Abstract
The etiology of mental retardation/developmental delay (MRDD) remains a challenge to geneticists and clinicians and can be correlated to environmental and genetic factors. Chromosomal aberrations are common causes of moderate to severe mental retardation and may represent 10% of these occurrences. Here we report the case of a boy with development delay, hypoplasia of corpus callosum, microcephaly, muscular hypotonia, and facial dysmorphisms. A deletion of 7q36.1 → 36.3 and duplication of 9p22.3 → 23 was detected as a result of an unbalanced translocation of paternal origin. Breakpoint delimitation was achieved with array comparative genomic hybridization assay. Additional multiplex ligation dependent probe amplification (MLPA) analyzes confirmed one copy loss of 7q36.3 region and one copy gain of 9p24.3 region. Patient resultant phenotype is consistent with the already described findings for both 7q deletion and 9p duplication syndromes.
Similar content being viewed by others
Background
Mental retardation/developmental delay (MRDD) is a relevant public health concern, since it may represent one of the major causes of child disability, with prevalence estimated in 1-3% [1]. In Brazil, the Governmental Census conducted in 2010 estimated that MRDD can be found in 1.4% of population [2], but this number may be underestimated. Actually, there are deficiencies not only in evaluating MRDD prevalence around the world but also in methodology for screening and impairment degree stratification [3, 4]. MRDD causes are heterogeneous and may have both environmental and genetic bases. It is estimated that 65-80% of patient’s MRDD etiology remains unknown [5].
9p duplication syndrome is considered one of the most frequent autosomal anomalies in life born together with trisomies 13, 18 and 21 [6]. More than 150 cases of 9p duplication are reported in literature [7] and common features include mental retardation, failure to thrive, hypotonia, microcephaly, low set malformed ears, upward-slanted eyes, small palpebral fissures, congenital heart disease, skeletal and genitourinary anomalies, enophthalmos or microphthalmos, broad base nose and prominent nasal tip, downward slanting mouth, among others characteristics [7, 8].
Similarly, deletion of 7q is a described syndrome [9] and clinical findings comprise low birth weight, mental retardation, development delay, facial dysmorphisms and genitourinary malformations [10–19]. Additionally, more severe phenotypes, as sacral agenesis and holoprosencephaly (HPE), may occur [9, 14–17, 20]. Here we report the case of a boy exhibiting 7q deletion and 9p duplication due to an unbalanced translocation t(7;9) from paternal origin.
Case presentation
The patient (male) is the first child of a 31-year-old mother and her unrelated 28-year-old husband. Mother was Caucasian and father Japanese descent. Ultrasonography on 33 weeks of gestation revealed intrauterine growth retardation (IUGR) and diminished vascular sport. The mother reported only vitamins ingestion during pregnancy. The proband was born by cesarean section (37 weeks), Apgar were reportedly 5-8, weight of 1950 g, length of 41 cm and occipitofrontal head circumference 29.0 cm.
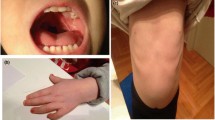
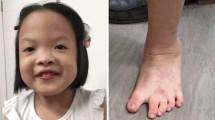
The newborn presented peculiar facies, severe microcephaly, and generalized hypotonia. He also developed jaundice without ABO incompatibility and phototerapy was required. Echocardiography with 2 days of life disclosed normal heart function. At the age of 5 months the child weight (5345 g), length (58 cm) and occipitofrontal head circumference (36.5 cm) were below the second percentile. Dysmorphic features included anterior fontanel with dimension like one finger, bilateral epicanthal folds, upslanting palpebral fissures, bulbous nasal tip, enlarged columela, large symmetric and posteriorly rotated ears. Limbs, hands and feet were symmetric. Hallux vagus and overlapping of the left third toe on the second toe were observed. Finger and toe nails were hyperconvex. Thorax and lombo-sacral radiographies revealed no abnormalities.
At 2 years old, patient presented seizure without fever (10 minutes duration) while sleeping. EEG disclosed focal epileptiform activity expanding to bilateral fronto central and medial region, with left side predominance. MRI scan of the brain showed hypoplasia of corpus callosum and white matter reduction. At age of 3 years old and 5 months child exhibited failure to thrive (weight = 9.5 kg, p < 3; length = 86.0 cm, 9p < 5), psychomotor and language delay, severe microcephaly (OFC = 43.5 cm, p < 3) and craniofacial dysmorphisms became more evident. Patient also presented micropenis (3.5 cm (p < 10). Patient phenotype at 5 years old can be assessed in Figure 1.
