withdrawal and heat shock. These stimuli provoke the mitochondrial outer membrane permeabilization (MOMP) and the release of proteins from the intermembrane space, such as cytochrome-c, Smac/DIABLO, Omi/HtrA2 and AIF to the cytosol [3]. This release allows the assemble of a multiprotein complex, the apoptosome, that includes cytochrome-c, procaspase-9, dATP and cytosolic apoptosis inductor factor-1 (Apaf-1) [4]. The apoptosome activates caspase-9, which in turn induces the activation of effector caspases-3, -6 and -7 [5]. The effector caspases cleave their cellular specific substrates and generate the typical morphology of apoptosis.
The activity of mature caspases is negatively regulated by their interaction with inhibitor of apoptosis proteins (IAPs) [6, 7]. This protein family is comprised by X-linked inhibitor of apoptosis (XIAP), cellular IAP-1 (c-IAP1), cellular IAP-2 (c-IAP2), Testis specific IAP (Ts-IAP), survivin, livin and BRUCE/Apollon [8]. The more studied member is XIAP, formed by three BIR (Baculoviral IAP Repeat) domains located in the NH2-terminus and one RING (Really Interesting New Gene) domain in the CO2H-terminus. The linker region between the BIR1 and BIR2 is implicated in the inhibition of caspase-3 and -7 whereas the BIR2 domain inhibits caspase-7 in a non-competitive manner [9]. Caspase-9 activity is inhibited by its association with the BIR3 domain of XIAP [10]. In addition, it has been determined that the RING domain of XIAP has E3 ubiquitin ligase activity toward caspases, provoking their degradation by the proteasome after their interaction [11, 12].
Smac (Second mitochondria-derived activator of caspase) protein, also known as DIABLO (Direct Inhibitor of Apoptosis-Binding protein with LOw pI), is codified by a nuclear gene. Its protein presents an NH2-terminus that serves as mitochondrial targeting signal (MTS). The mature form of Smac/DIABLO is originated by the cleavage of this signal. In the presence of apoptotic stimuli, mature Smac/DIABLO is release to the cytosol [13]. There, Smac/DIABLO has a pro-apoptotic effect that is mediated by its interaction with IAPs and the release of caspases from them. Structural data had established that Smac/DIABLO requires to form homodimers to interact with IAPs [14]. A particular NH2- terminal motif, consisting of four amino acids, Ala-Val-Pro-Ile, is responsible for the interaction with IAPs [14, 15]. It has been demonstrated that Smac/DIABLO interacts with the BIR2 and BIR3 domains of XIAP, allowing the release of caspase-3 [14] and caspase 9 [16], respectively. Caspase-9 has a similar tetrapeptide motif in its NH2-terminus, so both compete for the BIR3 domain of XIAP [15]. Capase-3 is released by the interaction between NH2-terminus of Smac/DIABLO and BIR2 domain of XIAP [17].
