Abstract
Background
Schistosomiasis japonica, caused by infection with Schistosoma japonicum, is still recognized as a major public health problem in the Peoples’ Republic of China. Mathematical modelling of schistosomiasis transmission has been undertaken in order to assess and project the effects of various control strategies for elimination of the disease. Seasonal fluctuations in transmission may have the potential to impact on the population dynamics of schistosomiasis, yet no model of S. japonicum has considered such effects. In this paper, we characterize the transmission dynamics of S. japonicum using a modified version of Barbour’s model to account for seasonal variation (SV), and investigate the effectiveness of the control strategy adopted in Liaonan village of Xingzi county, Jiangxi Province.
Methods
We use mathematical tools for stability analysis of periodic systems and derive expressions for the basic reproduction ratio of S. japonicum in humans; we parameterise such expressions with surveillance data to investigate the conditions for persistence or elimination of the disease in the study village. We perform numerical simulations and parametric sensitivity analysis to understand local transmission conditions and compare values of the basic reproductive ratio with and without seasonal fluctuations.
Results
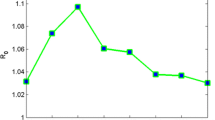
The explicit formula of the basic reproduction ratio for the SV-modified Barbour’s model is derived. Results show that the value of the basic reproduction ratio, R0, of Liaonan village, Xingzi county is located between 1.064 and 1.066 (very close to 1), for schistosomiasis transmission during 2006 to 2010, after intensification of control efforts.
Conclusions
Our modified version of the Barbour model to account for seasonal fluctuations in transmission has the potential to provide better estimations of infection risk than previous models. Ignoring seasonality tends to underestimate R0 values albeit only marginally. In the absence of simultaneous R0 estimations for villages not under control interventions (such villages do not currently exist in China), it is difficult to assess whether control strategies have had a substantial impact on levels of transmission, as the parasite population would still be able to maintain itself at an endemic level, highlighting the difficulties faced by elimination efforts.
Similar content being viewed by others
Background
Human schistosomiasis japonica is caused by infection with Schistosoma japonicum, a parasitic flatworm (Platyhelminth: Trematoda), and a significant cause of morbidity in oriental Asia, including the People’s Republic of China (P.R. China), the Republic of the Philippines and Indonesia [1]. The disease is a serious threat to human health and contributes to poverty in endemic regions, and has been prevalent in P.R. China for more than two millennia [2]. Schistosomiasis was endemic in 12 provinces of P.R. China, with an estimated 11.6 million people infected as indicated by epidemiological data from the 1950s [3]. Over the last few decades, the prevalence and burden of disease in P.R. China has dropped significantly as a result of sustained efforts and updated intervention strategies within the context of the national schistosomiasis control programme [4, 5]. However, the programme is facing new challenges due to the rebound of transmission in some areas, probably influenced by changes in environmental and social factors, particularly in lake regions, which exhibit more ecological complexity than other regions [6]. One of the obvious complexities of S. japonicum is that it is a multiple host–parasite system, with many possible non-human, mammal (wild and domestic) reservoirs potentially playing a role in transmission [7]. The results of a nationwide schistosomiasis survey in 2003 revealed that there were still more than 800,000 people infected with S. japonicum in P.R. China, with an average infection prevalence of 2.5% [8]. Since then, the national control programme has intensified its efforts, implementing an integrated control strategy aiming to interrupt transmission by focusing on the elimination of infectious sources [9].
The possibility to eliminate schistosomiasis transmission in P.R. China has spurred much debate and discussion on the operational feasibility of such an endeavour. At the same time, mathematical modelling of S. japonicum has been crucial to help understand its transmission dynamics and the effects on such dynamics of the interventions implemented [10–14]. Further investigations on the threshold of schistosomiasis transmission, measured by the basic reproduction ratio (R0) have been undertaken to understand the feasibility of, and effort involved in the shifting of the current strategy from transmission and morbidity control to elimination of the infection reservoir [15, 16].
The concept of the basic reproduction ratio can be traced back to the work of Alfred Lotka, Ronald Ross, and others [17–20], but its first application in modern epidemiology was by George MacDonald in 1952, who constructed population models of the spread of malaria [21]. It is often denoted by R0 and has received much attention in mathematical epidemiology [22–27]. The basic reproduction ratio is typically defined in the epidemiology literature as the average number of secondary cases resulting from a single infected primary case, introduced into a completely susceptible population, during the entire infectious period [28]. This threshold criterion states that when R0< 1, each infected individual produces, on average, less than one new infected individual, and the infection would be cleared from the population. If R0> 1, the infection will become endemic in the population providing that susceptibles are replenished. In an endemic infection, we can determine which control measures, and at what magnitude, would be most effective in reducing R0 below one, providing important guidance for public health initiatives [29].
Mathematical modeling of schistosomiasis transmission, which began with the work of MacDonald in 1965 [30], has been undertaken extensively over the years, both from a theoretical standpoint [31–35], and from the perspective of developing operational research tools for evaluation of control programmes [36–38]. By simulating control interventions, models are used to make theoretical predictions and/or projections about the future trends of schistosomiasis.
