Abstract
Introduction
Emergency treatment of major sub-/total traumatic amputations continue to represent a clinical challenge due to high infection rates and serious handicaps. Effective treatment is based on two columns: surgery and antimicrobial therapy. Detailed identification of pathogen spectrum and epidemiology associated with these injuries is of tremendous importance as it guides the initial empiric antibiotic regimen and prevents adverse septic effents.
Methods
In this retrospective study 51 patients with major traumatic amputations (n = 16) and subtotal amputations (n = 35) treated from 2001 to 2010 in our trauma center were investigated. All patients received emergency surgery, debridement with microbiological testing within 6 h after admission and empircic antimicrobial therapy. Additionally to baseline patient characteristics, the incidence of positive standardized microbiologic testing combined with clinical signs of infection, pathogen spectrum, administered antimicrobial agents and clinical complications were analyzed.
Results
70.6% of the patients (n = 36) acquired wound infection. In 39% wounds were contaminated on day 1, whereas the mean length of duration until first pathogen detection was 9.1 ± 13.4 days after injury. In 37% polymicrobial colonization and 28% Pseudomonas were responsible for wound infections during hospitalization. In 45% the empirc antimicrobial therapy focussed on Gram positive strains did not cover the detected bacteria, according antimicrobial resistogram. It was significantly more often found in infections associated with Pseudomonas (p 0.02) or polymicrobial wound infections.
Conclusions
This epidemiologic study reveals a pathogen shift from Gram-positive to Gram-negative strains with high incidence of Pseudomonas and polymicrobial infections in sub-/total major traumatic amputations. Therefore, empiric antimicrobial treatment historically focussing on Gram-positive strains must be adjusted. We recommend the use of Piperacillin/Tazobactam for these injuries. As soon as possible antimicrobial treatment should be changed from empiric to goal directed therapy according to the microbiological tests and resistogram results.
Similar content being viewed by others
Background
The emergency treatment of subtotal and total traumatic amputations (type IIIC open fractures according to the Gustilo/Anderson and type IV according to Tscherne/Oestern classification) with extensive soft tissue injury represents a clinical challenge [1–3]. Although sub-/total traumatic amputations are rare injuries, the potential risk for individual detrimental consequences are serious, including limb loss, infections, osteomyelitis, delayed or non-union, bone segmental defects, soft tissue defects and sensomotor deficits, which in turn cause major socioeconomic costs [4–7].
The successful management of open fractures and traumatic amputations is limited due to high infection rate (<50%), poor soft tissue coverage, impaired fracture healing, non-union and secondary amputation. Traumatic amputations are known to be almost always contaminated with any sort of pathogen, predominately Gram-positive strains [8]. The infection rates are associated with the severity of soft tissue damage (muscle injury, wound contamination, blood supply, reperfusion injury), application of tourniquet in the field and need of fasciotomy in compartment syndrome [1, 2, 6, 9–12]. In particular, sub-/total traumatic amputations with ischemia are more susceptible to infections. Moreover, following vascular repair and reperfusion, severe reperfusion edema and post-ischemic compartment syndrome are observed. Reperfusion syndrome additionally impairs the restored microcirculatory perfusion and local delivery of antibiotics due to endothelial leakage and pre-capillary shunting. Furthermore, traumatic coagulopathy and coagulation factor deficiency (e.g. factor XIII) are known to additionally impair wound healing and predispose towards wound infection [13].
The two cornerstones for successful sub-/total traumatic amputation treatment are emergency surgery with bleeding control, radical debridement, temporary stabilization and early, sufficient empiric antimicrobial therapy [7, 14]. Despite high infection rates up to 66%, the most effective type or combination of empiric antimicrobial agent in open fractures and traumatic amputations are still a controversy [2, 6, 15–18]. In the last decades Staphylococcus aureus, Staphylococcus epidermidis and Gram-negative strains have been the predominate pathogens in these injuries [8]. Empiric antimicrobial therapy is focusing on epidemiologic data, plausible pathogens and patient’s specific comorbidities (e.g. allergy, diabetes). But current epidemiologic data for sub-/total traumatic amputations are not available and microbiological spectrum and corresponding bacterial resistances are changing over the time.
Therefore, this study aimed to retrospectively analyze the characteristics, epidemiologic microbiological pathogen spectrum, incidence of multi-resistant pathogens, the efficiency of antimicrobial therapy, the incidence of multiple trauma and coagulopathy of all sub-/total traumatic amputations, treated in our center.