Peripheral blood karyotype (Phytohemagglutinin -PHA-stimulated lymphocyte with G banding-500 bands) from propositus and progenitors were studied. Abnormalities were described according to ISCN (2009) [21]. Father analysis showed a 46, XY, der(7)t(7;9)(q36;p22), 9 ph karyotype, while none structural or numeric alterations were observed for mother. Patient karyotype was 46, XY, der(7)t(7;9)(q36;p22), 9 ph (Figure 2).
Additional aCGH and MLPA investigations were conducted. Patient and progenitors DNA samples were extracted from blood with Qiamp Dna Blood Midi (Qiagen, Germany). aCGH procedures were performed as recommended by manufacturer (Perkin Elmer, Norwalk). Constitutional Chip 4.0 (Perkin Elmer, Norwalk) was employed and was comprised of 5000 BAC clones spotted in duplicate, with resolution lower than 650 kb and segments arranged from 100 to 300 kb of human DNA, distributed through whole genome.
Slides were scanned with InnoScann 710 (Innopsys, Carbonne, France) and MAPIX 4.5 software was used for GPR files generation. Data analysis was conducted with SpectralWare® v2.3.3 aCGH Analysis System software [22] from Perkin Elmer. The computation parameters used were pin linear for normalization; threshold between 0.7/1.3; Lowess alpha of 0.1 normalization and confidence level of 95%. Median data were used for interpretation of results. For chromosome region size determination and CNV search, Database of Genomics Variants was consulted [23]. Analyses of proband DNA revealed a 7q deletion (7q36.1 -- > qter) and 9p duplication (9p22.3-- > 23) (Table 1). In 7q deleted segment, 80 genes are present, although the BAC clones of aCGH array comprised less than 20% of them. In a similar way, duplicated region of 9p encloses 42 genes, however only two genes were detected through hybridization with BAC clones of the chosen platform. None alteration was observed in maternal DNA analysis. Losses of two clones (14q32.33 and 16p13.13) were detected in paternal DNA analysis, both unrelated to child’s phenotype.
Multiplex ligation-dependent probe amplification (MLPA) assay was performed with SALSA MLPA P036-E1 Human Telomere-3 probemix kit (MRC-Holland; Amsterdam, The Netherlands). Reaction was conducted as recommended by manufacturer. Fragments were separated on an ABI3100 genetic analyzer (Applied Biosystems). Genotyper Software (version 2.0) and Coffalyser V9.4 Software [24] were employed for data analyses. None alteration was observed for both parents. One copy loss of VIPR2 gene (7q36.3) and one copy gain of DMRT1 gene (9p24.3) were detected in the patient DNA analysis (Figure 3).
Discussion
Deletion of terminal portion of chromosome 7 long arm is an established syndrome. More than 50 cases are reported and in most of them deletion of 7q32 → qter is involved [20, 25]. Microcephaly, short stature, mental and growth retardation, alobar and (semi)lobar HPE, facial dysmorphisms and genital hypoplasia in males are generally observed [9]. Clinical findings also include malformed ears, hypoplasia of corpus callosum, abnormal fingers, overriding toes, caudal deficiency sequence and chest abnormalities, as pectum excavatum [12, 25]. Ocular defects are also described [12, 19, 26–28]. Table 2 summarizes data of 8 patients with one copy deletion of 7q as well as those of present case.
Although HPE was not pointed as a feature of the case here shown, MRI scan of the brain of the patient disclosed hypoplasia of corpus callosum, which may be considered a minimal finding of HPE [20]. In fact, 7q36.3 region encloses the gene SHH- Sonic Hedgehog, which haploinsufficiency was previously associated with HPE [29, 30], microcephaly and cerebral midline defects [31]. Some characteristics as mental retardation, microcephaly, hypotonia and failure to thrive are common features of 9p duplication syndrome, also exhibited by this patient.
Duplication of 9p was recognized as a syndrome in 1970 [8], since then, more than one hundred cases were described and additional findings comprise mental retardation, failure to thrive, hypotonia, microcephaly, hypertelorism, deep set eyes in non-horizontal position, cup-shaped ears, kyphoscholiosis, cryptorchidism in males and syndactily of toes [32]. Moreover, 9p duplication was already associated to HPE [33] and epilepsy [34], also observed in this case.