Broadly, mathematical models for schistosomiasis can be divided into those describing the intensity of infection (the worm burden per host), such as the early frameworks of MacDonald (1965) and others [28, 34, 39, 40], and those describing the prevalence of infection [32, 41–44]. In particular, Barbour (1996), to which we refer here as the Barbour’s model, compares the performance of both approaches for the calculation of R0 and concludes that intensity of infection frameworks which include density dependence in the snail component alone may lead to its underestimation. The interactions between definitive hosts and snail intermediate host in multihost–parasite systems are more complex than that has been described in Barbour’s model [41] (Table 1). In model (1.1), a and b are composite parameters. Parameter a, involving much biology and sociology, represents the rate at which a single definitive host becomes infected at unit density of infected snails and is difficult to estimate. Parameter b is the rate at which snails become infected. For each definitive host, Barbour [41] indicates that the force of infection acting upon a single definitive host species is equal to aΔy, where Δ is the density of snails, and y is the prevalence of infected snails (Table 2). Barbour [41] defines R0′ = abΣ/(gμ) as the basic reproduction ratio for the autonomous dynamical system (1.1), where Σ is the density of hosts, g the recovery rate for definitive host infections, and μ the per capita mortality rate of snails. It is shown that if R′0 < 1, the only equilibrium is , with and denoting, respectively, the average prevalence of infection in hosts and snails, and no endemic infection is possible. If R′0 > 1, infection leads to an endemic steady state. An extended Barbour’s two-host model system that was also described by Barbour 1996 for S. japonicum, was used to study human–bovine transmission of schistosomiasis in Jiangxi Province, P.R. China [14]. The analysis showed that treatment of humans that eliminates a fraction of adult worms according to drug efficacy and coverage has an immediate and large effect on human prevalence of infection, whereas treatment or vaccination of bovines with an anti-S japonicum fecundity vaccine of imperfect efficacy impacts on human incidence in the longer term [2, 45].
In the aforementioned framework, the coefficients of the Barbour’s model and its extensions are reasonably simplified. Those coefficients are considered as constants, which are approximated by average values. Our field observations indicate, by contrast, that infection of definitive hosts occurs at different rates every month of a year in the endemic areas, with a high re-infection rate [46]. In fact, natural factors, such as seasonal changes in moisture and temperature, affect the abundance and activity of the intermediate snail host, Oncomelania hupensis, and the transmission dynamics of schistosomiasis are in a constant state of flux [47]. Moreover, there are many social factors related to human behaviours accounting for the change of schistosomiasis incidence, such as marked changes of contact rates caused by daily production activities [48]. Therefore, periodic fluctuations in schistosomiasis transmission occur and vary according to local settings, yet such fluctuations have not yet been incorporated into mathematical models of schistosomiasis japonica.
The objectives of the present study are, therefore, (i) to modify the prevalence framework presented by Barbour by incorporating seasonally fluctuating dynamics, (ii) to derive expressions for the basic reproduction ratio with seasonality and compare them with those ignoring seasonality, (iii) to parameterize the model with surveillance data from a study village in Liaonan in Xingzi county, Jiangxi Province, and (iv) to discuss the impact of the interventions implemented on the stability and persistence of S. japonicum in the study area.
Methods
To incorporate seasonal variation with multiple factors into mathematical models, a reasonable method is to assume that some parameters of the model behave as periodic functions [3, 49]. In order to overcome the shortages of Barbour’s model as discussed above, we modify the mathematical model for S. japonicum transmission by including periodic coefficients. Taking the rates of change with respect to time of infected humans and of infected snails as our variables of interest (thus ignoring non-human definitive hosts), the following assumptions are made: (i) the human population is considered a constant closed system in which it is taken that the death rate is equal to the birth rate and is much smaller than the recovery rate, and hence it is ignored in the corresponding equation, (ii) a snail, whether infected once or several times, releases cercariae at the same rate, (iii) the infectivity of an infected definitive host is not influenced by the number of subsequent infections or by the current parasite burden of such a host. Under these assumptions our aim is to assess the effect of the new integrated control strategy, which has been implemented since 2005, in terms of temporal trends in the magnitude of schistosomiasis transmission measured by R 0 , based on surveillance data collected at our study site.
Mathematical formulation
The incidence of schistosomiasis in the real world tends to vary due to seasonal ecological fluctuations. With the aim of assessing the effect of periodic fluctuations on transmission of schistosomiasis, we modify the transmission coefficients, a and b of Barbour’s framework to reflect seasonality, namely they become functions of time rather than fixed constants, a(t) and b(t). Our mathematical model is given by the following equations, with P denoting the prevalence of infection in humans and y the prevalence of infection in snails (and the remaining parameters as defined above and in Table 1) to keep consistency with Barbour’s model,
Here, the incidence rate at which a single definitive host acquires infection at unit density of infected snails, a(t), and the rate at which an infected definitive host causes snail infections, b(t), are periodic positive continuous functions of t with period ω = 365 (days). System (2.1) is called the seasonal variation (SV)-modified Barbour’s model. In this study, we choose the composite functions as follows,
with and . Thus, , , and , .
The basic reproduction ratio (R0)
For the SV-modified Barbour’s model, we use operator theory in functional analysis and the monodromy matrix of linear periodic system theory to derive a biologically meaningful threshold index, the basic reproduction ratio R0. The method, which is motivated by the work of Wang and Zhao [50], presents the R0 formulation for the SV-modified Barbour’s model first. Numerical computation of the basic reproduction ratio was then conducted using the mathematical programming language MATLAB 7.1.
