Abstract
Malaria continues to be a major health problem in more than 100 endemic countries located primarily in tropical and sub-tropical regions around the world. Malaria transmission is a dynamic process and involves many interlinked factors, from uncontrollable natural environmental conditions to man-made disturbances to nature. Almost half of the population at risk of malaria lives in forest areas. Forests are hot beds of malaria transmission as they provide conditions such as vegetation cover, temperature, rainfall and humidity conditions that are conducive to distribution and survival of malaria vectors. Forests often lack infrastructure and harbor tribes with distinct genetic traits, socio-cultural beliefs and practices that greatly influence malaria transmission dynamics. Here we summarize the various topographical, entomological, parasitological, human ecological and socio-economic factors, which are crucial and shape malaria transmission in forested areas. An in-depth understanding and synthesis of the intricate relationship of these parameters in achieving better malaria control in various types of forest ecosystems is emphasized.
Similar content being viewed by others
Background
Stratification of global malaria
Malaria is an infectious disease caused by parasites belonging to the genus Plasmodium. It is endemic in 104 tropical and subtropical countries, comprising half of the world’s population (3.4 billion people) [1], of which 2.57 billion are at risk for P. falciparum[2], and 2.5 billion for P. vivax[3]. P. malariae and P. ovale contribute a very small proportion of malaria infections but the population at risk of P. malariae is distributed all over sub-Saharan Africa, most parts of Southeast Asia, western Pacific islands, and Amazonian Basin [4, 5]. P. ovale is prevalent in Africa [5], and it is also reported from Asia-Pacific regions [6]. P. knowlesi, the fifth human parasite [7] is essentially a primate malaria species that is being reported from remote forested areas of Southeast Asian countries [8–11].
Forest malaria
Definition of “forest”
The ‘forest ecotype’ is defined by UNESCO as terrain with a tree canopy cover of more than 10% and an area of more than 0.5 hectares, including natural forests and plantations [12] with a minimum tree height of 5 m, including coffee, rubber, cork oak, and fruit tree plantations, wind break and shelter belts more than 20 m width [13]. Forest vegetation is categorized as rain forest, deciduous forest, scrub forest, highland rain forest, and highland alpine forest [14]. The former three are usually distributed in low to mid latitude and the last two are part of the high altitude biome.
Impact of forest malaria
Forest ecosystems are well known to support transmission of malaria, significantly contributing to the global disease burden. A global assessment reports that “closed forests within areas of malaria risk cover approximately 4.8 million km2”[12]. Almost half the malaria risk is estimated to occur among people living in forested areas (1.4 billion) accounting for 11.7, 18.7, 35.1 and 70.1 million population respectively from 1.5 million km2 in the Amazon region, 1.4 million km2 in Central Africa, 1.2 million km2 in the Western Pacific, and 0.7 million km2 in South–East Asia [15, 16]. Corresponding forest areas containing these malaria risk zones are 11.16 million to 15.71 million km2, 6.53 million– 7.80 million km2, 1.93 million– 5.19 million km2, 2.70 million–2.72 million km2[12, 15, 16]. Controlling malaria in these forested regions of the world has been a major challenge [17].
Summarizing hidden risks of malaria in forests
Most studies of forest malaria are focused on local factors associated with malaria transmission. These include distance from forest, impact of deforestation and reforestation, effect of forest on microclimate, vector bionomics, Plasmodium species survival, and human activities in forests. In this review we analyze the underlying factors influencing transmission of malaria in forests worldwide. Mosquito vectors vary according to forest locality and their behavior changes with the forest micro-climate [18], human population, and their social behaviors [19, 20]. Forest communities are generally tribal and cope with poor infrastructure. Certain practices like slash and burn cultivation, overnight stays within forests in order to collect forest produce, hunting, wide open household construction, and cattle ranching, increase vulnerability to malaria. It can be challenging to educate forest communities about malaria control, and without their cooperation it is difficult to control malaria [21]. Now, worldwide malaria communities are aiming at malaria eradication/elimination [22, 23], a proposition, which is impractical without prevention of re-introduction/re-emergence from hidden foci/uncontrolled forest malaria [22, 24]. Malaria had declined during the previous eradication era in many regions of the world, some of which subsequently experienced resurgence and suffered from its consequences [23, 25–27]. The problem of malaria in forests is compounded by hidden reservoirs of malaria infections that are not fully addressed [28, 29]. The origin and evolution of drug and insecticide resistance are often found associated with the forest and near forest areas [24, 30–32]. In addition to existing asymptomatic infections, the presence of primate malaria parasites and their zoonotic vectors might pose additional challenges to human health in the forest and forest fringe areas [33–35], where malaria surveillance is generally poor [33, 36]. Occasional focal outbreaks (unstable transmission) might occur when malaria transmission extends from the forest shade (nidus) to peri-urban and urban areas [37], where much higher density of human population and presence of vectors could fuel large epidemics.
Key factors which make forest different from other ecosystems
The major factors, that differentiate forest from other ecosystems in relation to malaria transmission dynamics, are the influence of forest on temperature buffering, rainfall [38], humidity [39–41], tree canopy [42], flora, fauna [43], high organic content in breeding pools [44], and lack of infrastructure [45]. It is difficult to develop infrastructure in forests due to their uneven land forms, presence of streams, and dense vegetation. Additionally, poor communication hinders malaria control activities particularly during the rainy season [21, 45]. Furthermore, forests with hilly land forms are more malariogenic, as their slopes form small rapid streams that facilitate breeding of efficient malaria vectors [46]. Forest influences vector distribution and bionomics, and also the distribution of the malaria parasites. Forested areas are primarily inhabited by tribes [47, 48], whose illiteracy and strong beliefs in age old traditions and practices and a fear of outside world leads to reliance on indigenous treatment for malaria [49].
These factors are discussed in detail in the following sections.
Review
Influence of topographic parameters in forested regions
Topographic factors influence malaria transmission dynamics in forests differently compared to other regions [38, 40, 50–52] as forests harbor forest adapted malaria vectors [53, 54] which respond differentially to these parameters due to their genetic makeup and presence of forest influences their ecology to a great extent in comparison to less or no forest areas [18, 28, 52, 55].
Temperature, rainfall, and humidity
In forested highlands of East Africa, average temperature in the previous month and rainfall in the previous two months have shown a linear-quadratic relationship with Anopheles gambiae density [51]. Another study in the same region showed that the ratio of rainfall over precipitation/potential evapo-transpiration was the driving force for An. gambiae and An. arabiensis population increase [40]. The same vector studied in The Gambia showed rapid population increase towards the end of the dry season, and maximally after onset of rains when humidity increases [56]. Generally trees in the forests add moisture in the air by transpiration and help in lowering temperature, thus increasing precipitation. The moist environment and breeding sites created by rainfall increase vector population, their longevity and hence increase malaria transmission [19].
Vegetation
Vegetation near human habitation increases the population of forest malaria vectors and thus increases malaria transmission [57–59]. Villages with more broadleaf forests, and wetland vegetation in Belize and in forested villages of Bangladesh have higher malaria rates [60, 61] due to effective density of forest vectors [61]. Forest vectors usually prefer tree canopy coverage [42, 62] and are known to take shelter in tree holes [63, 64]. Forest flora and sugar availability have also been shown to be crucial determinants of vectorial capacity. The availability of plant sugar increased egg numbers [43, 65] and survival potential of An. gambiae beyond ages at which they are old enough to transmit malaria [66]. In addition, leaves falling into larval habitats assure sustainable micro-climatic conditions and food for larvae, which favor vectors like An. dirus in South-East Asia [52].
Bodies of water
Mosquitoes mature in bodies of water (their larval habitat) and disperse according to their flight range. For example An. gambiae and An. funestus populations were observed decreasing with increasing distance from the Yala river in Kenya [42]. Even a small change in the distance from bodies of water can influence malaria transmission [19, 67]. Anopheles fluviatilis[68], Anopheles maculatus[68, 69] and An. minimus[52, 68, 69] are prevalent near streams of water in forested areas having cooler climate and tree canopy [70], but An. dirus larvae grow well in small, clear and stagnant bodies of water in forested areas of Asia [52, 68]. In Africa, An. gambiae s.s larvae grow better in bodies of water under dense forest canopy rather than sparse forest coverage [71]. Generally, larvae of forest vectors develop better in bodies of water under tree canopy where the water temperature is buffered and usually 3–3.5 degrees Celsius lower than that of sun-exposed bodies of water [71].