Methods
In this retrospective study we included 51 sub-/total traumatic amputations treated at the Campus Virchow Klinikum, Charité - Universitätsmedizin between 2001 and 2010. Due to the retrospective and observational character of the study no approval by our local ethic committee has been necessary.
Inclusion criteria
-
Major sub-/total traumatic amputation (type IV Gustilo/Anderson Anderson and Tscherne/Oestern; n = 16) [2, 3]
-
Severe open fractures with vascular injury and large soft tissue injury (>5 cm defect or more than loss of 3/4 of circumference; type IIIC Gustilo/Anderson and type IV Tscherne/Oestern; n = 35) [2, 3].
Exclusion criteria
-
Type IIIc open fractures with <5 cm soft tissue defect or <3/4 of circumference [2]
-
Minor traumatic amputations (finger, toe)
-
Survival <24 hours or patients dying before obtaining microbiological test
-
Lack of substantial information in medical record.
The baseline characteristics (gender, age, trauma mechanism, injury type, anatomical fracture location (AO classification) and following parameters were recorded:
-
Abbreviated Injury Scale (AIS) and Injury Severity Score (ISS)
-
Hanover Polytrauma Score (PTS)
-
Clinical signs of wound infection (rubor, calor, dolor, wound secretion)
-
Microbiological testing (swab, tissue, wound secretion)
-
Pathogen type (Gram positive/negative)
-
Laboratory findings (white blood count (WBC), C-reactive protein (CRP))
-
Type and sufficiency of antimicobial therapy
-
Risk factors for infection (diabetes, immune deficiency, compartment syndrome, arterial occlusive disease, nicotine/alcohol abuse)
-
Length of hospital stay and critical care
-
Type and number of surgical procedures, revisions and use of negative pressure wound therapy (NPWT/VAC)
-
Late complications (osteomyelitis, removal of osteosynthesis materials due to infection, secondary amputation).
The leading injury was defined as the body region (head, face/neck, thorax, abdomen, extremities) with the highest AIS score. Polytrauma was classified according to ISS >15 and severe polytrauma ISS >25.
Traumatic coagulopathy on admission, negatively influencing wound healing, microcirculation and the clinical course of open fractures, was defined by INR >1.2 and/or platelet count <100 000/nl [13, 19].
Microbiological assessment and definition of wound infections
All microbiological tests of the 51 patients were screened for pathogen type, number of simultaneous pathogens, resistance towards administered antimicrobial agent and time until positive pathogen proof.
Microbiological specimens (i.e. wound swabs, tissue samples, drain fluids) were obtained during surgery before debridement and administration of antibiotic therapy. Within operation at least 2 wound swabs (superficial and deep) were obtained. Based on surgeon’s decision additionally tissue samples were obtain under sterile conditions. Drains were standardized removed within 2 days after surgery except high amount of wound secretion. Therefore, drain fluids were tested in case of persisting secretion >48 hours after surgery. Initial surgical debridement was performed within 2 hours after injury, followed by staged surgical debridement until conditioning of soft tissue or plastic surgery, depending on the clinical situation. Specimens were sent native (i.e. without transport media) and cultivated within 6 hours of sampling, except patient arrived at night, then samples were cultivated next morning. Samples were plated on sheep blood agar plates and incubated for 7 days under aerobic and anaerobic conditions at 37°C; an aliquot was in addition inoculated in thioglycolate broth. Identification and susceptibility testing of grown microorganisms were performed as standard microbiological methods. Wound cultures on day 1 refer to intraoperative microbiological samples collected before the first surgical debridement and antibiotic therapy, reflecting the incidence and spectrum of initial wound contamination.
Wound infection was defined as the proof of at least one pathogenic species in microbiological testing combined with clinical signs of infection (fever, pus, rubor, odor, macerated soft tissue) and elevated laboratory infectious parameter (WBC, CRP). A wound infection occurring within 14 days after trauma was defined as an early infection. Simultaneous detection of more than one pathogen species in the same sample was classified as polymicrobial wound infection. The initially applied antibiotic therapy was considered to be insufficient, if proven so by the microbiological resistance determination via antibiogram.
