Abstract
Background
α-Mangostin was extracted with methanol from the rind of mangosteen fruit and purified by using silica gel column chromatography technique. The compound is characterised using infrared, 13C and 1H NMR as well as UV–vis spectroscopy. The α-mangostin dispersion in colloidal systems was studied by incorporating it with an ionic microgel, poly (N-Isopropylacrylamide)-co-2VP at different pH.
Result
The DLS result showed the size of microgel-α-mangostin mixture declined from 548 nm to 200 nm upon the increment of the pH. Moreover, it was found the morphology of loaded compound depended largely on the nature of the continuous phase of the microgel system. Interestingly, by manipulating the pH, α-mangostin tends to form crystal at extremely low pH and transforms into spherical shapes at pH 6.
Conclusion
This research shows different structures of the α-mangostin particle that are attributed by adjusting the pH using microgel systems as a template.

Similar content being viewed by others
Background
Mangosteen, the ‘queen’ of all fruits is a plant native to Southeast Asia that is used as traditional remedy to treating skin infections wound, improve muscle and bone pain, eating disorder, diarrhea and accelerating wound healing [1, 2]. Among the essential phytonutrients found in the rind of the mangosteen, α-mangostin or 1,3,6-trihydroxy-7-methoxy, 2-8-bis (3-methyl-but-2-enyl)-xanthen-9-one stands alone in its impressive benefits. Since it was first discovered by W. Schmid in 1855 [3], this compound has attracted many researchers due to its biological active properties such as antioxidant [4], anti bacteria [5], antifungal [6], anti inflammatory [7], anti cancer [8] and anti tuberculosis [9], therapeutic drugs [10] and also being used as mosquitoes larvicide [11]. Furthermore, it has been commercialised as supplement in food products and natural dyes in fabric industries, which are readily available in the worldwide market. However, its poor solubility in aqueous solution and low oral bioavailability are the limiting factors for many applications. Therefore, it is a challenge to the scientists to solve these problems in order to fully utilise this compound deemed suitable for the human body systems. So far, there is only one reported study on the enhancement of the α-mangostin solubility and oral bioavailability by Aisha et al [12]. on the solid dispersion of α-mangostin in water soluble carriers using polyvinylpyrrolidone (PVP). Although this technique showed an improvement on the dissolution of α-mangostin, its commercial use is still very limited, primarily due to manufacturing difficulties and stability problems [13]. As an alternative, a responsive polymer has being used for α-mangostin dispersions.
Much attention has been given to responsive polymers (smart polymers) due to their response ability to external stimuli such as temperature [14], electrolyte, light and pH [15]. One of them is microgel, a polymer colloid particle with three-dimensional network structure that offers many applications from the viewpoint of drug delivery. It can be manipulated as nanoreactor which controls the size property, from macrometers to nanometers [13–15]. Moreover, this polymer is responsive and having large surface network area enables to incorporate with bio-related molecules. This ‘smart’ system is used for bioactive molecules entrapments including drugs, proteins, carbohydrates and DNA [14–16]. Its applications are not only limited for biomedical purposes but are widely applied for the incorporation of inorganic nanocrystals, quantum dots [16, 17], magnetic nanoparticles [18, 19], optical imaging for living cells and photodynamic therapy [20].
Ionic microgels are formed when at least one of the co-monomer becomes charged specifically when the pH reaches the pKa of that species. Generally monomer such as 2-vinylpyridine [21], acrylic acid [22] and methacrylic acid [23] are used to produce a pH responsive microgel, which can be synthesized via emulsion polymerisation or surfactant free emulsion polymerisation [24]. Xu et al [25] reported on the utilization of poly (ϵ-caprolactone)-pluronic–poly-(ϵ-caprolactone)-dimethylacrylic (PCFC-DMA) as a vitamin B12 carrier. These modified microgels were discovered to be very sensitive towards pH. They found the vitamin B12 could be released from the microgel faster at pH 7.4 than at pH 12. Wang et al [26]. studied the utilization of poly (N-isopropylacrylamide-co-acrylamide) as bleomycin drug carrier. The result showed the releasing rates of bleomycin from the microgel exhibited diffusion control at human body temperature.