Smac/DIABLO sensitizes tumor cells to die by apoptosis
Several studies have shown that overexpression of Smac/DIABLO sensitizes neoplastic cells to apoptotic death [18, 19]. These findings prompted the development of peptides derived from NH2-terminal of smac/DIABLO and small molecules that mimic Smac/DIABLO functions as therapeutic agents in order to induce death or to increase the apoptotic effect of chemotherapeutic agents. Permeable NH2-terminal peptides of Smac/DIABLO sensitize Hodgkin lymphoma cells to apoptosis mediated by B granzyme [18] and induce caspase-3 activation mediated by cytochrome- c [19]. Moreover, NH2-terminal peptides of Smac/DIABLO fused to Drosophila antennapaedia penetratin sequences enhance apoptosis mediated by different antineoplastic agents in breast cancer [20] and glioblastoma cell lines [21]. Similarly, a small Smac-mimic compound is able to increase the apoptotic effects of death factors such as TRAIL and TNF-α [22]. It is interesting to note that this small molecule induces apoptosis by itself in MDA-MB-231 breast cancer cells, which have high expression levels of XIAP and c-IAP1. In contrast, it only sensitizes MDA-MB-452 and T47D cells, which have low IAPs expression, to TRAIL or etoposide. [23]. Recently, it has been described that compounds that mimic Smac/DIABLO induce the activation of the NF-kB pathway eliciting TNF-α-dependent apoptosis via caspase-8 activation [24]. This activation depends on c-IAP1 and -2 degradation [25]. This was replicated in vivo using a malignant glioma xenograft mice model, in which co-administration of Smac/DIABLO peptides and TRAIL sensitized glioma cells to apoptotic death and induced tumor regression [26]. In the same line, tumor regression was observed when nude mice with xenografted hepatocarcinoma tumors where locally treated with an adenovirus expressing Smac/DIABLO and 5-Fluorouracyl [27]. Taken together, these results show that the NH2-terminal Smac/DIABLO derivatives and small molecules that mimic its function could be useful as adyuvant therapy in tumors with low levels of IAPs and as stand-alone therapy in tumors with high expression of IAPs. Further work is needed to address the potential use of these drugs in humans. Nevertheless, these studies show that particular expression levels of IAPs are key to the cellular response to these peptides or small molecules and underline the posible contribution of Smac/DIABLO levels to the intrinsic resistance to antineoplastic drugs.
Smac/DIABLO and cancer progression
Due to the importance of Smac/DIABLO in determining the sensitivity of cancer cells to apoptotic death induced by diverse stimuli, it is important to investigate if its expression levels could be negatively regulated during the initiation or progression of cancer. This information could be useful as a prognostic or therapeutic marker or to provide new targets for drug design. As mentioned before, it could be expected that cells with lower Smac/DIABLO and therefore, higher apoptosis resistance, should be selected during cancer progression. This selection would contribute to the higher intrinsic resistance in more advanced cancer stages. All the studies have been summarized in Table 1.
Inverse correlation between Smac/DIABLO expression and cancer progression
Two recent studies of Renal Cell Carcinoma (RCC) patients showed that Smac/DIABLO expression at both mRNA and protein level were not associated with stage or grade of tumor [28, 29]. However, another study demonstrated a significative inverse correlation between Smac/DIABLO protein expression level and both stage and histologic grade of RCC [30]. Regardless of this discrepancy, a clear correlation with progression was made in the three studies: patients with metastasic disease had lower Smac/DIABLO levels than those with localized disease [28–30]. In addition, it was shown that patients that undergo radical nephrectomy for RCC with positive Smac/DIABLO expression had a longer disease-specific survival when compared with those with negative expression [30]. Moreover, RCC patients with low Smac/DIABLO expression had a four times higher risk to die than those with high expression [29].
Similarly, Sekimura et al have shown that Smac/DIABLO mRNA levels decreased significantly during lung cancer progression [31]. Since it has been shown that IAPs levels are upregulated in non small cell lung cancer [32], this reduction should increase further the apoptotic threshold of these cells. As expected from these results, decreasing XIAP expression in vitro sensitized lung cancer cells to apoptosis induced by diverse stimuli [33].
It has also been shown that testicular germ tumors with more advanced malignant phenotype presented lower Smac/DIABLO expression levels [34]. Moreover, the XIAP-Smac/DIABLO ratio increased significatively in clinical stage III tumors when compared with stages I or II. In this study an inverse correlation between Smac/DIABLO expression levels and cancer progression was also demonstrated [34].