Surgical management
Our algorithm for limb salvage and surgical decision making was published in 2010 and performed with the help of the Mangled Extremity Severity Score ≥7, injury severity (polytrauma: “life before limb”) and physiologic parameters (base deficit, lactate, blood pressure, and coagulopathy [6, 20].
Out of 42 cases (82%) of limb salvage, revascularization was successfully performed in 37 cases (73%). In 18% primary (n = 9), 14% (n = 7) macro-replantation and 10% (n = 5) secondary amputation was performed.
Our soft tissue management is guided by the degree of open fracture, contusions, contamination and defect size. According to secondary swelling with progressive soft tissue necrosis and compartment syndrome primary wound closure in type III open fractures was an exception. In our protocol we performed open amputations in case of primary amputation. Criteria for stump formation were clean wean, no pathogen proof obtained in the microbiological test in the prior operation and no need for surgical debridement. We covered the soft tissue defects in type III open fractures with Epigard® + dry dressing in 44 patients, 5 open wound treatment with initial dry dressing and 1 primary NPWT. In 1 case primary wound closure was possible. The dressings were changed at least once every day. NPWT was used in 25 patients (49%) and were changed between 2–5 days according to wound conditions while surgery and results of microbiological testing.
Multiple trauma patients were treated according to their injury severity and hemodynamic constitution with life before limb principle for in extremis patients and damage control orthopedic surgery for hemodynamic unstable patients.
Statistic
Statistical analysis was conducted by use of PASW statistics 21.0 (IBM, USA; Mann–Whitney-/ Wilcoxon test). Continuous variables were described with standard variation (±) and p-values <0.05 were considered as statistically significant.
Results
Characteristics
51 patients were included in this study (male/female: n = 42/9; 82/18%, mean age: 37.5 ± 13.4 years, 62.7% coagulopathy at admission, 7.8% mortality rate). The length of hospitalization in non-survivors (mean 39 ± 42.4 days; range 16–103 days) compared to survivors (mean 39.5 ± 20.4 days; range 7–103 days) showed no statistical significant difference. Therefore, non-survivors were not excluded from analysis.
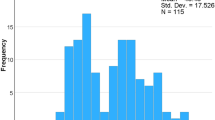
The mean injury severity was 24.7 ± 13.0 points for ISS and 34.8 ± 20.7 points for PTS. 62% were polytraumatized (n = 31, ISS > 15 points) and 52.9% suffered a severe polytrauma (n = 27, ISS >25 points). The trauma mechanisms are shown in Table 1.In polytraumatized patients the most common injury according to the AIS was extremity trauma (n = 39; 76.5%), followed by chest trauma (n = 9; 17.6%) and abdomen trauma (n = 3; 5.9%). The anatomic location of the open fractures are shown in Figure 1.
Risk factors for infection
Risk factors for wound infection were negligible (Table 2). We found no significant difference for the rate of wound infections in patient’s with-/out risk factors or/and compartment syndrome.
Injuries
68.6% of the patients sustained subtotal (n = 35) and 31.4% total traumatic amputation (n = 16). No statistical significant differences was found for subtotal and total traumatic amputation referring injury severity, hospitalization (39.1 ± 22.4, range 7–103 days), ICU-days (11.9 ± 14.2, range 0–78 days), survival, acute (OF:51%/n = 18; amputation: 69%/n = 11) and total infection rate (OF:66%/n = 23; amputation: 81%/n = 13), type of infection, number and type of needed surgical interventions. Also no differences for the above listed parameters were detected for limb salvage (72.6%; n = 37), primary (17.6%; n = 9) and secondary amputation (9.8%; n = 5). But the mean ISS showed a trend to primary (27.9 ± 12.5 points) and secondary amputation (28.8 ± 15.1 points) compared to limb salvage group (23.3 ± 13 points). Severe polytrauma (ISS > 25) was more frequent in primary (67%) and secondary amputation (60%) compared to limb salvage group (49%).
The number of surgical interventions was 4.4 ± 3.6 for limb salvage, 4.3 ± 3.2 for primary amputation and 9.2 ± 4.4 for secondary amputations. We observed a higher number operations in the secondary compared to the primary amputation and limb salvage group (p 0.04).
The analysis of common laboratory testing (CRP, leucocyte count) revealed no association with the incidence and type of infection.
The traumatic coagulopathy was significantly associated with higher injury severity (PTS p 0.003, ISS p 0.008), severe polytrauma (p 0.02), increased number of surgical revisions (p 0.003) and most relevant, significantly higher infection rate (p 0.03).