Deforestation
Reduction of dense tree shade increases exposure of vector breeding sites and resting places to sunlight, hence altering vector habitats. Studies have shown preference for forest shade by, An. dirus[52, 72], An. fluviatilis[72, 73], An. minimus[72, 74], and An. funestus[74, 75], An. darlingi[72] and contrastingly, preference for sunlight is shown by some of the species of An. gambiae[72, 75], and An. maculatus[68, 69, 76]. Changing density of anophelines due to deforestation has been reported worldwide, and its relation to niche width and sunlight preference were reviewed in meta-analysis/tabulation [72], and it was found that changes in anophelines density and malaria incidence varied by type of development, agriculture, and locality [72]. It was predicted that deforestation in central Africa and tropical America might increase malaria [12, 77], whereas in Asia deforestation would result in reduction in malaria [78]. As predicted in the Sahara region, malaria incidence increased due to deforestation as a consequence of increased vector density of An. gambiae and An. arabiensis[72], and increase in An. funestus and An. gambiae population in Sub-Saharan Africa [72]. Similarly deforestation increased the population of the South American vectors An. darlingi and An. aquasalis[72], accompanied by increased malaria in Guyana and Amazonia [72]. The predicted reduction in malaria in deforested regions of Asia may be due to a decrease in forest-loving (halophobic) vectors like An. dirus in Thailand and An. fluviatilis in India [73, 79]. However, malaria transmission was accelerated by An. minimus due to deforestation in Thailand and India [80], as well as An. culicifacies in Nepal and Sri Lanka [72], and An. philippinensis, An. annularis, and An. varuna in India [80]. A risk of increased malaria in response to deforestation exists if vectors like An. darlingi are present in a deforested habitat [81], as the biting rate of An. darlingi has been estimated to increase 278 times in the deforested regions [82]. Thus deforestation affects malaria transmission depending upon the vector diversity of a particular region.
Entomological parameters of forested regions
Impact of forest and forestation on vector abundance
The vector species in forest ecoregions reflect their preference or adaptability to the forest ecotype. Malaria transmission dynamics in forests may be the output of more than one vector [83]. Different vector species or sympatric sibling species may be present in a particular region whose populations fluctuate according to seasons [52, 84]. In the dense, hilly forested Thai-Myanmar border region, more An. dirus were found at the start while more An. baimaii were found towards the middle of the wet season [52]. In the less forested southern part of Thailand, however, An. cracens dominated over An. baimaii at the beginning of the wet season though by the end of the wet season An. scanloni dominated An. cracens[52, 84]. In the subtropical mountainous forest of northwestern Argentina, An. argyritarsis was more abundant than An. pseudopunctipennis and both the vectors attained their peaks during spring [85]. Among the species of the Gambiae Complex, An. gambiae s.s. is less adapted to hotter conditions than An. arabiensis[86, 87], hence the former is more abundant in forest than desert in comparison with the latter species, as reflected by their spatial and temporal distribution in Africa [29, 54, 88–90]. During dry periods when the primary forest vector (e.g., An. gambiae s.s.) population decreases, the secondary vector (e.g., An. arabiensis) takes over the transmission of malaria.
Manmade forests including significantly large plantation areas or reforestation also cause habitat change and influence malaria vector abundance leading to changes in malaria transmission scenarios. For example malaria increased due to a coffee plantation in Thailand [91], palm plantations in Cameroon [92], Papua New Guinea [93] and Malaysia [94]; rubber plantation in Cameroon [95], Thailand [91] and orchard plantations in Thai-Myanmar and other South-East Asia regions [91, 96, 97]. Commercial plantations and reforestation, which increase human insurgence, increases man-vector encounter and malaria transmission in those areas [78, 91, 96, 97].
Behavior of forest-adapted vector forms
Some non-forest vectors have distinct forest forms that exhibit alteration in chromosomal banding pattern and show altered bionomics compared to their non-forest forms [53, 54, 98]. The variation in vector forms is accompanied by differences in vectorial capacity, biting habits and differential resistance to insecticides, thus influencing vector control strategies [99, 100] in response to malaria transmission [98, 101]. Obsomer et al., 2007 in their review on An. dirus in Asian forested zones, emphasized forest environment, human behavior, and insecticide usage over vector genetics to account for the vector’s behavioral heterogeneity [52], which also supports the earlier views of Trung and co-workers [18, 28]. They reviewed behavioral heterogeneity of anophelines in South-East Asia with reference to forest, hill, and other factors and found that early evening shift in the human biting rhythm of An. dirus A (An. dirus) and An. minimus A (An. minimus Theobald), and higher degree of exophagy are inversely related to distance from forests and hills [52]. The forest form of An. gambiae has shown stronger exophily in southern Sierra Leone whereas the Savannah form was mostly endophilic [98]. Daytime biting by An. dirus was also observed in the forest, where very little sunlight penetrates through the tree canopy [52, 102].
Non-forest vectors showed altered bionomics in the forest. For example An. culicifacies which is mainly endophilic [103] but in dense forests of central India, it is reported mainly exophilic in nature [104]. Similarly An. gambiae was observed to be highly exophilic and anthropophagic in a forested region compared to a non-forested region of southern Sierra [55]. The highest anthropophagic index and sporozoite positivity was observed in the savanna forest region for all four major malaria vectors An. gambiae, An. funestus, An. arabiensis, and An. moucheti in Nigeria [105], and for An. gambiae in Southern Ethiopia [106] and Madagascar [107] in comparison with the less forested rainforest region of south-western Nigeria, where An. arabiensis was largely zoophagic, whereas An. gambiae, An. melas and An. moucheti remained predominantly anthropophagic [108]. The impact of forest/deforestation on vector populations, their bionomics and malaria incidence is summarized in Table 1.
Parasitological factors in relation to vector and host
Plasmodium species distribution in forested regions
Plasmodium in humans is little influenced by forest factors, as its secondary lifecycle is completed in a homeotherm. However, the primary life cycle occurs in an ectothermic vector, which is very much influenced by environment. Intrinsic incubation period is triggered by unknown phenomena [115] but extrinsic incubation period is inversely related to temperature and also depends upon Plasmodium species and the vector [116]. P. vivax and P. falciparum have shorter extrinsic incubation periods and are also the most common human malaria parasites [117]. P. vivax can survive in places like the Central Andes where the weak vector An. pseudopunctipennis and fluctuating environmental conditions prevail and are compensated by the short extrinsic incubation period of P. vivax, long intrinsic incubation periods in the human liver [118], and by forest cover that increases the life expectancy of the vector [109]. Plasmodium species have evolved to fit local vectors, as observed in P. falciparum in rural Cameroon by shortening sporogony [119] as survival of the vector influences Plasmodium distribution [120]. An increase in vectorial capacity of An. gambiae was reported in deforested areas of Kenya as deforestation led to a decrease in duration of sporogony of P. falciparum[109]. Plasmodium malariae, P. knowlesi and P. ovale cases are rare and mainly confined to remote forested areas and are usually underreported as these are often misidentified [121–123].
Risk of primate malaria to humans in forest regions
The presence of a non-human primate Plasmodium species in forest foci poses a constant risk of host switching to nearby human populations due to deforestation, with its associated insurgence of the human population [124]. Anopheles in subgenus Kerteszia (An. Kerteszia cruzii, An. Kerteszia bellator), are vectors of human and simian plasmodia in areas like Atlantic forest in South America [33, 34]. It is possible that zoonosis may be present in such areas, as the parasites found in monkeys (P. simium and P. brasilianum) are genetically similar/related to human plasmodia (P. vivax and P. malariae) [35]. Such cases occur, albeit infrequently, inside the forest or on its edges whose identity may be confused with human Plasmodia being morphologically similar [33]. The simian Plasmodium could switch over to humans as appears to have occurred in the case of P. cynomolgi in India [125], P. simium in Brazil [126, 127], P. knowlesi in Malaysia [20, 124]. The presence of asymptomatic human reservoirs together with infected monkeys could maintain malaria transmission in a situation where routine malaria surveillance and control are difficult [33, 36].