Study area and estimation of model parameters
Liaonan village in Xingzi county, Jiangxi province, was selected as our study area. This village had implemented an integrated control strategy, with emphasis on infection source control, since 2005. Calibration of the model was based on parameters from the literature and preliminary analysis of the data collected. The data were obtained from the annual report surveillance data, and included data from 2003 to 2010. Therefore, data from 2003 to 2005 represent a period prior to implementation of the integrated control strategy, with data from 2006 to 2010 representing the period of intensified intervention. The prevalence data of human, bovine and snail were estimated based on the routine surveillance [51]. In the routine surveillance, the residents were screened by antibody-based indirect hemagglutination assay (IHA) and then the positives with dilution titers over 1:10 in IHA were confirmed by Kato-Katz stool examination [52, 53]. Infected bovines were detected by the faecal hatching test, briefly, bovine faecal samples (50 g per cattle) were stirred and precipitated with clean water for several times, water was added to the supernatant in a flask and transferred to an incubator maintained at 25±3°C for observation after 1–3 hours to check if any miracidium had hatched in the top surface of water in the flask [54]. The snail intermediate host, O. hupensis, was detected on the marshland near the study village connecting to the Poyang Lake, using the methods of randomized sampling with a sampling iron-frame (0.1 m2). All of snails inside each sampling iron-frame were collected and dissected under a microscope to check whether the snails were infected with cercaria of S. japonicum[55]. The infection rate of humans or snails was calculated based on how many infected residents or infected snails there were out of the numbers examined [56]. The densities of human and bovines were collected from census data in human and bovines, respectively, and snail density was estimated based on the average number of snails in each sampling iron-frame.
For models (1.1) and (2.1), composite parameters a and b, and composite functions, a(t) and b(t) are difficult to measure directly, as they involve contact rates between hosts and snails and the probability of the infection establishing successfully in humans and snails. The difficulty in estimating these composite parameters for schistosomiasis lies in the large amount of unobserved data inherent in the disease process. Here, we use the approach of Wu [57] to define composite parameters as annual average values. Expressions for the functions a(t) and b(t) are difficult to determine from reported data, so in Eqn (2.1) they are represented by periodic functions to signify seasonal variation. In our study, optimized expressions of a(t) and b(t) are obtained by using the trigonometric functions shown above, with a period 365 days. The values of parameters a and b are equivalent to the average values of functions a(t) and b(t) in one year (365 days), respectively.
The reciprocal of the recovery rate, 1/g, is equivalent to the average duration of infection in human hosts. The death rate of infected snails was estimated as the reciprocal of the snails’ life expectancy [57]. The life expectancy of infected snails and the duration of infection of humans were estimated based on data from [14]. It was assumed that the average duration of infection in humans is 4 years. Snail infections were assumed to last an average of 6 months and since snails do not recover from infection, this was assumed to be the life expectancy of infected snails. Thus, we have g = 1/4 per year = 1/(4 × 365) per day= 0.00068 per day, and μ = 1/6 per month = 1/(6 × 30) per day= 0.0055 per day.
The density of snails, Δ(/m2), and the density of definitive hosts, Σ(/m2), were estimated from annual report surveillance data (Table 3). The surveillance data was collected in July or August each year, rather than monthly, this was one month after mass drug administration with praziquantel for the residents. This yearly data is often used as an annual average value (therefore masking any monthly fluctuations that may have occurred). Therefore, for the purpose of modelling (1.1), it is assumed that schistosomiasis transmission remains in a steady state throughout the year in endemic areas.
By setting equations of the model (1.1) to zero we find steady-state (equilibrium) values for the prevalence of infection in humans and snails,
It is assumed that is the equilibrium infected snail prevalence and is the equilibrium prevalence of infection in humans for each year.
Results
Calculation of R0
The mathematical details of the derivation of expressions for the basic reproduction ratio can be found in the Additional file 1: Derivation of R0 and the proof of the main results.
Prevalence of infection in humans and snails
The values for the equilibrium infected snail prevalence and the equilibrium prevalence of infection in humans are shown in Table 3. Combining these with the steady-state values of the model, composite parameters a and b of model (1.1) can be derived from equations (2.3) and (2.4) and are shown in Table 4.
Numerical simulation and sensitivity analysis
Based on the annual report data in Xingzi county during 2003 to 2010, where a control strategy which focused on control of the infection source was implemented in 2005 [58, 59], we investigated the effectiveness of the strategies adopted in Liaonan village of Xingzi county. Table 5 gives the results from the calculation of the basic reproduction ratios for each year comparing the values obtained by using model 1.1 (denoted R′0) with those obtained with model 2.1 (denoted R0). The values of R0 are consistently (but not markedly) higher than those of R′0. The values of R0 are located in the range from 1.064 to 1.089 for schistosomiasis transmission from 2003 to 2010 in the Liaonan village of Xingzi county, which are close to 1 (Figure 1). The values of R′0 obtained with the non-SV model lay between 1.020 and 1.081.
Furthermore, the sensitivity analysis on parameters a and b, and parameters a0 and b0 are related to the magnitudes of the seasonal fluctuation a(t) and b(t), respectively, showing that parameters, a and b vary with the basic reproduction ratio. It is also shown that R0 is linearly related to both a and b with the pattern that R0 decreases to a relatively small value (less than 1) only when a and b are very small (Figure 2).