In 47.1% (n = 24) late complications occurred (45.8% osteomyelitis, 25% early implant removal due to infection, 29.2% secondary amputation).
Microbiological findings
In total more than two thirds of the patients (70.6%, n = 36) acquired a wound infections during their hospitalization. The rate of polymicrobial wound infections and Pseudomonas infections in all patients (n = 51) during hospitalization were 37.3% (n = 19) and 27.5% (n = 14), respectively. 14 out of 36 patients with wound infections had a positive Pseudomonas wound swab (38.9%) during hospitalization, in which 50%/n = 7 were detected within 72 hours after admission. The distribution of 14 pseudomonas species have been: 9 P. aeruginosa, 2 P. putida and 1 P. oryzihabitans, P.fluorescens and Aeromonas hydrphilia, respectively.
First pathogen proof
In 38.9% (n = 14) the first microbiologic test, obtained under sterile conditions within 2 hours after trauma, was positive, indicating that nearly 40% are admitted with contaminated wounds. In contrast, the first positive pathogen proof within several surgical debridement was detected in mean 9.1 ± 13.4 days (range 1–61 days) after injury. Epidemiologic analyses of all pathogen revealed polymicrobial infections (25.5%/n = 13) and Pseudomonas (23.5%/n = 12) as the most frequently detected pathogen in the first positive microbiological testing. Polymicrobial infections assembled in 51.9% from gram-negative and positive strains, 25.9% more than one gram-negative strain and 7.4% more than one gram-positive strain.
Early infections
In 56.9% (n = 29) patients fulfilled the criteria of an early infection within 14 days after trauma. In 25.5% (n = 13) Pseudomonas species and 23.5% polymicrobial infection (n = 12) were detected in the first positive microbiologic test within 14 days.
An overview of the epidemiology of pathogens in open fractures and amputations during hospitalization is shown in Table 3 and the temporal distribution of positive pathogen proof in Figure 2.
Temporal incidence of positive polymicrobial and Pseudomonas pathogen proof. Temporal distribution of positive polymicrobial and Pseudomonas pathogen proof in sub-/total traumatic amputations; y-axis: days after trauma; x-axis: admission: Specimen obtain within emergency surgery; early infect: positive pathogen proof within 14 days after trauma; total: incidence of positive pathogen proof within hospitalization period.
Patients with acute Pseudomonas wound infections within 14 days after trauma (n = 13, NPWT 85%, p 0.01) and Pseudomonas wound infections while hospitalization (n = 14, NPWT 79%, p 0.03) were significantly more often treated with negative pressure wound therapy (NPWT) compared to no acute (n = 38, NPWT 37%) or no Pseudomonas wound infection (n = 37, NPWT 38%). We observed no methicillin-resistant Staphylococcus aureus (MRSA) but 13.7% (n = 7) carbapenem-resistant Gram-negative strains. The carbapenem-resistant Gram-negative pathogens were detected at mean 17 ± 10 days (Range 3-31days) after injury.
Neither the incidence of early wound infection nor the fact of completed sufficient initial antibiotic therapy did show a significant effect on the length of critical care treatment and hospitalization period. Also the number of revision surgeries conducted to reach wound closure, soft tissue coverage and skeletal reconstruction did also not significantly differ in patients with early wound infections compared to patients without wound infection. Furthermore, no relation was found between the injury severity and infection rate. Additionally, the injury severity according to ISS and PTS had no significant impact on the incidence of a Pseudomonas or polymicrobial wound infection. We found also no statistical significant differences referring the infection rate, acute infection, Pseudomonas infection, combined infections and appropriate antimicrobial treatment in polytrauma (ISS > 15 points) and severe polytrauma (ISS >25 points) compared to non polytraumatized patients.
Antimicrobial therapy
All 51 patients received empiric antibiotic therapy after admission. The most common antibiotic agents and frequency of application are shown in Table 4.
Initial antimicrobial mono-therapy (aminopenicillin plus a beta-lactamase inhibitor) was performed in 66.7% (n = 33) while 29.4% (n = 15) received a double antibiotic therapy. In 45.1% the empiric antibiotic therapy was insufficient according to the later reported microbiological findings and antibiogram. Additionally, the insufficient antibiotic therapy according antibiogram was significantly associated with an increase of Pseudomonas wound infection (<14 days p 0.05; during hospitalization p 0.02).