In fact, double imbalances, as the present one, may confer phenotypic variability to described syndromes, turning it difficult to predict the characteristics that evolved as a result of the global gene imbalance, caused by the concomitant duplication and deletion, and the contribution of each isolated alteration to resulting phenotype.
Actually, chromosomes 7 and 9 are grouped among those frequently involved in karyotype alterations leading to pregnancies losses, mental retardation and multiple congenital abnormalities [35]. Chromosome 7 is also observed as one of the three preferentially involved in reciprocal [36] and complex translocations [37], while 9p23-24 is suggested as hotspot for chromosome breaking [38].
Interestingly, reciprocal translocation t(7;9) leading to 7q33 → qter duplication and pter → 9p23 deletion was also described and phenotype involved prominent metopic suture, epicanthal folds, strabismus, low-set ears, microretrognathia, large anterior fontanel, bilateral simian creases, muscular hypotonia, severe psychomotor retardation [39] and hermaphroditism [40]. Additional cases may help to evaluate if t(7;9) is a recurrent translocation correlated to miscarriages, MRDD and congenital birth defects.
Aneuploidy together with segmental aneusomy constitute the mainly cause of pregnancy losses in humans. Additionally, among 5% of all pregnancies present aneusomies [41] and the resulting children can show malformations and MRDD. It is estimated that 1 out of 150 liveborn babies present chromosomal abnormalities [42], but this number might rise with the employment of arrays and next generation sequencing. In fact, the advent of these high throughput techniques has permitted whole genome scanning with finer resolution, leading to detection of subtelomeric rearrangements and microalterations previously undetectable through conventional approaches, as G-band karyotyping and FISH [43]. Furthermore, DNA arrays have been useful for breakpoint site determination and copy number evaluation improving correlation among chromosomal region alteration and resulting phenotype.
Conclusions
We present the case of a patient exhibiting deletion of 7q36.1 → 36.3 and duplication of 9p22.3 → 23 as a result of an unbalanced translocation from paternal origin. Breakpoint delimitation was achieved with array comparative genomic hybridization analyzes and MLPA assays confirmed the alterations found. Patient presented development delay and facial dysmorphisms, among other abnormalities, consistent with both 7q deletion and 9p duplication syndromes. Reciprocal translocation leading to 9p deletion and 7q duplication had already been reported in two unrelated cases, indicating that t(7;9)(q36;p23) may occur more frequently than expected. Further cases may confirm these results. Finally, the increasing use of high throughput platforms for genomic studies tends to refine chromosome breakpoint analyses, evaluate critical regions involved in genetic disorders and disclose micro rearrangements firstly inaccessible through conventional approaches.
Consent
Written informed consent was obtained from patient’s progenitors for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
Chelly J, Khelfaoui M, Francis F, Cherif B, Bienvenu T: Genetics and pathophysiology of mental retardation. Eur J Hum Genet 2006, 14: 701–713. 10.1038/sj.ejhg.5201595
Preliminary results of 2010 demographic censusAccessed in http://www.ibge.gov.br/english/estatistica/populacao/censo2010/default.shtm
Kerker BD, Owens PL, Zigler E, Horwitz SM: Mental health disorders among individuals with mental retardation: challenges to accurate prevalence estimates. Public Health Rep 2004, 119(4):409–417. 10.1016/j.phr.2004.05.005
Belfer M, Nurcomb B: The epidemiology and burden of child and adolescent mental disorder. In Front matter, in the mental health of children and adolescents: an area of global neglect. Edited by: Remschmidt H, Nurcombe B, Belfer ML, Sartorius N, Okasha A. Ltd, Chichester, UK: John Wiley & Sons; 2007.
Rauch A, Hoyer J, Guth G, Zweier C, Kraus C, Becker C, Zenker M, Hüffmeier U, Thiel C, Rüschendorf F, Nürnberg P, Reis A, Trautmann U: Diagnostic yield of various genetic approaches in patients with unexplained developmental delay or mental retardation. Am J Med Genet 2006, 140A: 2063–2074. 10.1002/ajmg.a.31416
Centerwall WR, Beatty-DeSana JW: The Trisomy 9p Syndrome. Pediatrics 1975, 56(5):748–755.
Wilson GN, Raj A, Baker D: The phenotypic and cytogenetic spectrum of partial trisomy 9. Am J of Med Genet 1985, 20: 277–282. 10.1002/ajmg.1320200211
Rethore MO, Hoehn H, Rott HD, Couturier J, Dutrillaux B, Lejeune J: Analyse de la trisomie 9p par denaturation menagee. Humangenetik 1973, 18: 129–138. 10.1007/BF00291480
Harris EL, Wappner RS, Palmer CG, Hall B, Dinno N, Seashore MR, Breg WR: 7q deletion syndrome (7q32 → 7qter). Clin Genet 1977, 12: 233–238.