Both experiments discussed on the successful of using microgel as a carrier for commercially bioactive molecules yet, there was no attempt of incorporating microgel with any organic bioactive compounds. Therefore, this paper discussed on the characterisations of extracted α-mangostin and the attempt of its dispersion in an ionic microgel system, poly (N-isopropylacrilamide) PNIPAM with a co-monomer 2-vinylpyridine (PNIPAM-co-2VP). The stability over a range of pH in microgel system is also reported
Results and discussion
α-Mangostin characterisation
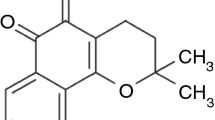
α-mangostin as in Figure 1 was obtained as a yellow crystalline solid and had a melting point of 175–177°C. The infrared spectrum showed the stretching of hydroxyl (OH) and carbonyl (C = O) group at 3256 and 1639 cm-1 respectively. The band at 1460 cm-1 showed the presence of aromatic C = C group while the band at 1077 cm-1 represents C-O ether bond. The stretching of C-H bond at 2856, 2925 and 2962 cm-1 was consistent with the presence of methyl groups in this compound. The infrared spectrum indicated the functional groups in the extracted compound have a similarity to xanthone [27]. NMR (DMSO-d6, 400 MHz) δH (ppm): 1.59 (6H, s, H-14 and H-15),1.70 (6H, s, H19 and H20), 2.50 (4H, d, H11 and H16), 3.18 (3H, s, -OCH3), 5.13 (2H, d, H12), 6.33 (1H, s, H4), 6.78 (1H, s, H5), 13.69 (3H, s, C1-OH,C3-OH and C6-OH). 13C NMR (DMSO-d6, 400 MHz) δC (ppm): 17.97 (C-14 and C-20), 18.28 (C-15 and C-19), 25.79 (C-11 and C-16), 60.49 (−OCH3), 92.54 (C-4), 102.03 (C-5 and C9a), 109.98 (C-2), 110.19 (C-8a), 122.65(C-12), 123.86 (C-17), 130.94 (C-13 and C-18), 136.73 (C-8), 143.63 (C-7), 154.47 (C-4a), 157.20 (C-6), 158.9 (C-10a), 160.11 (C-1), 162.59 (C-3), 181.59 (C-9). The NMR data are consistent with the literature [28].
The UV–vis spectra of α-mangostin as seen in Figure 2 showed maximum absorption peaks at 243, 317 and 352 nm. These values are in agreement with the reported values [28]. Absorption peak at 243 nm represents the C = C chromophore with the excitation energy π → π* transition while the peak at 317 nm is related to C = O chromophore with excitation energy n → π* transition. There is one shoulder at 265 nm indicating the C-O-C with lone pair chromophore denoting n → σ* transition. The existence of shoulder might be due to the interaction of the compound with the protic solvent used. However, when α-mangostin is mixed with PNIPAM-co-2VP microgel, only peaks at 317 and 352 nm are observed while peak at 243 nm and the shoulder disappeared. The absorbance intensity of the mixture peaks is also decreased. This showed an interaction between α-mangostin and the microgel system.
Incorporating α-mangostin into PNIPAM-co-2VP dispersions
The hydrodynamic diameter of 0.1 wt% cross-linked PNIPAM-co-P2VP as native microgel particles (without) and with additional α-mangostin (with concentration of 5 x 10-5 M) as a function of pH is shown in Figure 3. Under acidic solution conditions, the PNIPAM-co-2VP microgel particles have a large hydrodynamic diameter, however with increasing the pH, their diameter decreases. The largest change in hydrodynamic diameter occurs at approximately pH 4. At low pH, the pyridine groups in the polymer framework are protonated resulting in charged microgel particles. Consequently, it caused electrostatic repulsion between the polymer chains and increased the osmotic pressure within the particles in the network hence led the microgel particles to swell. As a result of the hydrophilic nature of PNIPAM-co-2VP, swelling begins when the pH is a couple of units above the pKa value of 2VP (approximately 4) and shows a gradual swelling profile with decreasing pH. These results are consistent with observations reported by Daly & Saunders [29] and Lazim et. al [30].. Upon adding the α-mangostin, the hydrodynamic diameter profile for microgels decreased nearly half of the original size, which suggests the α-mangostin has infiltrated the networks. In a significant research reported by Lazim et. al [30], if any compounds are most likely to be adsorbed to the surface of the microgel particles and not infiltrated into the microgel network, it would be shown by a fully swollen in hydrodynamic diameter particularly at low pH where positively charged microgels have maximum charge.