Discussion
Open fractures and major traumatic amputations are typically observed among high-energy velocity victims. Appropriate therapeutic management of these severe and rare injuries, including radical debridement, microsurgical revascularization and replantation belongs to the most advanced techniques in orthopedic surgery and requires enormous experience.
Injuries and coagulopathy
The predominantly injured body region in our collective was the lower leg, which confirms the findings of various other studies [5, 18, 21–27]. Notably, most of the studies exclusively investigated open fractures (type I-III) of the lower leg, whereas our study includes all major traumatic amputations and only type IIIC open fracture within the study period [9, 11, 28–31]. More than half of the patients included in our study were severely polytraumatized (ISS >25 points). We could not find a significant relation between injury severity and infection rate in our study, confirming previously published data for battlefield injuries [12]. We postulate that the individual posttraumatic immune response predispose for infection due to immune depression or pro-inflammatory reactions, not measurable by an anatomical injury severity score [32]. We definitely suggest a specific subgroup of polytrauma patients to be susceptible to acquire infection. Therefore, we need immunological tools to detect and characterize posttraumatic immune response and allocate an individual risk guiding decision-making in polytrauma [32]. In contrast, traumatic coagulopathy was significantly associated with higher infection rate and higher injury severity. Our data suggest that traumatic coagulopathy predisposes for wound infections underscoring the recent detected cross talk of complement system and coagulation factors [33]. We found no statistically significant differences for wound infections in subtotal traumatic amputation and primary major traumatic amputations. It is conceivable, that at this high level of severe soft tissue damage with ischemia in both injury types the already high risk of wound infection does not further increase. Comparison with previous publications is critical, because most studies include all types of open fractures and no traumatic amputation injuries [34–36].
Microbiology
Due to exclusively type IIIc open fracture the rate of wound infections (70.6%) in our study exceeds the infection rates of previously published data (5-40%) supporting the fact, that the infection rate is strongly related to the extend of the soft tissue injury and vascularization [2, 6, 15, 16, 37].
The epidemiologic part of this study revealed a shift from Gram-positive to Gram-negative pathogens in subtotal and total traumatic amputation compared to literature [1, 8]. We observed a high rate of Pseudomonas and polymicrobial wound infections already at admission to the hospital within emergency operation less then 2 hours after trauma and within hospitalization. This fact indicates the change of natural pathogen environment. Especially Pseudomonas was considered as a late hospitalization infection [12]. We detect a unique high Pseudomonas infection rate of 39% compared to the literature [8, 11, 34]. Furthermore, in our clinical experience we observe more and more primary Gram-negative contaminations or infections also in type II/IIIa/IIIb open fractures. For us, there is a clear trend towards Gram-negative strains in open fractures.
The use of NPWT is a controversy with positive and negative effects [31, 38–40]. In our study we found a significant association between Pseudomonas wound infection and treatment with NPWT. A study revealed the NPWT foam as a hatchery for especially Pseudomonas. Therefore, NPWT has also disadvantages and should not be abused to delay or substitute plastic surgery in traumatic amputations [41]. Temporary soft tissue coverage with artificial skin coverage (Epigard®) or dry dressings can induce moistening and maceration of the wound. The humid wound atmosphere, fluid collections, bradytrophic tissue in an environment of Gram-positive focused empiric antimicrobial therapy might lead to a survival advantage and secondary selection of Gram-negative pathogens. Additionally the abuse of antimicrobial therapy combined with NPWT has the potential to select multiresistant pathogens, e.g. the carbapenem-resistant Gram-negative strains in our study. All multiple resistances were detected after hospitalization and under antimicrobial therapy at mean 10 days after trauma.
In contrast, the initial temporary vacuum-assisted wound coverage followed by early wound closure or plastic surgery has the potential to lower the secondary infection rates with Gram-negative rods, especially Pseudomonas, because of droughty effects on the wound side and removal of fluid collections predisposed for bacterial secondary colonization with hospitality pathogens in the future.
Antimicrobial therapy
The change of pathogen spectrum to Gram-negative strains has tremendous impact on the choice of empiric antimicrobial therapy and morbidity of our patients. Former empiric therapy focused on the reported epidemiologic data from 1990s with predominate Gram-positive strains. Therefore, we detect in 45% of our patients the empiric antibiotic regimen as insufficient and were significantly associated with Pseudomonas or polymicrobial wound infections. The data of this study clearly indicate the need to adjust the empiric antimicrobial therapy for type IIIc open fractures and traumatic amputations. However, remarkable controversy exists about the use of antibiotic therapy and guidelines similar to e.g. the 2013 WSES guidelines for intra-abdominal infections are absent [42, 43].