Parasite reservoir and drug resistance
Stability of malaria transmission in endemic areas is also reported to be associated with asymptomatic P. falciparum and P. vivax reservoirs and hypnozoite reservoirs of P. vivax[128, 129]. Asymptomatic malaria is often associated with forested regions of the world [130–133]. Due to the asymptomatic reservoir [131], ‘stable endemic malaria’ is maintained continuously in forested areas [134], and in non-forested areas with unstable environments the reservoir plays a very important role in bridging transmission seasons, as human reservoirs help the parasite in escaping from a harsh environment during which the vector population also decline below the critical transmissible level [134]. For example, intense perennial transmission through an asymptomatic malaria reservoir was reported in the forested riverine areas of Tanzania [131] and Gabon [130]. Asymptomatic patients having sub microscopic presence of parasites [129, 135] act as a reservoir [132, 136, 137] and ready source of infection in vectors [136] and are one of the hidden obstacles in malaria control in forests.
Patients infected with drug-resistant Plasmodium carry transmissible gametocytes for a very long time, and act as reservoirs. Plasmodium falciparum and P. vivax are reported to be more often associated with drug resistance in forested areas where intensity of malaria transmission is higher and malaria control often neglected. For these reasons the forested Thai-Cambodia border is believed to be the “epicenter” for the origin of chloroquine resistance and evolution of multidrug resistance [24, 30, 31]. Recently, partial artemisinin-resistance in P. falciparum has emerged from the same area [24, 32]. The faster dissemination of drug resistant strain is likely due to the presence of some of the very efficient forest vectors in South-East Asia such as An. dirus and An. minimus; these vectors showed 66% and 44% susceptibility to a drug-resistant strain of P. falciparum infected patient blood respectively [28, 52], and the number of oocysts of the drug-resistant type was reportedly higher in An. dirus[52]. Chloroquine-resistant foci have also been found associated with An. dirus in South Asia [52, 138].
Human ecological and socioeconomic factors
Known malariogenic practices of forest natives
Deep forest areas are primarily inhabited by indigenous populations of ethnic minorities and tribals [47, 48] that are in little touch with outer world and mostly dependent on the forest for sustenance [139]. Such communities are mostly illiterate, prone to superstitious beliefs, and poor at communicating with malaria control workers. Tribes inhabiting forests have conserved traditions and practices that have remarkable impacts on malaria transmission [17] thus these forest dwelling people are vulnerable to malaria [21]. Slash and burn is a functional element of forest area farming practices in many parts of the world [17, 140] leading to deforestation and succession of halophilic vectors, hence changes in the malaria transmission pattern [17]. In this type of cultivation, 1–2 members of the family stay in a hut near the farm overnight, which in turn exposes them to malaria vectors [17, 140]. For example, malariogenic conditions are created in the central mountainous and forested part of Vietnam where the Rag Lays tribes practice slash and burn [17, 140], and also by commercial logging, and cattle ranching [141]. Certain ethnic groups in India ritually plaster their houses with fresh cow dung and mud that masks the insecticide on treated walls and render it ineffective for vector control [142]. Traditionally women cover more of their bodies and perhaps for this reason were found to be at lower malaria risk than men [17, 61, 143]. In many tribal cultures both men and women consume alcoholic beverages on a regular basis and this practice results in reduced self-protection against mosquito bites. Interestingly, it has been found that beer consumption increases human attractiveness to An. gambiae in experiments conducted in Burkina Faso [144].
Population migration in forest areas
Populations move within and out of the forest for a variety of reasons and this helps in malaria dissemination [145, 146]. Daily short-distance movement is done for cattle grazing, hunting, fishing, farming, collecting forest products like leaf, wood, fruit, flower and honey, etc.[147]. Such movements increase the contact with efficient malaria vectors when night halt is done in the forest [145, 148, 149] and even in the daylight where vectors like An. dirus prevails [52, 140]. Short-term movement of forest inhabitants to medium distances is observed during sowing and harvesting seasons [38]. Generally this type of movement draws malaria from forested areas to plain field areas [145]. For example, increase in movement of people both within the highlands of New Guinea and also between holo and hyper-endemic lowland areas and the highlands increased malaria spread [150]. Non-forest populations also visit forest areas for animal grazing and wood collection [151]. Refugees have been settled and many resettlement programs have been launched in forest areas, for example, in India, Bangladeshi refugees were shifted to the forested Chittagong hill district and the forested area of the Orissa–Chhattisgarh border near Bastar under ‘Dandakaranya’ project. The refugees contracted malaria from native tribes, which led to epidemics in Bastar [152, 153]. According to Lindsay et al., “Many of the first European settlers in Africa who sought refuge from the heat and diseases of the plains by moving to the cool and salubrious highlands also carried malaria with them” [154].
Poor infrastructure and communication
Forest inhabitants usually construct houses with mud, and infection increased among those living in muddy or poorly constructed houses near vector breeding places in Egypt [155], Ethiopia [156] and Kenya [42]. An. minimus A in central Vietnam exhibits a high anthropophilic and endophagic ratio, most likely influenced by the largely open houses with incomplete walls that allow it to easily detect human stimuli and enter into houses [157].
Health infrastructure and surveillance are neglected in remote forest areas [158] and it becomes impractical in the rainy season where road infrastructure is poor or absent [159]. Unfortunately, the rainy season is also the peak transmission period in most malarious areas [160] when adequate surveillance is required as patients find it difficult to access health facilities due to climatic and communication problems [21, 45]. Thus the indigenous population generally relies on the health practices of local faith healers and/or quacks [161–163]. Modern health infrastructure among sparsely distributed forest settlements is far from adequate [139, 164]. It is reported that treatment seeking behavior is inversely related to the distance from a health facility and communication problems [21, 165, 166].
People’s conceptions and cooperation
Different perceptions about malaria have been reported among tribal groups in different parts of the world. Certain tribes believe that malaria is caused by spirits, angry deities, black magic, or consider it a self-limiting fever in countries like India [167] and southwest Ethiopia [165]. Low cost treatment with traditional medicines, good accessibility and good communication with quacks are preferred most in remote forest areas far from government health centers as reported in rural Ethiopia [168]. In the forest areas health seeking is directly related to culture, faith and affordability of the health care [21, 165]. It is observed that “health services may be underutilized and several health care instructions may be ineffective or ignored in traditional and transitional societies where people’s ideas and behavioral patterns conflict with the knowledge being passed to them” [161]. Generally poverty is the next most important factor in accessing a distant healthcare facility besides illiteracy, superstition, and cultural faith among the indigenous populations of most forest regions in Bangladesh [143]. Poor forest inhabitants do not own a bed net primarily due to a lack of availability of affordable nets in spite of the fact that they know the benefits of nets and would want to use them [169, 170]. Hence not having bed nets, poor people cover their body and face with blankets, burn wood and shrubs to ward off mosquitoes, and due to lack of affordable modern medical facility they practice traditional remedies [171]. Surveillance is poor in remote forested areas [172] and presumptive treatment using antimalarials in low doses is taken for all sorts of fever [173], accelerating the development of drug resistance [174, 175]. Thus the origin and evolution of drug resistance was reported first in forested remote areas like Cambodia and the Thai-Myanmar border areas [176].
Suggestions for overcoming major challenges to curbing malaria transmission in forest ecosystems
Forests escape malaria control efforts mainly due to inadequate roads and poor communication [21]. Communication infrastructure development should be the first priority as this will open new ways and opportunity to the inhabitants and boost their socio-economic status. Establishing and development of accessible health facilities and making them available to inhabitants by reducing gaps between locals and health providers can improve the situation. Social awareness for malaria control and involvement of traditional health providers and NGOs may help in filling the gaps in backward forested areas [21, 162].
Malaria transmission respects no political boundaries and in forested borders people often migrate across the borders and carry malaria as seen in Thai-Myanmar border. The population migrations in forested regions are due to various reasons but mainly due to availability of work [49, 97]. Military camps, radar stations, police, and other armed forces camps and big development projects like road and other infrastructure development, mining, agricultural activities like tea, rubber, coffee plantation and construction of dams employ large numbers of migrant workers. These people acquire malaria easily from natives [152]. The vulnerable migrants and refugees need to be screened and treated for malaria promptly. The military and other camps in forested region are required to put large efforts to fight against malaria together with the local people.