In both cases, the particle size reached its optimal de-swollen state at pH 5 (Dh ~200 nm). Any further increase in pH had no significant effect on the particle size. The exact mechanism by which the α-mangostin associates with the microgel is still unclear. However, Bradley et.al [31] proposed that the step involved would be the electrostatic interaction between the negatively charged of the additional compound with the cationic groups on the microgel network. This would act to reduce the electrostatic repulsion screening of the charges that would otherwise exists in the network [31].
TEM imaging
The mixtures of microgels and α-mangostin were characterised using the transmission electron microscope (TEM), which allowed a direct morphology dispersion investigation in the systems without staining the necessary process. Figure 4 shows TEM images of up taking α-mangostin into PNIPAM-co-2VP microgel systems at pH 2 and pH 6. It has been established that the microscopic gel systems were used as promising templates to prepare nanoparticles, composite with non-classical shapes of morphology [32, 33]. It can be clearly seen that at pH2 α-mangostin showed formation of nanocrystals in PNIPAM-co-2VP microgel systems with average sizes of 148 nm. At low pH, an initially fluid-like system slowed down gradually due to the formation of crystalline structures, hence nucleation occurred homogeneously throughout the systems [34]. Upon increasing the pH by adding the NaOH, the negatively charge concentration increases and neutralization occurs. As a result, an additional attractive force between the microgel particles is introduced [35]resulting the α-mangostin agglomerated. In comparison with acidic ambience, the size of α-mangostin particle at pH 6 is larger with an average size of 217 nm (Figure 4b).
Conclusion
Yellow compound was successfully extracted from the rind of Garcinia Mangostana Linn., which has similarity with α-mangostin characteristics and supported by data that is highly significant with previous literature. The α-mangostin dispersions in microgel systems as a function of pH were investigated. The DLS results showed that there was an interaction in the presence of α-mangostin in PNIPAM-co-2VP microgel systems in swollen state, where the neutralization occurred which then affected the particle sizes. On the contrary, at the collapsed state (pH 6 and higher) there was no contribution to any significant changes to the structure of PNIPAM-co-2VP microgels since it was only a surface interaction. Interestingly, the extreme of pH might not only affect the colloidal dispersion but also the α-mangostin morphology. At pH 2, it resulted as crystal-like shape. However, by increasing the pH value it turned to be agglomerated as big spherical forms. This result showed PNIPAM-co-2VP could be potentially used as a controlled-reactor triggered by pH for α-mangostin particles. Over a variation of pH, interaction of the microgel polymers with distinct crystallographic planes or area of the growing nuclei permits control of the size, shape and structure of the organic compounds. Moreover, it can also be used to overcome its weakness in the aqueous solubility. The future research for investigating the retention, release, the kinetics and its mechanism might be useful for the application of PNIPAM-co-2VP as drug delivery agent for α-mangostin particles to the body system.
Methods
Material and instrumentation
All experiments utilized purified water which was milli-Q water standard (PureLab, Elga), with resistivity of 18.2 MΩ cm. Dialysis tubing (Fisher) with a Mw cut-off of 12,000-14,000 Daltons was used for microgel purification. For all samples, pH was measured by using a waterproof pH meter (HI98127, pHep Hanna). For poly (N-isopropylacrylamide) co-2-vinylpyridine (PNIPAM-co 2VP) microgel synthesis, the cross-linking monomers divinylbenzene (DVB, Aldrich, 80%) and N, N-methylenebisacrylamide (BA, Aldrich, 99%), were used without further purification. The initiator used for the cationic microgels was 2, 2’-azobis (2-methylpropionamidine) dihydrochloride (V50, Waco, 95%). Aqueous solutions of HCl and NaOH were used to adjust pH. All chemicals and solvents used were reagent grade and used without further purification. Infrared spectra were recorded on a Perkin Elmer GX Spectrometer by using potassium bromide pellet. The size and morphology of the sample was investigated by using Transmission Electron Microscope (TEM) Philips CM12 model.