Frühmesser A, Haberlandt E, Judmaier W, Schinzel A, Utermann B, Erdel M, Fauth C, Utermann G, Zschocke J, Kotzot D: Effects of deletion and duplication in a patient with a 46, XX, der(7)t(7;17)(q36;p13)mat karyotype. Am J Med Genet 2012, 158A(9):2239–2244. 10.1002/ajmg.a.35450
Ponnala R, Dalal A: Partial monosomy 7q. Indian Pediatr 2011, 48(5):399–401.
Vorsanova SG, Iourov IY, Voinova-Ulas VY, Weise A, Monakhov VV, Kolotii AD, et al.: Partial monosomy 7q34-qter and 21 pter q22.13due to cryptic unbalanced translocation t(7;21) but not monosomy of the whole chromosome 21: a case report plus review of the literature. Molecular Cytogenetics 2008, 1: 13. 10.1186/1755-8166-1-13
Ahn JM, Koo DH, Kwon KW, Lee YK, Lee YH, Lee HH, Nam KH, Lee KH: Partial trisomy 2q(2q37.3 → Qter) and monosomy 7q(7q34 → qter) due to paternal reciprocal translocation 2;7: a case report. J Korean Med Sci 2003, 18: 112–113.
Nowaczyk MJ, Huggins MJ, Tomkins DJ, Rossi E, Ramsay JA, Woulfe J, Scherer SW, Belloni E: Holoprosencephaly, sacral anomalies, and situs ambiguus in an infant with partial monosomy 7q/trisomy 2p and SHH and HLXB9 haploinsufficiency. Clin Genet 2000, 57(5):388–393.
Chen CP, Devriendt K, Lee CC, Chen WL, Wang W, Wang TY: Prenatal diagnosis of partial trisomy 3p(3p23– > pter) and monosomy 7q(7q36– > qter) in a fetus with microcephaly alobar holoprosencephaly and cyclopia. Prenat Diagn 1999, 19(10):986–989. 10.1002/(SICI)1097-0223(199910)19:10<986::AID-PD672>3.0.CO;2-H
Savage NM, Maclachlan NA, Joyce CA, Moore IE, Crolla JA: Isolated sacral agenesis in a fetus monosomic for 7q36. 1 –pqter. J Med Genet 1997, 34: 866–868. 10.1136/jmg.34.10.866
Morichon-Delvallez N, Delezoide AL, Vekemans M: Holopresencephaly and sacral agenesis in featus with terminal deletion 7q36–7qter J Med. Gen 1993, 30: 521–524.
Nielsen KB, Egede F, Mouridsen I, Mohr J: Familial partial 7q monosomy resulting from segregation of an insertional chromosome rearrangement. J Med Genet 1979, 16: 461–466. 10.1136/jmg.16.6.461
Hara S, Yamada T, Nakai H, Ohtani A, Mizuno K: A case of partial 5q trisomy associated with partial 7q monosomy. Br J Ophthalmol 1986, 70: 630–633. 10.1136/bjo.70.8.630
Frints SG, Schoenmakers EF, Smeets E, Petit P, Fryns JP: De novo 7q36 deletion: breakpoint analysis and types of holoprosencephaly. Am J Med Genet 1998, 13(2):153–158.
Shaffer LG, Slovak ML, Campbell LJ (Eds): ISCN: an International System for Human Cytogenetic Nomenclature. Basel: S. Karger; 2009.
SpectralWare® v2.3.3 ACGH analysis system softwareavailable at http://www.lifescisoft.com/spectralware.html
Database of genomics variantsavailable at http://projects.tcag.ca/variation/
Coffalyser V9.4 Software. MRC-Holland, Amsterdam, The Netherlands http://www.mrcholland.com
Schinzel A: Catalogue of unbalanced chromosome aberrations in man, 2nd edn. Walter de Gruyter, Berlin; 2001.
Lukusa T, Vermeesch JR, Fryns JP: De novo deletion 7q36 resulting from a distal 7q/8q translocation: phenotypic expression and comparison to the literature. Genet Couns 2005, 16(1):1–15.
Reynolds JD, Golden WL, Zhang Y, Hiles DA: Ocular abnormalities in terminal deletion of the long arm of chromosome seven. J Pediatr Ophthalmol Strabismus 1984, 21: 28–32.