Tribals in forest areas, often hidden from outer world, are generally conservative and reluctant in treatment seeking. Social inhibitions, ignorance, superstitions, and negligence promote tolerance in symptomatic or asymptomatic malaria carriers, who do not seek treatment themselves and act as reservoirs of malaria parasites [21, 143]. Mass screening may be useful in order to assess malaria sero-positivity among communities where asymptomatic malaria prevails and people are less prompt in seeking treatment [23]. Antigen based species specific rapid diagnostic test kits should be used in forested areas for instant on-site detection and treatment. Primitive nomadic tribes need to be accessed by the healthcare providers and should be given place in social structure and are required to be encouraged to use healthcare and other facilities.
Forests are reported to be the epicenter of drug resistance spread and low attainment rates in malaria control. Therefore, strictly controlled administration of antimalarials with periodic assessment of drug resistance status is suggested in forested areas. Molecular markers associated with antimalarial resistance need to be evaluated in the high transmission forested areas and can help in saving the valuable antimalarials for posterity [176].
Deforestation and climate change can conspire with other factors of forest-malaria to cause a boom in vector species and increases in their vectorial dimensions [20, 82]. Ecological succession of malaria vectors attributable to climate and ecological changes needs to be explored and frequent inspection of abundance of vector species and an update on their distribution pattern, bionomics and behavioral changes in forested areas are justified for effective vector control measures.
Conclusion
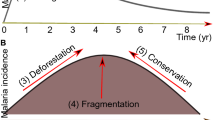
Strong links exist between various factors influencing malaria transmission dynamics in forest ecosystems. Slight change in any of the factors affects the others, culminating in a different transmission pattern. Change in the vegetation cover and deforestation alters the distribution and behavior of malaria vectors. Human ecological and socioeconomic traits also affect exophagy, anthropophagy, biting rhythm, and resting behavior of vectors. The genetic traits of communities residing in forest areas and their health-seeking behaviors are crucial for parasite prevalence and precipitation of drug resistance. Moreover, insurgence of human populations and developmental activities in forests are important in altering the transmission pattern. Thus malaria transmission in forest areas is a complex process involving interplay between topographical, entomological, parasitological and human factors (Figure 1). Studies carried out in forested areas in different parts of the world generally focus on one or a few of the many factors which may not be adequate in understanding complexities of the malaria situation arising out of interaction of several factors. This review can help understand the complex interlinks between different factors acting simultaneously to influence malaria transmission. Predictive models of malaria transmission can be worked out for forest areas of different ecoregions by taking into account all relevant weighted factors. Based on in-depth understanding of the intricate relationship of various parameters, situation specific vector/malaria control strategies can be developed and implemented to address malaria problem in the forests. Although implementation of such strategies is primarily a responsibility of the government and local health authorities, NGO workers, local medicine practitioners and traditional faith healers would be important as these are acceptable to the communities residing in remote forested areas. At the same time establishing a good rapport through interaction between implementers and the communities is essential for the success and sustenance of malaria control programs in forest ecosystems.
References
WHO: World Malaria Report: 2013. 2013, Switzerland: World Health Organization, 1-199.
Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, Tatem AJ, Hay SI: A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011, 10: 378-10.1186/1475-2875-10-378.
Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI: A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012, 6: e1814-10.1371/journal.pntd.0001814.
Collins WE, Jeffery GM: Plasmodium malariae: parasite and disease. Clin Microbiol Rev. 2007, 20: 579-592. 10.1128/CMR.00027-07.
Obare P, Ogutu B, Adams M, Odera JS, Lilley K, Dosoo D, Adhiambo C, Owusu-Agyei S, Binka F, Wanja E, Johnson J: Misclassification of Plasmodium infections by conventional microscopy and the impact of remedial training on the proficiency of laboratory technicians in species identification. Malar J. 2013, 12: 113-10.1186/1475-2875-12-113.
Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, Dolecek C, Hien TT, Do Rosario VE, Arez AP, Pinto J, Michon P, Escalante AA, Nosten F, Burke M, Lee R, Blaze M, Otto TD, Barnwell JW, Pain A, Williams J, White NJ, Day NP, Snounou G, Lockhart PJ, Chiodini PL, Imwong M, Polley SD: Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010, 201: 1544-1550. 10.1086/652240.
White NJ: Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis. 2008, 46: 172-173. 10.1086/524889.
Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S: Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J Infect Dis. 2009, 199: 1143-1150. 10.1086/597414.
Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ: A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004, 363: 1017-1024. 10.1016/S0140-6736(04)15836-4.
Van den Eede P, Van HN, Van Overmeir C, Vythilingam I, Duc TN, Hung Le X, Manh HN, Anne J, D’Alessandro U, Erhart A: Human Plasmodium knowlesi infections in young children in central Vietnam. Malar J. 2009, 8: 249-10.1186/1475-2875-8-249.
Vythilingam I, Noorazian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, Norparina I, Noorrain A, Lokmanhakim S: Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors. 2008, 1: 26-10.1186/1756-3305-1-26.
Guerra CA, Snow RW, Hay SI: A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006, 100: 189-204. 10.1179/136485906X91512.
Snelder DJ, Lasco RD, NetLibrary I: Smallholder Tree Growing for Rural Development and Environmental Services Lessons from Asia. 2008, Dordrecht: Springer, 1-493.
Breckle S-W, Walter H: Walter’s Vegetation of the Earth: The Ecological Systems of the Geo Biosphere. 2002, Berlin [u.a.]: Springer, 1-236.
Achard F, Eva HD, Stibig HJ, Mayaux P, Gallego J, Richards T, Malingreau JP: Determination of deforestation rates of the world’s humid tropical forests. Science. 2002, 297: 999-1002. 10.1126/science.1070656.
Mayaux P, Holmgren P, Achard F, Eva H, Stibig HJ, Branthomme A: Tropical forest cover change in the 1990s and options for future monitoring. Philos Trans R Soc Lond B Biol Sci. 2005, 360: 373-384. 10.1098/rstb.2004.1590.
Erhart A, Ngo DT, Phan VK, Ta TT, Van Overmeir C, Speybroeck N, Obsomer V, Le XH, Le KT, Coosemans M, D’Alessandro U: Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005, 4: 58-10.1186/1475-2875-4-58.
Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJ, Coosemans M: Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health. 2005, 10: 251-262. 10.1111/j.1365-3156.2004.01378.x.
Walker M, Winskill P, Basanez MG, Mwangangi JM, Mbogo C, Beier JC, Midega JT: Temporal and micro-spatial heterogeneity in the distribution of Anopheles vectors of malaria along the Kenyan coast. Parasit Vectors. 2013, 6: 311-10.1186/1756-3305-6-311.
Alias H, Surin J, Mahmud R, Shafie A, Mohd Zin J, Mohamad Nor M, Ibrahim AS, Rundi C: Spatial distribution of malaria in Peninsular Malaysia from 2000 to 2009. Parasit Vectors. 2014, 7: 186-10.1186/1756-3305-7-186.
Ribera JM, Hausmann-Muela S: The straw that breaks the camel’s back. Redirecting health-seeking behavior studies on malaria and vulnerability. Med Anthropol Q. 2011, 25: 103-121. 10.1111/j.1548-1387.2010.01139.x.
Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI: Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010, 376: 1579-1591. 10.1016/S0140-6736(10)61301-3.
Hamainza B, Moonga H, Sikaala CH, Kamuliwo M, Bennett A, Eisele TP, Miller J, Seyoum A, Killeen GF: Monitoring, characterization and control of chronic, symptomatic malaria infections in rural Zambia through monthly household visits by paid community health workers. Malar J. 2014, 13: 128-10.1186/1475-2875-13-128.
Salud OM: Global Malaria Control and Elimination: Report of a Meeting on Containment of Artemisinin Tolerance, 19 January 2008, Geneva, Switzerland. 2008, Geneva: WHO, 1-29.
Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW: The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004, 4: 327-336. 10.1016/S1473-3099(04)01043-6.