UV determination
The Ultra-violet spectra were determined by Shimadzu UV–vis Spectrophotometer (UV 2400PC series). The sample was dissolved in ethanol and the solution was scanned from 200 to 450 nm. Ethanol was used as the background for α-mangostin sample while a mixture of ethanol and pnipam-co-2VP microgel was used for α-mangostin and microgel mixture.
NMR analysis
The 1H and 13C nuclear magnetic resonance spectra were measured with Jeol JNM-ECP 400 NMR Spectrometer. Samples were dissolved in dimethylsulfoxide (DMSO)-d 6 and chemical shifts were given in parts per million (ppm) relative to tetramethylsilane (TMS) as an internal standard.
DLS and zeta potential characterisation
Microgel particle sizes and polydispersities index (PDI) were determined by dynamic light scattering (DLS) using a Zetasizer Nano-S (Malvern,PA). The electrophoretic mobility (μe) for α-mangostin and microgel is determined as a function of pH for dispersions at 25°C. The α-mangostin μe values remain negative across the entire pH range from 2 to 10, whereas the PNIPAM-co-2VP microgel μe values remain positive which are consistent with the cationic polymer remaining with positive charge.
Extraction of α-mangostin
Dried mangosteen rind samples were collected from Terengganu, Malaysia and the extraction of α-mangostin was carried out by following the normal procedure of isolating natural products as previously reported [36]. The grinded mangosteen rind was extracted with methanol for three weeks and then separated by column chromatography eluted with the mixture of dichloromethane-hexane (6:4) giving a fine yellow powder.
Synthesis of PNIPAM-co-2VP microgel
The PNIPAM-co-2VP microgels were synthesized by a surfactant free polymerization technique as previously reported [37]. Briefly, 800 ml of purified water was purged with nitrogen for 30 minutes in a 1 L, five-neck round bottom flask fitted with a mechanical stirrer, which operated at 150 rpm. About 0.50 g cationic initiator 2, 2’-azobis (2-methylpropionamidine) dihydrochloride (V50, Waco) was then added to the reaction flask and stirred. In a beaker 200 ml of purified water (milli-Q standard) (PureLab, Elga), 3.75 g of NIPAM and 0.55 g of BA together with 1.25 g of 2-vinylpyridine (2VP) were added together and stirred for 15 minutes. This solution was then added to the reaction vessel with the temperature raised to 70°C. The polymerization reaction was left to proceed for 6 hours with continuous stirring (~150 rpm). The outcome of the dispersion was filtered through glass wool followed by extensive dialysis against milli-Q water for one week with two changes of water per day.
References
D’Orazio D, Raederstorff D, Schueler G, Wang-Schmidt Y, Wolfram S: Novel use of organic compounds. Patent US. 2009, 0221693: A1-
Mahabusarakam W, Wiriyachitra P, Taylor WC: Chemical constituents of garcinia mangostana. J Nat Prod. 1987, 50: 474-478. 10.1021/np50051a021.
Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM: Review- medicinal properties of mangosteen (Garcinia Mangostana). Food Chem Toxicol. 2008, 46: 3227-3239. 10.1016/j.fct.2008.07.024.
Suvarnakuta P, Chaweerungrat C, Devahastin S: Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011, 1: 240-247.
Nguyen PTM, Marquis RE: Antimicrobial actions of α-mangostin against oral streptococci. Can J Microbiol. 2011, 3: 217-225.
Ruchadaporn K, Kusuma J, Niratcha C: Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci. 2009, 51: 401-406. 10.2334/josnusd.51.401.
Chen L, Ling-Ling Y, Ching-Chiung W: Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008, 46: 688-693. 10.1016/j.fct.2007.09.096.
Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinumad M, Nozawaa Y: Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg Med Chem. 2005, 13: 6064-6069. 10.1016/j.bmc.2005.06.065.
Arunrattiyakorn P, Suksamrarn S, Suwannasai N, Kanzaki H: Microbial metabolism of α-mangostin isolated from Garcinia mangostana L. Phytochemistry. 2011, 72: 730-734. 10.1016/j.phytochem.2011.02.007.
Moffet A, Shah P: Pharmaceutical and therapeutic composition derived from garcinia mangostana L. Plant Patent US. 2006, 055688: A1-
Lan Q, Kim M: Use of α-mangostin as mosquito larvicide. Patent US. 2008, 0300300: A1-
Aisha AFA, Ismail Z, Abu-Salah KM, Majid AMSA: Solid dispersion of α-mangostin improve its aqueous solubility through self-assembly of nanomicelles. J Pharm Sci. 2011, 101: 815-825.
Serajuddin ATM: Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999, 88: 1058-1066. 10.1021/js980403l.
Lusançay G, Norvez S, Iliopoulos I: Temperature-controlled release of catechol dye in thermosensitive phenylboronate-containing copolymers: a quantitative study. Eur Polym J. 2010, 46: 1367-1373. 10.1016/j.eurpolymj.2010.03.020.
Yin J, Dupin D, Li J, Armes SP, Liu S: pH-induced deswelling kinetics of sterically stabilized poly(2-vinylpyridine) microgels probed by stopped-flow light scattering. Langmuir. 2008, 24: 9334-9340. 10.1021/la8014282.
Hasegawa U, Nomura SM, Kaul SC, Hirano T, Akiyoshi K: Nanogelquantum dot hybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun. 2005, 331: 917-921. 10.1016/j.bbrc.2005.03.228.
Fukui T, Kobayashi H, Hasegawa U, Nagasawa T, Akiyoshi K, Ishikawa I: Intracellular delivery of nanogel-quantum dot hybrid nanoparticles into human periodontal ligament cells. Drug Metab Lett. 2007, 1: 131-135. 10.2174/187231207780363570.
Gupta AK, Wells S: Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Trans Nanobiosci. 2004, 3: 66-73. 10.1109/TNB.2003.820277.
Chatterjee J, Haik Y, Chen CJ: Size dependent magnetic properties of iron oxide nanoparticles. J Magn Magn Mater. 2003, 257: 113-118. 10.1016/S0304-8853(02)01066-1.
Das M, Sanson N, Fava D, Kumacheva E: Zwitterionic poly(betaine-n-isopropylacrylamide) microgels:properties and applications. Langmuir. 2007, 23: 196-201. 10.1021/la061596s.
Loxley A, Vincent B: Equilibrium and kinetic aspects of the pH-dependent swelling of poly(2-vinylpyridine-co-styrene) microgels. Colloid Polym Sci. 2007, 275: 1108-1114.
Zhang J, Xu S, Kumacheva E: Polymer microgels: reactors for semiconductor, metal, and magnetic nanoparticles. J Am Chem Soc. 2004, 126: 7908-7914. 10.1021/ja031523k.
Saunders BR, Crowther HM, Vincent B: Poly[(methyl methacrylate)-co-(methacrylic acid)] microgel particles: swelling control using pH, cononsolvency, and osmotic deswelling. Macromolecules. 1997, 30: 482-487. 10.1021/ma961277f.
Tan JPK, Goh CH, Tam KC: Comparative drug release studies of two cationic drugs from pH-responsive nanogels. Eur J Pharm Sci. 2007, 32: 340-348. 10.1016/j.ejps.2007.08.010.
Xu X, Fu S, Wang K, Jia W, Guo G, Zheng X, Dong P, Guo Q, Qian Z: Preparation and characterization of vitamin-12 loaded biodegradable pH-sensitive microgels. J Microencapsul. 2009, 26: 642-648. 10.3109/02652040802610827.
Wang Q, Zhao Y, Yang Y, Xu H, Yang X: Thermosensitive phase behavior and drug release of in situ gelable poly(N-isopropylacrylamide-co-acrylamide) microgels. Colloid Polym Sci. 2007, 285: 515-521. 10.1007/s00396-006-1592-6.