Discussion
Recently, much attention has been given to developing schistosomiasis transmission models that can be used to assess and project the effects of control measures. A biologically-motivated model of S. japonicum transmission has been described in detail that incorporates human hosts, adult parasites, uninfected and infected snails, free-living miracidia, and free-living cercariae [60]. A mathematical model for the transmission dynamics of S. mansoni was also presented, in which the dynamics of miracidia and cercariae were incorporated [16]. The model was analyzed to gain insights into the qualitative features of endemic equilibrium and to allow for determination of the basic reproduction ratio (using the spectral radius of an appropriate matrix as described by Van den Driessche et al.[61]). In another study, thresholds for the survival of schistosomes were established after more than two human habitats sharing the same contaminated water resource were observed [15], then control strategies were discussed in the light of these thresholds [15]. Many of these studies, however, have not yet solved the problems associated with estimating R0, which is remarkably difficult, particularly for vector-borne and indirectly-transmitted diseases, based on field surveillance data [62, 63].
In general, the difficulty lies in the large number of parameters necessary to obtain or estimate the basic reproduction ratio. So decisions as to which parameters are the most important are crucial. In this study, we modified the framework presented by Barbour in 1996, adapted for S. japonicum transmission, by incorporating periodic functions into those parameters that include the contact rates, to evaluate the effect of control measures in one village in Xingzi county. Parameters g (the recovery rate from infection in the humans), μ (the mortality rate of snails), Δ (the density of snails per unit water area), and Σ (the density of definitive hosts), can be directly obtained from the reported data. However, there is no information available for the values of parameters a(t) and b(t), the transmission coefficients from snails to humans and from humans to snails, respectively, in model (2.1). For simplicity, we employed trigonometric functions for the expressions of a(t) and b(t). While detailed information is still lacking, there is little doubt that the composite parameters change over time. The method we used to estimate the composite parameters of model (2.1) in this paper takes into consideration realistic features of the disease and is preferable to the constant coefficients used in other models. However, although our values of the basic reproduction ratio obtained with the SV-model were consistently higher than those obtained with the non-SV model, the difference was only marginal.
Often, the basic reproduction ratio is a useful indicator of both the risk of an epidemic and the effort required to control an infection. Here, we define the basic reproduction ratio R0 of the SV-modified Barbour's model (2.1) and confirm that the disease would be controlled if R0 < 1 or sustained at an endemic level if R0 > 1. On the basis of the surveillance data collected, we observed that the basic reproduction ratio of S. japonicum in Liaonan village under control lies in the range of 1.064 to 1.066 after 2005, with slight year-to-year fluctuations. It is shown that although Liaonan village has achieved good results by implementing integrated control strategies, with emphasis on infection source control, the results are not very different from those between 2003 and 2005, before the intensification of control efforts, suggesting that the infection is still able to maintain itself at a low, yet quasi-steady state equilibrium. Other modelling studies have shown that after repeated treatment, and conditioned to non-elimination, the prevalence distribution, in those villages where infection persists at a very low level, it reaches a stationary (termed a quasi-stationary) distribution [31, 64–67]. Modelling studies for lymphatic filariasis have also shown that in the vicinity of transmission breakpoints, parasite resilience and the specificities of the host-parasite system and distribution among hosts may make elimination hard to achieve [68].
The composite parameters in our SV-modified Barbour’s model are naturally subject to fluctuation in time, that is, the SV-modified Barbour model is non-autonomous. It should be more realistic than the autonomous system, as seasonal variation in the dynamics of snails and humans occur in the field. Moreover, from our results of numerical computation, we can see that transmission elimination policies, based on computation of the basic reproduction ratio using time-averaged transmission coefficients, may underestimate (albeit slightly) the infectious risk inherent to periodic disease transmission.
Note that the values computed for the basic reproduction ratio depend on model parameters, which can be influenced by the control strategies implemented in the study areas. For better control and elimination of schistosomiasis japonica, it is necessary to obtain a better knowledge of the relationship between control strategies and model parameters, particularly those composite parameters that include the contact rates. The main interventions for schistosomiasis include preventive chemotherapy using praziquantel which impacts on the duration of infection, health education that affects contact rates, mollusciciding which decreases snail survival, environmental management that influences exposure, and sanitation improvement which decreases contamination rates of water sites. On the whole, elimination of schistosomiasis is based on protection of water sources, limitation of water contact, and eradication of intermediate snail hosts [69]. Treatment with praziquantel, which reduces egg output, should reduce contamination rates and impact on b (the rate at which humans infect snails). However, it has also been suggested that praziquantel treatment, by killing adult worms and releasing antigens otherwise not seen by the immune system [70], can have a ‘vaccination’ effect by these antigens eliciting some protective immunity (thus impacting on a) [71]. The frequency of contact with infected water by humans in the lake and embankment areas can be high. Health education can reduce the frequency of contact with possibly contaminated water, also impacting on a. Sanitation improvement should reduce the probability of faeces reaching water sites and therefore the probability of an egg developing into a miracidium, also decreasing b. However, sanitation improvement may also reduce exposure and hence a. It is well known that reducing the number of snails with extensive mollusciciding, and modifying snail habitats by draining rivers and ditches will reduce contact rates. Chemical mollusciciding in the study village was generally carried out annually. But snails re-appeared in certain environments still suitable for snail breeding. Thus, environmental modification was also carried out in an effort to control transmission more effectively [72].