Carter R, Mendis KN: Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002, 15: 564-594. 10.1128/CMR.15.4.564-594.2002.
Fuller DO, Parenti MS, Hassan AN, Beier JC: Linking land cover and species distribution models to project potential ranges of malaria vectors: an example using Anopheles arabiensis in Sudan and Upper Egypt. Malar J. 2012, 11: 264-10.1186/1475-2875-11-264.
Trung HD, Van Bortel W, Sochantha T, Keokenchanh K, Quang NT, Cong LD, Coosemans M: Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia. Trop Med Int Health. 2004, 9: 230-237. 10.1046/j.1365-3156.2003.01179.x.
Charlwood JD, Vij R, Billingsley PF: Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am J Trop Med Hyg. 2000, 62: 726-732.
Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, Min Lwin M, Zaw T, Annerberg A, De Radigues X, Nosten F: Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health. 2008, 13: 91-98. 10.1111/j.1365-3156.2007.01978.x.
Nosten F, ter Kuile F, Chongsuphajaisiddhi T, Luxemburger C, Webster HK, Edstein M, Phaipun L, Thew KL, White NJ: Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet. 1991, 337: 1140-1143. 10.1016/0140-6736(91)92798-7.
Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L: Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010, 8: 272-280.
Duarte AM, Pereira DM, de Paula MB, Fernandes A, Urbinatti PR, Ribeiro AF, Mello MH, Matos MO, Mucci LF, Fernandes LN, Natal D, Malafronte RS: Natural infection in anopheline species and its implications for autochthonous malaria in the Atlantic Forest in Brazil. Parasit Vectors. 2013, 6: 58-10.1186/1756-3305-6-58.
Deane LM, Ferreira Neto JA, Lima MM: The vertical dispersion of Anopheles (Kerteszia) cruzi in a forest in southern Brazil suggests that human cases of malaria of simian origin might be expected. Mem Inst Oswaldo Cruz. 1984, 79: 461-463. 10.1590/S0074-02761984000400011.
Waters AP, Higgins DG, McCutchan T: Evolutionary relatedness of some primate models of Plasmodium. Mol Biol Evol. 1993, 10: 914-923.
De Castro Duarte AMR, Malafronte RS, Cerutti C, Curado I, De Paiva BR, Maeda AY, Yamasaki T, Summa MEL, Neves DVDA, De Oliveira SG: Natural Plasmodium infections in Brazilian wild monkeys: reservoirs for human infections?. Acta Trop. 2008, 107: 179-185. 10.1016/j.actatropica.2008.05.020.
Rosenberg R: Forest malaria in Bangladesh. III. Breeding habits of Anopheles dirus. Am J Trop Med Hyg. 1982, 31: 192-201.
Rubio-Palis Y, Zimmerman RH: Ecoregional classification of malaria vectors in the neotropics. J Med Entomol. 1997, 34: 499-510.
Minakawa N, Sonye G, Mogi M, Githeko A, Yan G: The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002, 39: 833-841. 10.1603/0022-2585-39.6.833.
Koenraadt CJ, Githeko AK, Takken W: The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Trop. 2004, 90: 141-153. 10.1016/j.actatropica.2003.11.007.
Aragao MB: Some microclimatic measurements, in a forest of the “bromeliad-malaria” region, in Santa Catarina, Brazil. III. Conditions of humidity measured with hygrographs. Rev Bras Med. 1960, 12: 395-414.
Zhou G, Munga S, Minakawa N, Githeko AK, Yan G: Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg. 2007, 77: 29-35.
Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A: Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J. 2007, 6: 113-10.1186/1475-2875-6-113.
Okech BA, Gouagna LC, Yan G, Githure JI, Beier JC: Larval habitats of Anopheles gambiae s.s. (Diptera: Culicidae) influences vector competence to Plasmodium falciparum parasites. Malar J. 2007, 6: 50-10.1186/1475-2875-6-50.
Lim ES: Current status of malaria in Malaysia. Southeast Asian J Trop Med Public Health. 1992, 23 (Suppl 4): 43-49.
Rattanarithikul R, Green CA, Panyim S, Noigamol C, Chanaimongkol S, Mahapibul P: Larval habitats of malaria vectors and other Anopheles mosquitoes around a transmission focus in northwestern Thailand. J Am Mosq Control Assoc. 1995, 11: 428-433.
Mandal H, Mukherjee S, Datta A, India AS: India, an Illustrated Atlas of Tribal World. 2002, Kolkata: Anthropological Survey of India Calcutta, Ministry of Tourism and Culture, Dept. of Culture, Govt. of India, 1-106.
Kusel J, Adler E: Forest Communities, Community Forests. 2003, Lanham, Md.: Rowman & Littlefield Publishers, 1-276.
Pichainarong N, Chaveepojnkamjorn W: Malaria infection and life-style factors among hilltribes along the Thai-Myanmar border area, northern Thailand. Southeast Asian J Trop Med Public Health. 2004, 35: 834-839.
Nath DC, Mwchahary DD: Association between climatic variables and malaria incidence: a study in Kokrajhar district of Assam, India. Glob J Health Sci. 2013, 5: 90-106.
Kristan M, Abeku TA, Beard J, Okia M, Rapuoda B, Sang J, Cox J: Variations in entomological indices in relation to weather patterns and malaria incidence in East African highlands: implications for epidemic prevention and control. Malar J. 2008, 7: 231-10.1186/1475-2875-7-231.
Obsomer V, Defourny P, Coosemans M: The Anopheles dirus complex: spatial distribution and environmental drivers. Malar J. 2007, 6: 26-10.1186/1475-2875-6-26.
Carnevale P, Le Goff G, Toto JC, Robert V: Anopheles nili as the main vector of human malaria in villages of southern Cameroon. Med Vet Entomol. 1992, 6: 135-138. 10.1111/j.1365-2915.1992.tb00590.x.
Wanji S, Tanke T, Atanga SN, Ajonina C, Nicholas T, Fontenille D: Anopheles species of the mount Cameroon region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Int Health. 2003, 8: 643-649. 10.1046/j.1365-3156.2003.01070.x.
Bockarie MJ, Service MW, Toure YT, Traore S, Barnish G, Greenwood BM: The ecology and behaviour of the forest form of Anopheles gambiae s.s. Parassitologia. 1993, 35 (Suppl): 5-8.
Jawara M, Pinder M, Drakeley CJ, Nwakanma DC, Jallow E, Bogh C, Lindsay SW, Conway DJ: Dry season ecology of Anopheles gambiae complex mosquitoes in The Gambia. Malar J. 2008, 7: 156-10.1186/1475-2875-7-156.
Mouchet J, Carnevale P: Impact of changes in the environment on vector-transmitted diseases. Sante. 1997, 7: 263-269.
Pavlovsky EN: Natural Nidality of Transmissible Diseases: With Special Reference to the Landscape Epidemiology of Zooanthroponoses. 1966, Urbana: University of Illinois Press, 1-261.
O’Loughlin SM, Okabayashi T, Honda M, Kitazoe Y, Kishino H, Somboon P, Sochantha T, Nambanya S, Saikia PK, Dev V, Walton C: Complex population history of two Anopheles dirus mosquito species in Southeast Asia suggests the influence of Pleistocene climate change rather than human-mediated effects. J Evol Biol. 2008, 21: 1555-1569. 10.1111/j.1420-9101.2008.01606.x.
Hakre S, Masuoka P, Vanzie E, Roberts DR: Spatial correlations of mapped malaria rates with environmental factors in Belize. Central America Int J Health Geogr. 2004, 3: 6-10.1186/1476-072X-3-6.
Haque U, Sunahara T, Hashizume M, Shields T, Yamamoto T, Haque R, Glass GE: Malaria prevalence, risk factors and spatial distribution in a hilly forest area of Bangladesh. PLoS One. 2011, 6: e18908-10.1371/journal.pone.0018908.
Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G: Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2006, 74: 772-778.
Yadav RS, Sharma VP, Chand SK: Mosquito breeding and resting in treeholes in a forest ecosystem in Orissa. Indian J Malariol. 1997, 34: 8-16.
Gunasekaran K, Sahu SS, Parida SK, Sadanandane C, Jambulingam P, Das PK: Anopheline fauna of Koraput district, Orissa state, with particular reference to transmission of malaria. Indian J Med Res. 1989, 89: 340-343.