Successful treatment of open fractures and traumatic amputations has two cornerstones, sufficient empiric antimicrobial therapy in combination with immediate radical surgical debridement [1, 6, 10, 15, 18, 31]. We recommend the use of empiric antimicrobial therapy in sub-/total traumatic amputations with Piperacillin/Tazobactam (4.5 g i.v. 3 times per day) [44]. In case of penicillin allergy but no type I allergy (quincke edema, anaphylaxis) we use Cefepim (2 g i.v. 3 times per day). In type I penicillin allergy we use a combination of ether Vancomycin or Daptomycin in combination with Gentamicin or Ciprofloxacin. We always tried to preserve Ciprofloxacin as the only oral Pseudomonas active agent for ambulant long-term treatment of our patients. As soon as available we change from empiric to goal directed antimicrobial treatment according to the microbiological test and resistogram results.
Conclusions
This epidemiologic study reveals a pathogen shift from Gram-positive to Gram-negative strains with high incidence of Pseudomonas and combined pathogen infections in type IIIC open fractures and major traumatic amputations. Therefore, empiric antimicrobial treatment must be adjusted. We recommend the use of Piperacillin/Tazobactam in sub-/total traumatic amputations. As soon as available antimicrobial treatment must change from empiric to goal directed therapy according to the microbiological test and resistogram results.
References
Gustilo RB, Anderson JT: Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976, 58 (4): 453-458.
Gustilo RB, Mendoza RM, Williams DN: Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984, 24 (8): 742-746. 10.1097/00005373-198408000-00009.
Tscherne H, Oestern HJ: [A new classification of soft-tissue damage in open and closed fractures (author's transl)]. Unfallheilkunde. 1982, 85 (3): 111-115.
Dillingham TR, Pezzin LE, MacKenzie EJ: Incidence, acute care length of stay, and discharge to rehabilitation of traumatic amputee patients: an epidemiologic study. Arch Phys Med Rehabil. 1998, 79 (3): 279-287. 10.1016/S0003-9993(98)90007-7.
Court-Brown CM, Rimmer S, Prakash U, McQueen MM: The epidemiology of open long bone fractures. Injury. 1998, 29 (7): 529-534. 10.1016/S0020-1383(98)00125-9.
Schwabe P, Haas NP, Schaser KD: Fractures of the extremities with severe open soft tissue damage. Initial management and reconstructive treatment strategies. Unfallchirurg. 2010, 113 (8): 647-670. 10.1007/s00113-010-1801-0. quiz 671–642
Kleber CHN: Biologics in open fractures. Surg Orthop Traumatol. 2013, doi:10.1007/978-3-642-34746.7_199 Springer-Verlag Berlin Heidelberg 2013
Robinson D, On E, Hadas N, Halperin N, Hofman S, Boldur I: Microbiologic flora contaminating open fractures: its significance in the choice of primary antibiotic agents and the likelihood of deep wound infection. J Orthop Trauma. 1989, 3 (4): 283-286. 10.1097/00005131-198912000-00003.
Yokoyama K, Itoman M, Nakamura K, Uchino M, Nitta H, Kojima Y: New scoring system predicting the occurrence of deep infection in open upper and lower extremity fractures: efficacy in retrospective re-scoring. Arch Orthop Trauma Surg. 2009, 129 (4): 469-474. 10.1007/s00402-008-0675-x.
Lenarz CJ, Watson JT, Moed BR, Israel H, Mullen JD, Macdonald JB: Timing of wound closure in open fractures based on cultures obtained after debridement. J Bone Joint Surg Am. 2010, 92 (10): 1921-1926. 10.2106/JBJS.I.00547.
Khatod M, Botte MJ, Hoyt DB, Meyer RS, Smith JM, Akeson WH: Outcomes in open tibia fractures: relationship between delay in treatment and infection. J Trauma. 2003, 55 (5): 949-954. 10.1097/01.TA.0000092685.80435.63.
Brown KV, Murray CK, Clasper JC: Infectious complications of combat-related mangled extremity injuries in the British military. J Trauma. 2010, 69 (Suppl 1): S109-S115.