Conclusions
Limitations of our study
The SV-modified Barbour's model for schistosomiasis transmission proposed here has the following limitations. Firstly, in fact, S. japonicum is a zoonosis with over 40 species of mammals acting as possible reservoir hosts as stated earlier [73, 74]. Bovines are thought to be particularly important for harbouring and transmitting S. japonicum in lake and marshland regions of southern China [74–77]. Diagnosis and control of bovine schistosomiasis is vitally important for reducing the prevalence of the disease [14]. Thus, it will be important to be able to include transmission from non-human definitive hosts into the model for evaluation of the efficacy of control programmes in many other disease-endemic areas. Our present model attempts to incorporate seasonal fluctuations of transmission, but in the future we will extend Barbour's two-host model to investigate a multihost model with seasonal fluctuations. Secondly, the basic reproduction ratio completely determines the long-term behaviour of the disease only in theory. We computed yearly basic reproduction ratios according to the expressions derived from our model and the previous non-SV model. Although indicative, this is not sufficient; formulation of stochastic models that allow the feasibility of elimination to be explored, and analysis of empirical studies in near elimination scenarios to determine operational thresholds for elimination will also be required [78]. Thirdly, as transmission rates vary according to time and circumstances, it is difficult to obtain accurate estimates of time-varying composite parameters due to lack of data [79]. In this paper, trigonometric functions with a period of 365 days were proposed to simulate these composite functions. However, more accurate estimation of the composite functions will be important for describing and analyzing the behaviour of the model. Thus the model will be combined with time series data in the future [80].
Actually, a defensible set of parameter estimates is difficult to determine because the intensity of control strategies differentially affects model parameters. The credibility of our conclusions relating to control strategies is weakened by only using annual report data. However, the collected data under trial conditions with relatively stable intervention measures can help us to assess the effectiveness of control strategies. The evaluation of a control strategy usually involves a group with intervention and a group without intervention, yet there are no villages in P.R. China under no intervention. However, in our study village, the fact that our estimates before (2003–2005) and after (2006–2010) intensification of control intervention do not reveal a substantial trend, indicates that estimation of the basic reproduction ratio alone may be insufficient to assess the impact of control strategies effectively.
Finally, we assumed that the study village was a closed system, whilst the issue of connectivity between villages either by movement of hosts or snails (the latter through hydrological connectivity) is central to the discussion of control strategies in low transmission settings in P.R. China [81]. Feng et al. (2005) considered this issue in the literature [15], but the models proposed are complex and difficult to parameterise. However, there is no doubt that a metapopulation approach for the understanding of disease persistence in P.R. China will shed light on the landscape of schistosomiasis japonica elimination efforts.
References
The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium: The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009, 460: 345-351. 10.1038/nature08140.
Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J: The public health significance and control of schistosomiasis in China– then and now. Acta Trop. 2005, 96: 97-105. 10.1016/j.actatropica.2005.07.005.
Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, Dang H, Wang XH, Xu J, Zhu HQ, Wu GL, Li YS, Xu XJ, Chen HG, Wang TP, Zhu YC, Qiu DC, Dong XQ, Zhao GM, Zhang SJ, Zhao NQ, Xia G, Wang LY, Zhang SQ, Lin DD, Chen MG, Hao Y: Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg Infect Dis. 2007, 13: 1470-1476. 10.3201/eid1310.061423.
Wang TP, Cao ZG, Chen HG, Zhou XN: Changes of control strategy and improvement of schistosomiasis control in China. Chin J Schisto Contrl. 2009, 21: 241-242.
Li SZ, Luz A, Wang XH, Xu LL, Wang Q, Qian YJ, Wu XH, Guo JG, Xia G, Wang LY, Zhou XN: Schistosomiasis in China: acute infections during 2005–2008. Chin Med J (Engl). 2009, 122: 1009-1014.
Zhang ZJ, Clark AB, Bivand R, Chen Y, Carpenter TE, Peng WX, Zhou YB, Zhao GM, Jiang QW: Nonparametric spatial analysis to detect high-risk regions for schistosomiasis in Guichi, China. Trans R Soc Trop Med Hyg. 2009, 103: 1045-1052. 10.1016/j.trstmh.2008.11.012.
He YX, Salafsky B, Ramaswamy K: Host-parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001, 17: 320-324. 10.1016/S1471-4922(01)01904-3.
Xiao DL, Yu Q, Dang H, Guo JG, Zhou XN, Wang LY: Schistosomiasis situation in People’s Republic of China in 2003 [in Chinese]. Chin J Schisto Contrl. 2004, 16: 401-405.
Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN: A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009, 360: 121-128. 10.1056/NEJMoa0800135.
Gray DJ, Williams GM, Li Y, McManus DP: Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS ONE. 2008, 3: e4058-10.1371/journal.pone.0004058.
Liang S, Maszle D, Spear RC: A quantitative framework for a multi-group model of Schistosomiasis japonica transmission dynamics and control in Sichuan, China. Acta Trop. 2002, 82: 263-277. 10.1016/S0001-706X(02)00018-9.
Liang S, Spear RC, Seto E, Hubbard A, Qiu D: A multi-group model of Schistosoma japonicum transmission dynamics and control: model calibration and control prediction. Trop Med Int Health. 2005, 10: 263-278. 10.1111/j.1365-3156.2005.01386.x.