Gary RE, Foster WA: Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med Vet Entomol. 2004, 18: 102-107. 10.1111/j.0269-283X.2004.00483.x.
Okech BA, Gouagna LC, Killeen GF, Knols BG, Kabiru EW, Beier JC, Yan G, Githure JI: Influence of sugar availability and indoor microclimate on survival of Anopheles gambiae (Diptera: Culicidae) under semifield conditions in western Kenya. J Med Entomol. 2003, 40: 657-663. 10.1603/0022-2585-40.5.657.
Berti J, Zimmerman R, Amarista J: Adult abundance, biting behavior and parity of Anopheles aquasalis, Curry 1932 in two malarious areas of Sucre State, Venezuela. Mem Inst Oswaldo Cruz. 1993, 88: 363-369. 10.1590/S0074-02761993000300004.
Lindsay S: Environmental Management for Malaria Control in the East Asia and Pacific (EAP) Region. 2004, Washington: World Bank, 1-46.
Kobayashi J, Somboon P, Keomanila H, Inthavongsa S, Nambanya S, Inthakone S, Sato Y, Miyagi I: Malaria prevalence and a brief entomological survey in a village surrounded by rice fields in Khammouan province, Lao PDR. Trop Med Int Health. 2000, 5: 17-21. 10.1046/j.1365-3156.2000.00516.x.
Sahu SS, Parida SK, Sadanandane C, Gunasekaran K, Jambulingam P, Das PK: Breeding habitats of malaria vectors: A. fluviatilis, A. annularis and A. culicifacies, in Koraput district, Orissa. Indian J Malariol. 1990, 27: 209-216.
Tuno N, Okeka W, Minakawa N, Takagi M, Yan G: Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in western Kenya highland forest. J Med Entomol. 2005, 42: 270-277. 10.1603/0022-2585(2005)042[0270:SOAGSS]2.0.CO;2.
Yasuoka J, Levins R: Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007, 76: 450-460.
Sharma VP, Prasittisuk C, Kondrashin AV: Forest Malaria in Southeast Asia. Proceedings of an Informal Consultative Meeting WHO/MRC; New Delhi. Edited by: Sharma VP, Kondrashin AV. 1991, New Delhi: Malaria Research Centre, 29-53.
Service MW: Agricultural development and arthropod-borne diseases: a review. Rev Saude Publica. 1991, 25: 165-178.
Service MW: Demography and Vector-Borne Diseases. 1989, Boca Raton: CRC Press, 1-416.
Gilles HM, Warrell DA: Bruce-Chwatt’s Essential Malariology. 1999, London: A Hodder Arnold Publication, 1-348.
Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sanchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, Patz JA: Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009, 81: 5-12.
Walsh JF, Molyneux DH, Birley MH: Deforestation: effects on vector-borne disease. Parasitology. 1993, 106 (Suppl): S55-S75.
Taylor D: Seeing the forests for the more than the trees. Environ Health Perspect. 1997, 105: 1186-1191.
Das NG, Talukdar PK, Das SC: Epidemiological and entomological aspects of malaria in forest-fringed villages of Sonitpur district, Assam. J Vector Borne Dis. 2004, 41: 5-9.
Elliott R: The influence of vector behavior on malaria transmission. Am J Trop Med Hyg. 1972, 21: 755-763.
Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA: The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006, 74: 3-11.
Smith DL, Ruktanonchai N: Progress in modelling malaria transmission. Adv Exp Med Biol. 2010, 673: 1-12. 10.1007/978-1-4419-6064-1_1.
Baimai V, Kijchalao U, Sawadwongporn P, Green CA: Geographic distribution and biting behaviour of four species of the Anopheles dirus complex (Diptera: Culicidae) in Thailand. Southeast Asian J Trop Med Public Health. 1988, 19: 151-161.
Dantur Juri MJ, Claps GL, Santana M, Zaidenberg M, Almiron WR: Abundance patterns of Anopheles pseudopunctipennis and Anopheles argyritarsis in northwestern Argentina. Acta Trop. 2010, 115: 234-241. 10.1016/j.actatropica.2010.04.003.
Hogg JC, Thomson MC, Hurd H: Comparative fecundity and associated factors for two sibling species of the Anopheles gambiae complex occurring sympatrically in The Gambia. Med Vet Entomol. 1996, 10: 385-391. 10.1111/j.1365-2915.1996.tb00761.x.
Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Burkot TR, Harbach RE, Hay SI: A global map of dominant malaria vectors. Parasit Vectors. 2012, 5: 69-10.1186/1756-3305-5-69.
Kirby MJ, Lindsay SW: Responses of adult mosquitoes of two sibling species, Anopheles arabiensis and A. gambiae s.s. (Diptera: Culicidae), to high temperatures. Bull Entomol Res. 2004, 94: 441-448.
Coetzee M, Craig M, le Sueur D: Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000, 16: 74-77. 10.1016/S0169-4758(99)01563-X.
Ebenezer A, Okiwelu SN, Agi PI, Noutcha MA, Awolola TS, Oduola AO: Species composition of the Anopheles gambiae complex across eco-vegetational zones in Bayelsa State, Niger Delta region, Nigeria. J Vector Borne Dis. 2012, 49: 164-167.
Singhasivanon P, Thimasarn K, Yimsamran S, Linthicum K, Nualchawee K, Dawreang D, Kongrod S, Premmanisakul N, Maneeboonyang W, Salazar N: Malaria in tree crop plantations in south-eastern and western provinces of Thailand. Southeast Asian J Trop Med Public Health. 1999, 30: 399-404.
Tanga MC, Ngundu WI, Tchouassi PD: Daily survival and human blood index of major malaria vectors associated with oil palm cultivation in Cameroon and their role in malaria transmission. Trop Med Int Health. 2011, 16: 447-457. 10.1111/j.1365-3156.2011.02726.x.
Pluess B, Mueller I, Levi D, King G, Smith TA, Lengeler C: Malaria–a major health problem within an oil palm plantation around Popondetta. Papua New Guinea Malar J. 2009, 8: 56-
Chang MS, Hii J, Buttner P, Mansoor F: Changes in abundance and behaviour of vector mosquitoes induced by land use during the development of an oil palm plantation in Sarawak. Trans R Soc Trop Med Hyg. 1997, 91: 382-386. 10.1016/S0035-9203(97)90248-0.
Bigoga JD, Nanfack FM, Awono-Ambene PH, Patchoke S, Atangana J, Otia VS, Fondjo E, Moyou RS, Leke RG: Seasonal prevalence of malaria vectors and entomological inoculation rates in the rubber cultivated area of Niete. South Region of Cameroon Parasit Vectors. 2012, 5: 197-10.1186/1756-3305-5-197.
Basurko C, Demattei C, Han-Sze R, Grenier C, Joubert M, Nacher M, Carme B: Deforestation, agriculture and farm jobs: a good recipe for Plasmodium vivax in French Guiana. Malar J. 2013, 12: 90-10.1186/1475-2875-12-90.
Wangroongsarb P, Sudathip P, Satimai W: Characteristics and malaria prevalence of migrant populations in malaria-endemic areas along the Thai-Cambodian border. Southeast Asian J Trop Med Public Health. 2012, 43: 261-269.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Burkot TR, Harbach RE, Hay SI: The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010, 3: 117-10.1186/1756-3305-3-117.
Ngom EH, Ndione J-A, Ba Y, Konate L, Faye O, Diallo M, Dia I: Spatio-temporal analysis of host preferences and feeding patterns of malaria vectors in the sylvo-pastoral area of Senegal: impact of landscape classes. Parasit Vectors. 2013, 6: 332-10.1186/1756-3305-6-332.
Massebo F, Balkew M, Gebre-Michael T, Lindtjorn B: Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in South-West Ethiopia. Parasit Vectors. 2013, 6: 44-10.1186/1756-3305-6-44.
Subbarao SK, Sharma VP: Anopheline species complexes & malaria control. Indian J Med Res. 1997, 106: 164-173.
Rosenberg R, Maheswary NP: Forest malaria in Bangladesh. II Transmission by Anopheles dirus. Am J Trop Med Hyg. 1982, 31: 183-191.
Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, Mahmoodi M, Akbarzadeh K, Sartipi M: Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan province, south-east Islamic Republic of Iran. E Mediterr Health J = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2011, 17: 439-445.
Singh N, Singh OP, Sharma VP: Dynamics of malaria transmission in forested and deforested regions of Mandla District, central India (Madhya Pradesh). J Am Mosq Control Assoc. 1996, 12: 225-234.
Okwa OO, Akinmolayan FI, Carter V, Hurd H: Transmission dynamics of malaria in four selected ecological zones of Nigeria in the rainy season. Ann Afr Med. 2009, 8: 1-9. 10.4103/1596-3519.55756.
Tirados I, Costantini C, Gibson G, Torr SJ: Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006, 20: 425-437. 10.1111/j.1365-2915.2006.652.x.
Fontenille D, Lepers JP, Coluzzi M, Campbell GH, Rakotoarivony I, Coulanges P: Malaria transmission and vector biology on Sainte Marie Island, Madagascar. J Med Entomol. 1992, 29: 197-202.
Awolola TS, Okwa O, Hunt RH, Ogunrinade AF, Coetzee M: Dynamics of the malaria-vector populations in coastal Lagos, south-western Nigeria. Ann Trop Med Parasitol. 2002, 96: 75-82. 10.1179/000349802125000538.
Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G: Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerg Infect Dis. 2008, 14: 1533-1538. 10.3201/eid1410.070781.
Oyewole IO, Awolola TS, Ibidapo CA, Oduola AO, Okwa OO, Obansa JA: Behaviour and population dynamics of the major anopheline vectors in a malaria endemic area in southern Nigeria. J Vector Borne Dis. 2007, 44: 56-64.
Nanda N, Yadav RS, Subbarao SK, Joshi H, Sharma VP: Studies on Anopheles fluviatilis and Anopheles culicifacies sibling species in relation to malaria in forested hilly and deforested riverine ecosystems in northern Orissa, India. J Am Mosq Control Assoc. 2000, 16: 199-205.
Socheath S, Seng C, RathT S, Deesin V, Deesin T, Apiwathanasorn C: Study on bionomics of principal malaria vectors in Kratie province, Cambodia. Southeast Asian J Trop Med Public Health. 2000, 31 (Suppl 1): 106-110.
Charlwood JD: Biological variation in Anopheles darlingi Root. Mem Inst Oswaldo Cruz. 1996, 91: 391-398.
Tadei WP, Dutary Thatcher B: Malaria vectors in the Brazilian amazon: Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop Sao Paulo. 2000, 42: 87-94. 10.1590/S0036-46652000000200005.
Baird JK: Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev. 2009, 22: 508-534. 10.1128/CMR.00008-09.
Pampana E: A Textbook of Malaria Eradication. 1969, London: Oxford U.P., 1-593.
Oaks SC: Malaria: Obstacles and Opportunities: A Report of the Committee for the Study on Malaria Prevention and Control: Status Review and Alternative Strategies, Division of International Health, Institute of Medicine. 1991, Washington, D.C.: National Academy Press, 1-309.
Lardeux F, Loayza P, Bouchite B, Chavez T: Host choice and human blood index of Anopheles pseudopunctipennis in a village of the Andean valleys of Bolivia. Malar J. 2007, 6: 8-10.1186/1475-2875-6-8.
Robert V, Le Goff G, Toto JC, Essong J, Verhave JP: Early sporogonic development in local vectors of Plasmodium falciparum in rural Cameroon. Mem Inst Oswaldo Cruz. 1994, 89 (Suppl 2): 23-26.
Bellan SE: The importance of age dependent mortality and the extrinsic incubation period in models of mosquito-borne disease transmission and control. PLoS One. 2010, 5: e10165-10.1371/journal.pone.0010165.
Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B: Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008, 46: 165-171. 10.1086/524888.
Van der Palen M, Gillet P, Bottieau E, Cnops L, Van Esbroeck M, Jacobs J: Test characteristics of two rapid antigen detection tests (SD FK50 and SD FK60) for the diagnosis of malaria in returned travellers. Malar J. 2009, 8: 90-10.1186/1475-2875-8-90.
Bharti PK, Chand SK, Singh MP, Mishra S, Shukla MM, Singh R, Singh N: Emergence of a new focus of Plasmodium malariae in forest villages of district Balaghat, Central India: implications for the diagnosis of malaria and its control. Trop Med Int Health. 2013, 18: 12-17. 10.1111/tmi.12005.
Lee KS, Divis PC, Zakaria SK, Matusop A, Julin RA, Conway DJ, Cox-Singh J, Singh B: Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 2011, 7: e1002015-10.1371/journal.ppat.1002015.
Kalra NL: Emergence of malaria zoonosis of simian origin as natural phenomenon in Greater Nicobars, Andaman & Nicobar islands–a preliminary note. J Commun Dis. 1980, 12: 6-
Deane LM, Deane MP, Ferreira Neto J: Studies on transmission of simian malaria and on a natural infection of man with Plasmodium simium in Brazil. Bull World Health Organ. 1966, 35: 805-808.
Deane LM: Simian malaria in Brazil. Mem Inst Oswaldo Cruz. 1992, 87 (Suppl 3): 1-20.
da Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM, Ferreira MU: Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012, 121: 281-291. 10.1016/j.actatropica.2011.10.001.
Bousema T, Drakeley C: Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011, 24: 377-410. 10.1128/CMR.00051-10.
Nkoghe D, Akue JP, Gonzalez JP, Leroy EM: Prevalence of Plasmodium falciparum infection in asymptomatic rural Gabonese populations. Malar J. 2011, 10: 33-10.1186/1475-2875-10-33.
Drakeley CJ, Akim NI, Sauerwein RW, Greenwood BM, Targett GA: Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans R Soc Trop Med Hyg. 2000, 94: 472-476. 10.1016/S0035-9203(00)90056-7.
Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH: Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005, 42: 777-779. 10.1603/0022-2585(2005)042[0777:ACOPSA]2.0.CO;2.
Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP: High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002, 66: 641-648.
Babiker HA, Abdel-Muhsin AM, Ranford-Cartwright LC, Satti G, Walliker D: Characteristics of Plasmodium falciparum parasites that survive the lengthy dry season in eastern Sudan where malaria transmission is markedly seasonal. Am J Trop Med Hyg. 1998, 59: 582-590.
Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW: Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004, 3: 18-10.1186/1475-2875-3-18.
Pethleart A, Prajakwong S, Suwonkerd W, Corthong B, Webber R, Curtis C: Infectious reservoir of Plasmodium infection in Mae Hong Son Province, north-west Thailand. Malar J. 2004, 3: 34-10.1186/1475-2875-3-34.
Wells TN, Burrows JN, Baird JK: Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010, 26: 145-151. 10.1016/j.pt.2009.12.005.
Das SC, Baruah I: Incrimination of Anopheles minimus Theobald and Anopheles balabacensis balabacensis Baisas (A. dirus) as malaria vectors in Mizoram. Indian J Malariol. 1985, 22: 53-55.
Adak DK: Demography and Health Profile of the Tribals : A Study of M.P. 2003, New Delhi: Anmol Publications
Peeters Grietens K, Xuan XN, Van Bortel W, Duc TN, Ribera JM, Ba Nhat T, Van KP, Le Xuan H, D’Alessandro U, Erhart A: Low perception of malaria risk among the Ra-glai ethnic minority in south-central Vietnam: implications for forest malaria control. Malar J. 2010, 9: 23-10.1186/1475-2875-9-23.
Myers N: Tropical forests: present status and future outlook. Clim Change. 1991, 19: 3-32. 10.1007/BF00142209.
Yadav SP, Kalundha RK, Sharma RC: Sociocultural factors and malaria in the desert part of Rajasthan, India. J Vector Borne Dis. 2007, 44: 205-212.
Hossain SM, Bhuiya A, Rasheed S: Correlates of perceived malarial episodes and treatment-seeking behavior in a malaria-endemic rural area in Bangladesh. Southeast Asian J Trop Med Public Health. 2001, 32: 707-719.
Lefevre T, Gouagna LC, Dabire KR, Elguero E, Fontenille D, Renaud F, Costantini C, Thomas F: Beer consumption increases human attractiveness to malaria mosquitoes. PLoS One. 2010, 5: e9546-10.1371/journal.pone.0009546.