Boonnak N, Karalai C, Chantrapromma S, Ponglimanont C, Fun H, Kanjana-Opas A, Laphookhieo S: Bioactive prenylated xanthones and anthraquinones fromCratoxylum formosum ssp. Pruniflorum. Tetrahedron. 2006, 62: 8850-8859. 10.1016/j.tet.2006.06.003.
Yu L, Zhao M, Yang B, Zhao Q, Jiang Y: Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food Chem. 2007, 104: 176-181. 10.1016/j.foodchem.2006.11.018.
Daly E, Saunders BR: A study of the effect of electrolyte on the swelling and stability of poly (N-isopropylacrylamide) microgel dispersions. Langmuir. 2000, 16: 5546-5552. 10.1021/la991292o.
Lazim AM, Bradley M, Eastoe J, Trickett K, Mohamed A, Rogeus SE: Recovery of gold nanoparticles using pH-sensitive microgels. Soft Matter. 2010, 6: 2050-2055. 10.1039/c002511a.
Bradley M, Vincent B, Warren N, Eastoe J, Vesperinas A: Poly (vinylpyridine) core/poly (N-isopropylacrylamide) shell microgel particles: Their characterization and the uptake and release of an anionic surfactant. Langmuir. 2008, 24: 2421-2425. 10.1021/la703327v.
Vimala K, Samba Sivudu K, Murali Mohan Y, Sreedhar B, Mohana Raju K: Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly(acrylamide) and carbohydrates: a rational methodology for antibacterial application. Carbohydr Polym. 2009, 75: 463-471. 10.1016/j.carbpol.2008.08.009.
Ballauff M, Lu Y: “Smart” nanoparticles: preparation, characterization and applications. Polymer. 2007, 48: 1815-1823. 10.1016/j.polymer.2007.02.004.
Muluneh M, Sprakel J, Wyss HM, Mattsson J, Weitz DA: Direct visualization of pH-dependent evolution of structure and dynamics in microgel suspensions. J Phys Condens Matter. 2011, 23 (50): 505101-10.1088/0953-8984/23/50/505101.
Cho JK, Meng Z, Lyon LA, Breedveld V: Tunable attractive and repulsive interactions between pH-responsive microgels. Soft Matter. 2009, 5: 3599-3602. 10.1039/b912105f.
Pedraza-Chaverrí J, Reyes-Fermín LM, Nolasco-Amaya EG, Orozco-Ibarra M, Medina-Campos ON, González-Cuahutencos O, Rivero-Cruz I, Mata R: ROS scavenging capacity and neuroprottective effect of α-mangostin against 3-nitropropionicacid in cerebellar granule neurons. Exp Toxicol Pathol. 2009, 61: 491-501. 10.1016/j.etp.2008.11.002.
Hall RJ, Pinkrah VT, Chowdhry BZ, Snowden MJ: Heteroaggregation studies of mixed cationic co-polymer/anionic homopolymer microgel dispersions. Colloids Surf A. 2004, 233: 25-38. 10.1016/j.colsurfa.2003.06.004.
Acknowledgements
The authors would like to thank the Faculty of Science and Technology, Universiti Kebangsaan Malaysia for the provision of laboratory facilities and technical assistance. MA also gratefully acknowledges the scholarship from the National Science Fellowship (NSF), MOSTI. AML acknowledge funding from DIP 2012–11 and FRGS/1/2011/SG/UKM/01/25.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
There is no conflict of interest for all authors of this article.
Authors’ contributions
MA carried out the extraction, purification and characterization of the compounds as well as conducts the dispersion study. AML carried out the synthesis of microgel and drafting the manuscript. BMY conceived the study, participated in its design and revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ahmad, M., Yamin, B.M. & Mat Lazim, A. A study on dispersion and characterisation of α-mangostin loaded pH sensitive microgel systems. Chemistry Central Journal 7, 85 (2013). https://doi.org/10.1186/1752-153X-7-85
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-153X-7-85