Spear RC, Hubbard A, Liang S, Seto E: Disease transmission models for public health decision making: toward an approach for designing intervention strategies for schistosomiasis japonica. Environ Health Perspect. 2002, 110: 907-915. 10.1289/ehp.02110907.
Williams GM, Sleigh AC, Li YS, Feng Z, Davis GM, Chen H, Ross AGP, Bergquist R, McManus DP: Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the People’s Republic of China. Acta Trop. 2002, 82: 253-262. 10.1016/S0001-706X(02)00017-7.
Feng Z, Li CC, Milner FA: Schistosomiasis models with two migrating human groups. Math Comput Model. 2005, 41: 1213-1230. 10.1016/j.mcm.2004.10.023.
Chiyaka ET, Garira W: Mathematical analysis of the transmission dynamics of schistosomiasis in the human-snail hosts. J Biol Syst. 2009, 17: 397-423. 10.1142/S0218339009002910.
Dublin LI, Lotka AJ: On the true rate of natural increase. J Am Stat Assoc New Series. 1925, 150: 305-339.
Ross R: The prevention of malaria. 1911, London: John Murray
Sharp FR, Lotka AJ: A problem in age distribution. Phil Mag. 1911, 6: 435-438.
Heesterbeek JAP, Roberts MG:Threshold quantities for helminth infections. J Math Biol. 1995, 33: 415-434.
MacDonald G: The analysis of equilibrium in malaria. Trop Dis Bull. 1952, 49: 813-829.
Wang JY, WD : The effect of population dispersal on the spread of a disease. J Math Anal Appl. 2005, 308: 343-364. 10.1016/j.jmaa.2005.01.034.
Mukandavire Z, Chiyaka C, Garira W, Musuka G: Mathematical analysis of a sex-structured HIV/AIDS model with a discrete time delay. Nonlinear Anal-Theor. 2009, 71: 1082-1093. 10.1016/j.na.2008.11.026.
Gao SJ, Liu YJ, Nieto JJ, Andrade H: Seasonality and mixed vaccination strategy in an epidemic model with vertical transmission. Math Comput Simulat. 2011, 81: 1855-1868. 10.1016/j.matcom.2010.10.032.
Bacaër N, Dads EHA:On the biological interpretation of a definition for the parameterR0in periodic population models. J Math Biol. 2012, 65: 601-621. 10.1007/s00285-011-0479-4.
Heesterbeek JAP:A brief history ofR0and a recipe for its calculation. Acta Biotheor. 2002, 50: 189-204. 10.1023/A:1016599411804.
Inaba H: On a new perspective of the basic reproduction number in heterogeneous environments. J Math Biol. 2012, 65: 309-348. 10.1007/s00285-011-0463-z.
Anderson RM, May RM: Infectious Disease of Humans: Dynamics and Control. 1991, Oxford: Oxford University Press
Diekmann O, Heesterbeek JAP, Metz JAJ:On the definition and the computation of the basic reproduction ratioR0in models for infectious diseases in heterogeneous populations. J Math Biol. 1990, 28: 365-382.
MacDonald G: The dynamics of helminth infections, with special reference to schistosomes. Trans R Soc Trop Med Hyg. 1965, 59: 489-506. 10.1016/0035-9203(65)90152-5.
Nåsell I: On eradication of schistosomiasis. Theor Popul Biol. 1976, 10: 133-144. 10.1016/0040-5809(76)90011-3.
Cohen JE: Mathematical models of schistosomiasis. Annu Rev Ecol Syst. 1977, 8: 209-233. 10.1146/annurev.es.08.110177.001233.
May RM: Togetherness among the schistosomes: its effects on the dynamics of the infection. Math Biosci. 1977, 35: 301-343. 10.1016/0025-5564(77)90030-X.
Barbour AD: Macdonald's model and the transmission of bilharzia. Trans R Soc Trop Med Hyg. 1978, 72: 6-15. 10.1016/0035-9203(78)90290-0.
Anderson RM, May RM: Helminth infections of humans: mathematical models, population dynamics, and control. Adv Parasitol. 1985, 24: 1-101.
Chan MS, Guyatt HL, Bundy DAP, Booth M, Fulford AJC, Medley GF: The development of an age structured model for schistosomiasis transmission dynamics and control and its validation for Schistosoma mansoni. Epidemiol Infect. 1995, 115: 325-344. 10.1017/S0950268800058453.
Chan MS, Montresor A, Savioli L, Bundy DAP: Planning chemotherapy-based schistosomiasis control: validation of a mathematical model using data on Schistosoma haematobium from Pemba, Tanzania. Epidemiol Infect. 1999, 123: 487-497. 10.1017/S0950268899003167.
French MD, Churcher TS, Gambhir M, Fenwick A, Webster JP, Kabatereine NB, Basáñez MG: Observed reductions in Schistosoma mansoni transmission from large-scale administration of praziquantel in Uganda: A mathematical modelling study. PLoS Negl Trop Dis. 2010, 4: e897-10.1371/journal.pntd.0000897.
Woolhouse MEJ: On the application of mathematical models of schistosome transmission dynamics. I. Natural transmission. Acta Trop. 1991, 49: 241-270.
Woolhouse MEJ: On the application of mathematical models of schistosome transmission dynamics. II. Control. Acta Trop. 1992, 50: 189-204. 10.1016/0001-706X(92)90076-A.
Barbour AD: Modelling the transmission of schistosomiasis: an introductory view. Am J Trop Med Hyg. 1996, 55 (5 Suppl): 135-143.