Somboon P, Aramrattana A, Lines J, Webber R: Entomological and epidemiological investigations of malaria transmission in relation to population movements in forest areas of north-west Thailand. Southeast Asian J Trop Med Public Health. 1998, 29: 3-9.
Martens P, Hall L: Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000, 6: 103-109. 10.3201/eid0602.000202.
Sahu SS, Gunasekaran K, Jambulingam P: Bionomics of Anopheles minimus and An. fluviatilis (Diptera: Culicidae) in east-central India, endemic for falciparum malaria: human landing rates, host feeding, and parity. J Med Entomol. 2009, 46: 1045-1051. 10.1603/033.046.0511.
Cai X, Deng D, Wu K, Tang L, Lan C, Gu Z, He Y, Wang K, Wu D, Du J: A study on human behavior and socioeconomic factors affecting malaria transmission and control in Qiongzhong, Hainan. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi = Chin J Parasitol Parasit Dis. 1995, 13: 89-93.
Butraporn P, Sornmani S, Hungsapruek T: Social, behavioural, housing factors and their interactive effects associated with malaria occurrence in east Thailand. Southeast Asian J Trop Med Public Health. 1986, 17: 386-392.
Radford AJ, Van Leeuwen H, Christian SH: Social aspects in the changing epidemiology of malaria in the highlands of New Guinea. Ann Trop Med Parasitol. 1976, 70: 11-23.
Sanh NH, Van Dung N, Thanh NX, Trung TN, Van Co T, Cooper RD: Forest malaria in central Vietnam. Am J Trop Med Hyg. 2008, 79: 652-654.
Das NG, Bhuyan M, Das SC: Some observations on malaria in tribal areas of Bastar District, Chhattisgarh. J Commun Dis. 2003, 35: 300-305.
Kosinski LA: Governments’ perceptions and policies of population redistribution in East and South East Asia. Popul Geogr. 1981, 3: 4-15.
Lindsay SW, Martens WJ: Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998, 76: 33-45.
Dahesh SM, Bassiouny HK, El-Masry SA: Socioeconomic and environmental factors affecting malaria infection in Fayoum Governorate, Egypt. J Egypt Soc Parasitol. 2009, 39: 511-523.
Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P: Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000, 94: 17-21. 10.1016/S0035-9203(00)90424-3.
Garros C, Van Bortel W, Trung HD, Coosemans M, Manguin S: Review of the Minimus Complex of Anopheles, main malaria vector in Southeast Asia: from taxonomic issues to vector control strategies. Trop Med Int Health. 2006, 11: 102-114. 10.1111/j.1365-3156.2005.01536.x.
Singh N, Dash AP, Thimasarn K: Fighting malaria in Madhya Pradesh (Central India): are we losing the battle?. Malar J. 2009, 8: 93-10.1186/1475-2875-8-93.
Giglioli G: Ecological change as a factor in renewed malaria transmission in an eradicated area. A localized outbreak of A. aquasalis transmitted malaria on the demerara river estuary, British Guiana, in the fifteenth year of A. darlingi and malaria eradication. Bull World Health Organ. 1963, 29: 131-145.
Dossou-Yovo J, Ouattara A, Doannio JM, Diarrassouba S, Chauvancy G: Malaria surveys in a humid savannah region in Cote d’Ivoire. Med Trop (Mars). 1998, 58: 51-56.
Feyisetan BJ, Asa S, Ebigbola JA: Mothers’ management of childhood diseases in Yorubaland: the influence of cultural beliefs. Health Transit Rev. 1997, 7: 221-234.
Herndon CN, Uiterloo M, Uremaru A, Plotkin MJ, Emanuels-Smith G, Jitan J: Disease concepts and treatment by tribal healers of an Amazonian forest culture. J Ethnobiol Ethnomed. 2009, 5: 27-10.1186/1746-4269-5-27.
Sharma SK, Jalees S, Kumar K, Rahman SJ: Knowledge, attitude and beliefs about malaria in a tribal area of Bastar district (Madhya Pradesh). Indian J Public Health. 1993, 37: 129-132.
Manguin S: Biodiversity of Malaria in the World. 2008, Montrouge: John Libbey Eurotext
Adera TD: Beliefs and traditional treatment of malaria in Kishe settlement area, southwest Ethiopia. Ethiop Med J. 2003, 41: 25-34.
Alves MJ, Barata LC, Barata Rde C, de Almeida Mdo C, Gutierrez EB, Wanderley DM, de Andrade JC: Socioeconomic aspects of subjects with imported malaria in the metropolitan area of Sao Paulo, Brazil. I. Characterization of the population and knowledge about the disease. Rev Saude Publica. 1990, 24: 253-258. 10.1590/S0034-89101990000400001.
Dhillon HS, Kar SB: Malaria eradication and investigation of cultural patterns and beliefs among tribal population in india. Int J Health Educ. 1965, Ill: 31-40.
Deressa W, Ali A, Hailemariam D: Malaria-related health-seeking behaviour and challenges for care providers in rural Ethiopia: implications for control. J Biosoc Sci. 2008, 40: 115-135.
Elphick H, Elphick D: Factors that contribute to the low use of bed nets in a malaria endemic zone of sub-Saharan Africa: a questionnaire survey in a rural population in Zambia. Cent Afr J Med. 2003, 49: 87-89.
Pulford J, Hetzel MW, Bryant M, Siba PM, Mueller I: Reported reasons for not using a mosquito net when one is available: a review of the published literature. Malar J. 2011, 10: 83-10.1186/1475-2875-10-83.
Esse C, Utzinger J, Tschannen AB, Raso G, Pfeiffer C, Granado S, Koudou BG, N’Goran EK, Cisse G, Girardin O, Tanner M, Obrist B: Social and cultural aspects of ‘malaria’ and its control in central Cote d’Ivoire. Malar J. 2008, 7: 224-10.1186/1475-2875-7-224.
Moore SJ, Min X, Hill N, Jones C, Zaixing Z, Cameron MM: Border malaria in China: knowledge and use of personal protection by minority populations and implications for malaria control: a questionnaire-based survey. BMC Public Health. 2008, 8: 344-10.1186/1471-2458-8-344.
Gosling R, Drakeley C, Mwita A, Chandramohan D: Presumptive treatment of fever cases as malaria: help or hindrance for malaria control?. Malar J. 2008, 7: 132-10.1186/1475-2875-7-132.
Talisuna AO, Langi P, Bakyaita N, Egwang T, Mutabingwa TK, Watkins W, Van Marck E, D’Alessandro U: Intensity of malaria transmission, antimalarial-drug use and resistance in Uganda: what is the relationship between these three factors?. Trans R Soc Trop Med Hyg. 2002, 96: 310-317. 10.1016/S0035-9203(02)90108-2.
Hastings IM, Watkins WM: Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 2005, 94: 218-229. 10.1016/j.actatropica.2005.04.003.
Alam MT, Vinayak S, Congpuong K, Wongsrichanalai C, Satimai W, Slutsker L, Escalante AA, Barnwell JW, Udhayakumar V: Tracking origins and spread of sulfadoxine-resistant Plasmodium falciparum dhps alleles in Thailand. Antimicrob Agents Chemother. 2011, 55: 155-164. 10.1128/AAC.00691-10.
Acknowledgements
The authors are thankful to the Director, National Institute of Malaria Research, New Delhi for providing necessary facilities to undertake this review. We thank Dr. Lalitha Ramanathapuram for making available full text articles referred in this review and Dr. Steven Sullivan for editing and proof reading. This study was supported by NIH/National Institute of Allergy and Infectious Diseases award U19AI089676, and by an NIH/Fogarty International Center Global Infectious Disease research training grant D43TW007884. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health. This manuscript bears the NIMR publication screening committee approval no. 001/2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NPK mined the literature as a part of his PhD thesis work. NPK and NN wrote the manuscript. AK, OP, and JMC added and improved overall manuscript structure and contents. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kar, N.P., Kumar, A., Singh, O.P. et al. A review of malaria transmission dynamics in forest ecosystems. Parasites Vectors 7, 265 (2014). https://doi.org/10.1186/1756-3305-7-265
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-7-265