Stutz CM, O'Rear LD, O'Neill KR, Tamborski ME, Crosby CG, Devin CJ, Schoenecker JG: Coagulopathies in orthopaedics: links to inflammation and the potential of individualizing treatment strategies. J Orthop Trauma. 2013, 27 (4): 236-241. 10.1097/BOT.0b013e318269b782.
Gosselin RA, Roberts I, Gillespie WJ: Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev. 2004, 1: CD003764.
Patzakis MJ, Bains RS, Lee J, Shepherd L, Singer G, Ressler R, Harvey F, Holtom P: Prospective, randomized, double-blind study comparing single-agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. J Orthop Trauma. 2000, 14 (8): 529-533. 10.1097/00005131-200011000-00002.
Patzakis MJ, Wilkins J: Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989, 243: 36-40.
Stannard JP, Volgas DA, Stewart R, McGwin G, Alonso JE: Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009, 23 (8): 552-557. 10.1097/BOT.0b013e3181a2e2b6.
Al-Arabi YB, Nader M, Hamidian-Jahromi AR, Woods DA: The effect of the timing of antibiotics and surgical treatment on infection rates in open long-bone fractures: a 9-year prospective study from a district general hospital. Injury. 2007, 38 (8): 900-905. 10.1016/j.injury.2007.02.043.
Richardson VR, Cordell P, Standeven KF, Carter AM: Substrates of Factor XIII-A: roles in thrombosis and wound healing. Clin Sci (Lond). 2013, 124 (3): 123-137. 10.1042/CS20120233.
Helfet DL, Howey T, Sanders R, Johansen K: Limb salvage versus amputation. Preliminary results of the mangled extremity severity score. Clin Orthop Relat Res. 1990, 256: 80-86.
Barmparas G, Inaba K, Teixeira PG, Dubose JJ, Criscuoli M, Talving P, Plurad D, Green D, Demetriades D: Epidemiology of post-traumatic limb amputation: a national trauma databank analysis. Am Surg. 2010, 76 (11): 1214-1222.
Dellinger EP, Miller SD, Wertz MJ, Grypma M, Droppert B, Anderson PA: Risk of infection after open fracture of the arm or leg. Arch Surg. 1988, 123 (11): 1320-1327. 10.1001/archsurg.1988.01400350034004.
DeLong WG, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW: Aggressive treatment of 119 open fracture wounds. J Trauma. 1999, 46 (6): 1049-1054. 10.1097/00005373-199906000-00012.
Busse JW, Jacobs CL, Swiontkowski MF, Bosse MJ, Bhandari M: Complex limb salvage or early amputation for severe lower-limb injury: a meta-analysis of observational studies. J Orthop Trauma. 2007, 21 (1): 70-76. 10.1097/BOT.0b013e31802cbc43.
Harley BJ, Beaupre LA, Jones CA, Dulai SK, Weber DW: The effect of time to definitive treatment on the rate of nonunion and infection in open fractures. J Orthop Trauma. 2002, 16 (7): 484-490. 10.1097/00005131-200208000-00006.
Mirzayan R, Itamura JM, Vangsness CT, Holtom PD, Sherman R, Patzakis MJ: Management of chronic deep infection following rotator cuff repair. J Bone Joint Surg Am. 2000, 82-A (8): 1115-1121.
Seligson D, Ostermann PA, Henry SL, Wolley T: The management of open fractures associated with arterial injury requiring vascular repair. J Trauma. 1994, 37 (6): 938-940. 10.1097/00005373-199412000-00011.
Seekamp A, Regel G, Ruffert S, Ziegler M, Tscherne H: Amputation or reconstruction of IIIB and IIIC open tibial fracture. Decision criteria in the acute phase and late functional outcome. Unfallchirurg. 1998, 101 (5): 360-369. 10.1007/s001130050281.
Sirkin M, Sanders R, DiPasquale T, Herscovici D: A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004, 18 (8 Suppl): S32-S38.
Cole JD, Ansel LJ, Schwartzberg R: A sequential protocol for management of severe open tibial fractures. Clin Orthop Relat Res. 1995, 315: 84-103.
Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S: Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010, 18 (1): 10-19.