Riley S, Carabin H, Marshall C, Olveda R, Willingham AL, McGarvey ST: Estimating and modeling the dynamics of the intensity of infection with Schistosoma japonicum in villagers of Leyte, Philippines. Part II: Intensity-specific transmission of S. japonicum. The schistosomiasis transmission and ecology project. Am J Trop Med Hyg. 2005, 72: 754-761.
Riley S, Carabin H, Belisle P, Joseph L, Tallo V, Balolong E, Willingham AL, Fernandez TJ, Gonzales RO, Olveda R, McGarvey ST: Multi-host transmission dynamics of Schistosoma japonicum in Samar province, the Philippines. PLoS Med. 2008, 5: e18-10.1371/journal.pmed.0050018.
Ishikawa H, Ohmae H, Pangilinan R, Redulla A, Matsuda H: Modeling the dynamics and control of Schistosoma japonicum transmission on Bohol island, the Philippines. Parasitol Int. 2006, 55: 23-29. 10.1016/j.parint.2005.09.001.
Wang LD, Utzinger J, Zhou XN: Schistosomiasis control: experiences and lessons from China. Lancet. 2008, 372: 1793-1795. 10.1016/S0140-6736(08)61358-6.
Wang TP, Vang Johansen M, Zhang SQ, Wang FF, Wu WD, Zhang GH, Pan XP, Ju Y, Ornbjerg N: Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005, 96: 198-204. 10.1016/j.actatropica.2005.07.017.
Wang XH, Wu XH, Zhou XN: Bayesian estimation of community prevalences of Schistosoma japonicum infection in China. Int J Parasitol. 2006, 36: 895-902. 10.1016/j.ijpara.2006.04.003.
Vandemark LM, Jia TW, Zhou XN: Social science implications for control of helminth infections in Southeast Asia. Adv Parasitol. 2010, 73: 137-70.
Zhou XN, Malone JB, Kristensen TK, Bergquist NR: Application of geographic information systems and remote sensing to schistosomiasis control in China. Acta Trop. 2001, 79: 97-106. 10.1016/S0001-706X(01)00107-3.
Wang WD, Zhao XQ: Threshold dynamics for compartmental epidemic models in periodic environments. J Dyn Diff Equat. 2008, 20: 699-717. 10.1007/s10884-008-9111-8.
Tao B, Jiang QL, Luo CJ, Yin ZH, Wang JM: Endemic situation of schistosomiasis in Xingzi Country. Chin J Schisto Contrl. 2009, 21: 62-63.
Katz N, Chaves A, Pellegrino J: A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972, 14: 397-400.
Lin DD, Liu YM, Hu F, Li YF, Tao B, Yuan M, Xie SY, Huang MJ, Jiang QL, Li JY, Gao ZL, Wang JM: Evaluation on application of common diagnosis methods for schistosomiasis japonica in endemic areas of China. III. Analysis and evaluation of underestimation of prevalence of Schistosoma japonicum infection by routine Kato-Katz technique. Chin J Parasitol Parasitic Dis. 2011, 23: 642-647.
Ministry of Health, C: Manual of Schistosomiais Control and Prevention, in: Control, B.o.D. (Ed.). 2000, Shanghai: Shanghai Scientific and Technical Publishers, 72-76. 3
Xu J, Li SZ, Huang YX, Cao ZG, Tu ZW, Wu CG, Miu F, Dang H, Zhang LJ, Chen Z, Wang LY, Guo JG, Zhou XN: Risk evaluation of schistosomiasis japonica in potential endemic areas in China. Chin J Parasitol Parasitic Dis. 2012, 30: 428–33-437.
Ministry of Health: National guideline for snail survey, control, population infection screen and chemotherapy of schistosomiasis (Trial), in: Control, B.o.D. (Ed.). 2005, Beijing: Ministry of Health
Wu KC: Mathematical model and transmission dynamics of schistosomiasis and its application. Chin Trop Med. 2005, 5: 837-844.
Seto EYW, Remais JV, Carlton EJ, Wang S, Liang S, Brindley PJ, Qiu DC, Spear RC, Wang LD, Wang TP, Chen HG, Dong XQ, Wang LY, Hao Y, Bergquist R, Zhou XN: Toward sustainable and comprehensive control of schistosomiasis in China: lessons from Sichuan. PLoS Negl Trop Dis. 2011, 5: e1372-10.1371/journal.pntd.0001372.
Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, Zhang ZH, Steinmann P, Yang GJ, Wang SP, Wu ZD, Wang LY, Hao Y, Bergquist R, Utzinger J, Zhou XN: China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009, 14: 1475-1483. 10.1111/j.1365-3156.2009.02403.x.
Feng Z, Li CC, Milner FA: Effects of density and age dependence on the transmission dynamics of schistosomes. Math Biosci. 2002, 177–178: 271-286.
Van den Driessche P, Watmough J: Reproduction numbers and sub-threshold endemic equilibiria for compartmental models of disease transmission. Math Biol. 2002, 180: 29-48.
Dye C: Vectorial capacity: must we measure all its components?. Parasitol Today. 1986, 2: 203-209. 10.1016/0169-4758(86)90082-7.
Woolhouse MEJ, Hasibeder G, Chandiwana SK: On estimating the basic reproduction number for Schistosoma haematobium. Trop Med Int Health. 1996, 1: 456-463. 10.1046/j.1365-3156.1996.d01-88.x.