Finally, it has also been shown that Smac/DIABLO mRNA and protein expression is reduced in hepatocellular carcinoma (HCC) when compared with normal hepatic tissue [35]. This reduction directly correlated with progression. Moreover, in this study, the authors showed that the IAP survivin, increased in parallel with cancer progression [35]. All these studies showed that Smac/DIABLO expression inversely correlated with cancer progression, aggressive behavior, as shown by metastasic disease, and bad prognosis. These correlations could be due to an increased apoptotic threshold, which should allow cancer cells to withstand not only invasion and metastasis, but to provide a relative increased intrinsic resistance to chemo- or radiotherapy. Further in vivo experiments specific for each tumor should help to solve this question.
Direct correlation between Smac/DIABLO expression and cancer progression
Recently, our group analyzed the participation of Smac/DIABLO in cervical cancer patients. Surprisingly, we found that Smac/DIABLO expression did not correlate inversely with progression. As a matter of fact, normal cervix barely expressed the Smac/DIABLO RNA messenger. During cancer progression, a subset of cervical cancer samples expressed de novo this protein [36]. We could not find any correlation between Smac/DIABLO expression and stage or grade for both squamous cell carcinoma and adenocarcinoma [37]. However, higher immunostaining was directly associated with local recurrence in squamous cell carcinoma. We also established a positive correlation between Smac/DIABLO immunoreactivity and high microvascular density [37], which could be accounting for the recurrence results. More investigation is needed to clarify the relation between Smac/DIABLO and angiogenesis in cervical cancer.
A similar finding in gastric cancer patients supports these results [38]. Gastric adenocarcinomas showed a significant higher expression of Smac/DIABLO than non-neoplastic gastric mucosa [38]. However, no difference in the ratio XIAP-Smac/DIABLO between non-neoplasic gastric mucosa and gastric adenocarcinoma was found [38]. This discrepancy could be due to decrease in the expression of XAF1 (XIAP-associated factor-1) found in these samples [38]. XAF1 is a direct inhibitor of XIAP, so this decrease could be also be increasing the apoptotic threshold of these tumors [39]. Supporting these results, a recent report showed that a decrease of XIAP in gastric cancer cells increase the apoptotic response induced by cisplatin and mitomycin-c [40].
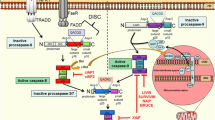
Analyzing a single protein such as Smac/DIABLO or a particular IAP in cancer samples, will only give a partial vision of the mitochondrial apoptotic threshold, since it is known that these proteins are regulated by themselves and, in a tissue-specific pattern, specific IAPs functions overlap. In the case of IAPs (Figure 1), although Smac/DIABLO is not able to degradate XIAP, it induces the ubiquitination and degradation of other members of the family, such as c-IAP1 and c-IAP2 [41]. In addition, a recently cloned Smac/DIABLO isoform, Smac3, induces the auto-ubiquitination and degradation of XIAP [42]. As mentioned before, another negative regulator of XIAP is XAF1. This protein antagonizes the inhibitory activity of XIAP toward caspase-3 [39]. Similarly to IAPs, Smac/DIABLO is also regulated by IAPs family members (Figure 2). Livin is able to ubiquitinate and degrade Smac/DIABLO [43]. Also, in a reciprocal manner, XIAP, c-IAP1, c-IAP2 and Apollon induce the ubiquitination and degradation of Smac/DIABLO [11, 44, 45]. Although in vivo relevance has not yet been demonstrated for these mechanisms, a complicated scenario emerges from these studies, underlying the need for more research focusing not only in Smac/DIABLO protein and isoforms, but also in their interaction partners in a tissue-specific manner.
Smac/DIABLO and other proteins are able to regulate the expression levels of inhibitors of apoptosis (IAPs). Smac/DIABLO-induced downregulation of c-IAP1 and c-IAP2 is mediated by ubiquitination and proteasomal degradation. Smac3, a Smac/DIABLO isoform generated by alternative splicing, induces the auto-ubiquitination and degradation of XIAP by the proteasome. Omi/HtrA2 degradates XIAP, c-IAP1 and c-IAP2. Another negative regulator of XIAP is XAF1.