Friedrich U, Osterballe O, Stenbjerg S, Jorgensen J: A girl with karyotype 46, XX, del (7) (pter-*q32). Hum Genet 1979, 51: 231–235. 10.1007/BF00287183
Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui L-C, Muenke M: Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nature Genet 1996, 14: 357–360. 10.1038/ng1196-357
Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HHQ, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW: Identification of Sonic Hedgehog as a candidate gene responsible for holoprosencephaly. Nature Genet 1996, 14: 353–356. 10.1038/ng1196-353
Lazaro L, Dubourg C, Pasquier L, Duff FL, Blayau M, Durou MR, Pintière AT, Aguilella C, David V, Odent S: Phenotypic and molecular variability of the holoprosencephalic spectrum. Am J Med Genet 2004, 129A: 21–24. 10.1002/ajmg.a.30110
Shinzel A: Trisomy 9p, a chromosome aberration with distinct radiologic findings. Radiology 1979, 130(1):125–133.
Gérard-Blanluet M, Danan C, Sinico M, Lelong F, Borghi E, Dassieu G, Janaud JC, Odent S, Encha-Razavi F: Mosaic trisomy 9 and lobar holoprosencephaly. Am J Med Genet 2002, 111: 295–300. 10.1002/ajmg.10481
Stern JM: The epilepsy of trisomy 9p. Neurology 1996, 47: 821–824. 10.1212/WNL.47.3.821
De Braekeleer M, Dao TN: Cytogenetic studies in couples experiencing repeated pregnancy losses. Hum Reprod 1990, 8(8):519–528.
Campana M, Serra A, Neri G, Reynolds JF: Role of chromosome aberrations in recurrent abortion: A study of 269 balanced translocations. Am J Med Genet 1986, 24: 341–356. 10.1002/ajmg.1320240214
Walker S, Howard PJ, Hunter D: Familial complex autosomal translocations involving chromosomes 7, 8, and 9 exhibiting male and female transmission with segregation and recombination. J Med Genet 1985, 22: 484–491. 10.1136/jmg.22.6.484
Christ LA, Crowe CA, Micale MA, Conroy JM, Schwartz S: Chromosome breakage hotspots and delineation of the critical region for the 9p-deletion syndrome. Am J Hum Genet 1999, 65(5):1387–1395. 10.1086/302606
Tuysuz B, Demirel A, Uysal S, Beyer V, Bartsch O: Boy with seizures (West syndrome) and distal 7q duplication and west syndrome due to an unbalanced 7q;9p translocation. Genet Couns 2008, 19(1):29–35.
Õunap K, Uibo O, Zordania R, Kiho L, Ilus T, Õiglane-Shlik E, Bartsch O: Three patients with 9p deletions including DMRT1 and DMRT2: A girl with XY complement, bilateral ovotestes, and extreme growth retardation, and two XX females with normal pubertal development. Am J Med Genet 2004, 130A: 415–423. 10.1002/ajmg.a.30269
Hassold T, Hall H, Hunt P: The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 2007, 16(R2):R203-R208. 10.1093/hmg/ddm243
Evans HJ: Chromosome anomalies among livebirths. J Med Genet 1977, 14: 309–312. 10.1136/jmg.14.5.309
Poot M, Eleveld MJ, van ’t Slot R, van Amstel HKP, Hochstenbach R: Recurrent copy number changes in mentally retarded children harbour genes involved in cellular localization and the glutamate receptor complex. Eur J Hum Genet 2010, 18: 39–46. 10.1038/ejhg.2009.120
Acknowledgments
We would like to thank patient and her parents for accepting participate in the research. ML Chauffaille received CNPq Produtividade em Pesquisa grant number 309932/2009-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PKO performed the aCGH experiments, MLPA assays, data analysis and interpretation, and participated in the preparation and revision of the manuscript. SS was responsible for the patient examination and clinical description, and participated in the preparation of the manuscript. CAL performed the karyotype analysis. LK performed the DNA extraction and participated in aCGH experiments. KF participated in writing of the discussion section and in the revision of the manuscript. CML coordinated the study, designed the project, was responsible for fund obtaining, and participated from data interpretation, manuscript writing and review. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pelegrino, K.d.O., Sugayama, S., Catelani, A.L. et al. 7q36 deletion and 9p22 duplication: effects of a double imbalance. Mol Cytogenet 6, 2 (2013). https://doi.org/10.1186/1755-8166-6-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-8166-6-2