Nåsell I: On the quasi-stationary distribution of the Ross malaria model. Math Biosci. 1991, 107: 187-207. 10.1016/0025-5564(91)90004-3.
Nåsell I: On the quasi-stationary distribution of the stochastic logistic epidemic. Math Biosci. 1999, 156: 21-40. 10.1016/S0025-5564(98)10059-7.
Jacquez JA, Simon CP: The stochastic SI model with recruitment and deaths. I. Comparison with the closed SIS model. Math Biosci. 1993, 117: 77-125. 10.1016/0025-5564(93)90018-6.
Ray KJ, Porco TC, Hong KC, Lee DC, Alemayehu W, Melese M, Lakew T, Yi E, House J, Chidambaram JD, Whitcher JP, Gaynor BD, Lietman TM: A rationale for continuing mass antibiotic distributions for trachoma. BMC Infect Dis. 2007, 7: 91-10.1186/1471-2334-7-91.
Gambhir M, Michael E: Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis. PLoS ONE. 2008, 3: e2874-10.1371/journal.pone.0002874.
Zhou XN, Bergquist R, Tanner M: Elimination of tropical diseases through surveillance and response. Inf Dis Poverty. 2013, 2: 1-10.1186/2049-9957-2-1.
Woolhouse ME, Hagan P: Seeking the ghost of worms past. Nat Med. 1999, 5: 1225-1227. 10.1038/15169.
Mutapi F, Ndhlovu PD, Hagan P, Spicer JT, Mduluza T, Turner CM, Chandiwana SK, Woolhouse ME: Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J Infect Dis. 1998, 178: 289-293. 10.1086/517456.
Zhou XN: Prioritizing research for “One health-One world”. Inf Dis Poverty. 2012, 1: 1-10.1186/2049-9957-1-1.
Gray DJ, Williams GM, Li YS, Chen HG, Forsyth SJ, Li RS, Barnett AG, Guo JG, Ross AG, Feng Z, McManus DP: A cluster-randomised intervention trail against Schistosoma japonicum in the People's Republic of China: bovine and human transmission. PLoS ONE. 2009, 4: e5900-10.1371/journal.pone.0005900.
Jin YM, Lu K, Zhou WF, Fu ZQ, Liu JM, Shi YJ, Li H, Lin JJ: Comparison of recombinant proteins from Schistosoma japonicum for schistosomiasis diagnosis. Clin Vaccine Immunol. 2010, 17: 476-480. 10.1128/CVI.00418-09.
McManus DP, Gray DJ, Ross AG, Williams GM, He HB, Li YS: Schistosomiasis research in the Dongting lake region and its impact on local and national treatment and control in China. PLoS Negl Trop Dis. 2011, 5: e1053-10.1371/journal.pntd.0001053.
Rudge JW, Lu DB, Fang GR, Wang TP, Basáñez MG, Webster JP: Parasite genetic differentiation by host species and habitat type: molecular epidemiology of Schistosoma japonicum in hilly and marshland areas of Anhui Province, China. Mol Ecol. 2009, 18: 2134-2147. 10.1111/j.1365-294X.2009.04181.x.
Lu DB, Wang TP, Rudge JW, Donnelly CA, Fang GR, Webster JP: Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China–a two-year longitudinal parasitological survey. Parasitology. 2010, 137: 99-110. 10.1017/S003118200999103X.
APOC: Conceptual and operational framework of onchocerciasis elimination with ivermectin treatment. African Programme for Onchocerciasis Control (WHO/APOC). 2010, Available: http://www.who.int/apoc/oncho_elimination_report_english.pdf (accessed 31 March 2013)
Butler CD: Infectious disease emergence and global change: thinking systemically in a shrinking world. Inf Dis Poverty. 2012, 1: 5-10.1186/2049-9957-1-5.
Lara-Ramírez EE, Rodríguez-Pérez MA, Pérez-Rodríguez MA, Adeleke MA, Orozco-Algarra ME, Arrendondo-Jiménez JI, Guo X: Time series analysis of onchocerciasis data from Mexico: a trend towards elimination. PLoS Negl Trop Dis. 2013, 7: e2033-10.1371/journal.pntd.0002033.
Gurarie D, Seto EY: Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities. J R Soc Interface. 2009, 6: 495-508. 10.1098/rsif.2008.0265.
Acknowledgements
The research of SJ Gao and YY He has been partially supported by The Natural Science Foundation of China (No.11261004), China Postdoctoral Science Foundation funded project (No. 2012M510039) and the National Key Technologies R & D Program of China (2009BAI78B01). XN Zhou was supported by the National S & T Major Program (No. 2012ZX10004-220), and by Shanghai S&T Committee (No. 11XD1405400). GJ Yang was supported by National Natural Science Foundation of China (No. 81102173).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SJG, YYH, YJL and XNZ were involved in all study processes including design, data acquisition, analysis and interpretation of the results as well as the drafting of the manuscript. SJG, GJY and XNZ initial study concept and revised all drafts of the manuscript. All authors read and approved the final version of the manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gao, SJ., He, YY., Liu, YJ. et al. Field transmission intensity of Schistosoma japonicum measured by basic reproduction ratio from modified Barbour’s model. Parasites Vectors 6, 141 (2013). https://doi.org/10.1186/1756-3305-6-141
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-6-141