The inhibitors of apoptosis (IAPs) regulate expression levels of Smac/DIABLO. Smac: Smac/DIABLO. IAPs downregulate Smac/DIABLO by ubiquitination and proteasomal degradation. XIAP, c-IAP1 and c-IAP2 action is mediated by their RING domain. Smac/DIABLO degradation by XIAP is inhibited when the protein NADE is associated with them. Survivin inhibits the release of Smac/DIABLO from mitochondria after apoptotic stimuli. In addition, Survivin prevents Smac/DIABLO degradation in the cytosol.
Conclusion
The balance of IAPs and Smac/DIABLO dictates the apoptotic response to microenvironment clues (e.g. invasion and metastasis) and therapy. Due to this, it is important to perform protein expression studies, which are more informative than mRNA expression analyses, to survey not only Smac/DIABLO but also IAP family members to establish the relevance of these molecules in cancer progression and as possible prognostic markers. Although there is much to learn, we believe that directed therapy aimed to inhibit IAPs functions could be more efficient in those cancers with IAP overexpression or low expression of Smac/DIABLO. Nevertheless, caution is needed in those tumors in which the Smac/DIABLO and IAPs association with progression and/or prognosis is not clear, such as cervical and gastric cancer.
Abbreviations
- Smac/DIABLO:
-
Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI
- IAP:
-
Inhibitor of Apoptosis Protein
- TNF-α:
-
Tumor necrosis factor-α
- FADD:
-
Fas Associated Death Domain
- TRADD:
-
TNF receptor associated-protein with death domain
- DISC:
-
Death Inducing Signal Complex
- MOMP:
-
Mitochondrial Outer Membrane Permeabilization
- RING:
-
Really Interesting New Gene
- BIR:
-
Baculovirus Inhibitor Repeat
- MTS:
-
Mitochondrial Targeting Signal
- XIAP:
-
X-linked inhibitor of apoptosis
- AIF:
-
Apoptosis inductor factor
- cIAP1:
-
cellular IAP-1
- cIAP2:
-
cellular IAP-2
- Ts-IAP:
-
Testis specific IAP
- XAF1:
-
XIAP-associated factor-1
- RCC:
-
Renal Cell Carcinoma
- Omi:
-
OmiHtrA2.
References
Hanahan D, Weinberg RA: The hallmarks of cancer. Cell. 2000, 100 (1): 57-70. 10.1016/S0092-8674(00)81683-9.
Vaux DL, Korsmeyer SJ: Cell death in development. Cell. 1999, 96 (2): 245-254. 10.1016/S0092-8674(00)80564-4.
van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P: The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002, 9 (10): 1031-1042. 10.1038/sj.cdd.4401088.
Zou H, Li Y, Liu X, Wang X: An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999, 274 (17): 11549-11556. 10.1074/jbc.274.17.11549.
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA: Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998, 94 (3): 325-337. 10.1016/S0092-8674(00)81476-2.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC: X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997, 388 (6639): 300-304. 10.1038/40901.
LaCasse EC, Baird S, Korneluk RG, MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998, 17 (25): 3247-3259. 10.1038/sj.onc.1202569.
Hunter AM, LaCasse EC, Korneluk RG: The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007, 12 (9): 1543-1568. 10.1007/s10495-007-0087-3.
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R: X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem. 2001, 276 (29): 27058-27063. 10.1074/jbc.M102415200.
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y: Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003, 11 (2): 519-527. 10.1016/S1097-2765(03)00054-6.
Morizane Y, Honda R, Fukami K, Yasuda H: X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J Biochem. 2005, 137 (2): 125-132. 10.1093/jb/mvi029.
Suzuki Y, Nakabayashi Y, Takahashi R: Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001, 98 (15): 8662-8667. 10.1073/pnas.161506698.
Du C, Fang M, Li Y, Li L, Wang X: Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000, 102 (1): 33-42. 10.1016/S0092-8674(00)00008-8.
Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y: Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000, 406 (6798): 855-862. 10.1038/35022514.
Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y: Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000, 408 (6815): 1008-1012. 10.1038/35050012.
Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, et al: A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001, 410 (6824): 112-116. 10.1038/35065125.
Gao Z, Tian Y, Wang J, Yin Q, Wu H, Li YM, Jiang X: A dimeric Smac/diablo peptide directly relieves caspase-3 inhibition by XIAP. Dynamic and cooperative regulation of XIAP by Smac/Diablo. J Biol Chem. 2007, 282 (42): 30718-30727. 10.1074/jbc.M705258200.
Kashkar H, Seeger JM, Hombach A, Deggerich A, Yazdanpanah B, Utermohlen O, Heimlich G, Abken H, Kronke M: XIAP targeting sensitizes Hodgkin lymphoma cells for cytolytic T-cell attack. Blood. 2006, 108 (10): 3434-3440. 10.1182/blood-2006-05-021675.
Kashkar H, Haefs C, Shin H, Hamilton-Dutoit SJ, Salvesen GS, Kronke M, Jurgensmeier JM: XIAP-mediated caspase inhibition in Hodgkin's lymphoma-derived B cells. J Exp Med. 2003, 198 (2): 341-347. 10.1084/jem.20021279.
Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH: Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002, 277 (46): 44236-44243. 10.1074/jbc.M207578200.
Mizukawa K, Kawamura A, Sasayama T, Tanaka K, Kamei M, Sasaki M, Kohmura E: Synthetic Smac peptide enhances the effect of etoposide-induced apoptosis in human glioblastoma cell lines. J Neurooncol. 2006, 77 (3): 247-255. 10.1007/s11060-005-9045-5.
Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG: A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004, 305 (5689): 1471-1474. 10.1126/science.1098231.
Bockbrader KM, Tan M, Sun Y: A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005, 24 (49): 7381-7388. 10.1038/sj.onc.1208888.
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et al: IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007, 131 (4): 682-693. 10.1016/j.cell.2007.10.037.
Wang L, Du F, Wang X: TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008, 133 (4): 693-703. 10.1016/j.cell.2008.03.036.
Fulda S, Wick W, Weller M, Debatin KM: Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002, 8 (8): 808-815.
Zhao J, Jin J, Zhang X, Shi M, Dai J, Wu M, Wang R, Guo Y: Transfection of Smac sensitizes tumor cells to etoposide-induced apoptosis and eradicates established human hepatoma in vivo. Cancer Gene Ther. 2006, 13 (4): 420-427. 10.1038/sj.cgt.7700910.
Yan Y, Mahotka C, Heikaus S, Shibata T, Wethkamp N, Liebmann J, Suschek CV, Guo Y, Gabbert HE, Gerharz CD, et al: Disturbed balance of expression between XIAP and Smac/DIABLO during tumour progression in renal cell carcinomas. Br J Cancer. 2004, 91 (7): 1349-1357. 10.1038/sj.bjc.6602127.
Kempkensteffen C, Hinz S, Christoph F, Krause H, Magheli A, Schrader M, Schostak M, Miller K, Weikert S: Expression levels of the mitochondrial IAP antagonists Smac/DIABLO and Omi/HtrA2 in clear-cell renal cell carcinomas and their prognostic value. J Cancer Res Clin Oncol. 2008, 134 (5): 543-550. 10.1007/s00432-007-0317-7.
Mizutani Y, Nakanishi H, Yamamoto K, Li YN, Matsubara H, Mikami K, Okihara K, Kawauchi A, Bonavida B, Miki T: Downregulation of Smac/DIABLO expression in renal cell carcinoma and its prognostic significance. J Clin Oncol. 2005, 23 (3): 448-454. 10.1200/JCO.2005.02.191.