Kleber C, Becker CA, Schmidt-Bleek K, Schaser KD, Haas NP: Are pentraxin 3 and transsignaling early markers for immunologic injury severity in polytrauma? a pilot study. Clin Orthop Relat Res. 2013, 471 (9): 2822-2830. 10.1007/s11999-013-2922-x. doi:10.1007/s11999-013-2922-x
Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M: Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010, 185 (9): 5628-5636. 10.4049/jimmunol.0903678.
Akinyoola AL, Oginni LM, Adegbehingbe OO, Orimolade EA, Ogundele OJ: Causes of limb amputations in Nigerian children. West Afr J Med. 2006, 25 (4): 273-275.
McIntosh J, Earnshaw JJ: Antibiotic prophylaxis for the prevention of infection after major limb amputation. Eur J Vasc Endovasc Surg. 2009, 37 (6): 696-703. 10.1016/j.ejvs.2009.01.013.
Sadat U, Chaudhuri A, Hayes PD, Gaunt ME, Boyle JR, Varty K: Five day antibiotic prophylaxis for major lower limb amputation reduces wound infection rates and the length of in-hospital stay. Eur J Vasc Endovasc Surg. 2008, 35 (1): 75-78. 10.1016/j.ejvs.2007.07.016.
Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE: Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage. 2009, 55 (8): 58-66.
Dedmond BT, Kortesis B, Punger K, Simpson J, Argenta J, Kulp B, Morykwas M, Webb LX: The use of negative-pressure wound therapy (NPWT) in the temporary treatment of soft-tissue injuries associated with high-energy open tibial shaft fractures. J Orthop Trauma. 2007, 21 (1): 11-17. 10.1097/BOT.0b013e31802cbc54.
Herscovici D, Sanders RW, Scaduto JM, Infante A, DiPasquale T: Vacuum-assisted wound closure (VAC therapy) for the management of patients with high-energy soft tissue injuries. J Orthop Trauma. 2003, 17 (10): 683-688. 10.1097/00005131-200311000-00004.
Labler L, Trentz O: The use of vacuum assisted closure (VAC) in soft tissue injuries after high energy pelvic trauma. Langenbecks Arch Surg. 2007, 392 (5): 601-609. 10.1007/s00423-006-0090-0.
Yusuf E, Jordan X, Clauss M, Borens O, Mader M, Trampuz A: High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound Repair Regen. 2013, 21 (5): 677-681. 10.1111/wrr.12088.
Hauser CJ, Adams CA, Eachempati SR: Surgical infection society guideline: prophylactic antibiotic use in open fractures: an evidence-based guideline. Surg Infect (Larchmt). 2006, 7 (4): 379-405. 10.1089/sur.2006.7.379.
Sartelli M, Viale P, Catena F, Ansaloni L, Moore E, Malangoni M, Moore FA, Velmahos G, Coimbra R, Ivatury R, Peitzman A, Koike K, Leppaniemi A, Biffl W, Burlew CC, Balogh ZJ, Boffard K, Bendinelli C, Gupta S, Kluger Y, Agresta F, Di Saverio S, Wani I, Escalona A, Ordonez C, Fraga GP, Junior GA, Bala M, Cui Y, Marwah S, et al: 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013, 8 (1): 3-10.1186/1749-7922-8-3.
Kleber CTA: Antibiotic prophylaxis and therapy in orthopedic and traumatology surgery _ What, When and How long to administer?. OP-Journal. 2014, 30: 8-10.
Acknowledgements
Contributions of Dr. med. C. Kleber were made possible by DFG funding through the Berlin-Brandenburg School for Regenerative Therapies GSC 203.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
There is no conflict of interest. The corresponding author affirms that he has no relationships with a company whose product is mentioned in the article or with one that sells a competitive product. The presentation is impartial and the content is independent of commercial influence.
Authors' contributions
MTG: conception and study design, data acquisition, manuscript drafting. PS: data acquisition, manuscript revision. FW: data analysis and interpretation, manuscript revision. AT: conception and study design, data analysis and interpretation, manuscript revision. CK: conception and study design, data analysis and interpretation, manuscript drafting. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Giesecke, M.T., Schwabe, P., Wichlas, F. et al. Impact of high prevalence of pseudomonas and polymicrobial gram-negative infections in major sub-/total traumatic amputations on empiric antimicrobial therapy: a retrospective study. World J Emerg Surg 9, 55 (2014). https://doi.org/10.1186/1749-7922-9-55
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1749-7922-9-55