Sekimura A, Konishi A, Mizuno K, Kobayashi Y, Sasaki H, Yano M, Fukai I, Fujii Y: Expression of Smac/DIABLO is a novel prognostic marker in lung cancer. Oncol Rep. 2004, 11 (4): 797-802.
Hofmann HS, Simm A, Hammer A, Silber RE, Bartling B: Expression of inhibitors of apoptosis (IAP) proteins in non-small cell human lung cancer. J Cancer Res Clin Oncol. 2002, 128 (10): 554-560. 10.1007/s00432-002-0364-z.
Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, Mayer LD, LaCasse EC: Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003, 9 (7): 2826-2836.
Kempkensteffen C, Jager T, Bub J, Weikert S, Hinz S, Christoph F, Krause H, Schostak M, Miller K, Schrader M: The equilibrium of XIAP and Smac/DIABLO expression is gradually deranged during the development and progression of testicular germ cell tumours. Int J Androl. 2007, 30 (5): 476-483. 10.1111/j.1365-2605.2006.00742.x.
Bao ST, Gui SQ, Lin MS: Relationship between expression of Smac and Survivin and apoptosis of primary hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006, 5 (4): 580-583.
Espinosa M, Cantu D, Lopez CM, De la Garza JG, Maldonado VA, Melendez-Zajgla J: SMAC is expressed de novo in a subset of cervical cancer tumors. BMC Cancer. 2004, 4: 84-10.1186/1471-2407-4-84.
Arellano-Llamas A, Garcia FJ, Perez D, Cantu D, Espinosa M, De la Garza JG, Maldonado V, Melendez-Zajgla J: High Smac/DIABLO expression is associated with early local recurrence of cervical cancer. BMC Cancer. 2006, 6: 256-10.1186/1471-2407-6-256.
Shibata T, Mahotka C, Wethkamp N, Heikaus S, Gabbert HE, Ramp U: Disturbed expression of the apoptosis regulators XIAP, XAF1, and Smac/DIABLO in gastric adenocarcinomas. Diagn Mol Pathol. 2007, 16 (1): 1-8. 10.1097/01.pdm.0000213471.92925.51.
Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW, Korneluk RG: Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol. 2001, 3 (2): 128-133. 10.1038/35055027.
Tong QS, Zheng LD, Wang L, Zeng FQ, Chen FM, Dong JH, Lu GC: Downregulation of XIAP expression induces apoptosis and enhances chemotherapeutic sensitivity in human gastric cancer cells. Cancer Gene Ther. 2005, 12 (5): 509-514.
Yang QH, Du C: Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem. 2004, 279 (17): 16963-16970. 10.1074/jbc.M401253200.
Fu J, Jin Y, Arend LJ: Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein. J Biol Chem. 2003, 278 (52): 52660-52672. 10.1074/jbc.M308036200.
Ma L, Huang Y, Song Z, Feng S, Tian X, Du W, Qiu X, Heese K, Wu M: Livin promotes Smac/DIABLO degradation by ubiquitin-proteasome pathway. Cell Death Differ. 2006, 13 (12): 2079-2088. 10.1038/sj.cdd.4401959.
Hu S, Yang X: Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem. 2003, 278 (12): 10055-10060. 10.1074/jbc.M207197200.
Hao Y, Sekine K, Kawabata A, Nakamura H, Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, et al: Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat Cell Biol. 2004, 6 (9): 849-860. 10.1038/ncb1159.
Acknowledgements
GMR is supported by a CONACyT doctoral fellowship. JM-Z work is supported by grant 45728 by CONACyT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GMR wrote the first draft, VM, GCC and JPRG contributed with specific sections, JM-Z reviewed the manuscript and wrote the final version. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Martinez-Ruiz, G., Maldonado, V., Ceballos-Cancino, G. et al. Role of Smac/DIABLO in cancer progression. J Exp Clin Cancer Res 27, 48 (2008). https://doi.org/10.1186/1756-9966-27-48
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-27-48