Abstract
Background and purpose
Targeted drugs have augmented the cancer treatment armamentarium. Based on the molecular specificity, it was initially believed that these drugs had significantly less side effects. However, currently it is accepted that all of these agents have their specific side effects. Based on the given multimodal approach, special emphasis has to be placed on putative interactions of conventional cytostatic drugs, targeted agents and other modalities. The interaction of targeted drugs with radiation harbours special risks, since the awareness for interactions and even synergistic toxicities is lacking. At present, only limited is data available regarding combinations of targeted drugs and radiotherapy. This review gives an overview on the current knowledge on such combined treatments.
Materials and methods
Using the following MESH headings and combinations of these terms pubmed database was searched: Radiotherapy AND cetuximab/trastuzumab/panitumumab/nimotuzumab, bevacizumab, sunitinib/sorafenib/lapatinib/gefitinib/erlotinib/sirolimus, thalidomide/lenalidomide as well as erythropoietin. For citation crosscheck the ISI web of science database was used employing the same search terms.
Results
Several classes of targeted substances may be distinguished: Small molecules including kinase inhibitors and specific inhibitors, antibodies, and anti-angiogenic agents. Combination of these agents with radiotherapy may lead to specific toxicities or negatively influence the efficacy of RT. Though there is only little information on the interaction of molecular targeted radiation and radiotherapy in clinical settings, several critical incidents are reported.
Conclusions
The addition of molecular targeted drugs to conventional radiotherapy outside of approved regimens or clinical trials warrants a careful consideration especially when used in conjunction in hypo-fractionated regimens. Clinical trials are urgently needed in order to address the open question in regard to efficacy, early and late toxicity.
Similar content being viewed by others
Background and purpose
Several new anti-cancer drugs have recently entered clinical practice in oncology. Among those, especially targeted drugs are promising therapeutic candidates with a comparatively low toxicity profile. At present, these drugs are often applied in palliative treatment situations for metastasized diseases. In addition, targeted agents are a substantial part of many multimodal oncologic treatment schedules. Thus the risk of parallel use of both radiotherapy and targeted drug is given. With few exceptions, the toxicity of any combination of targeted drugs with radiotherapy has not yet been studied in detail.
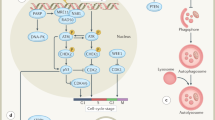
Key cellular signalling pathways [1] are responsible for the response of normal tissue and tumour cells to radiation therapy [2]. Although some of the anti-cancer targets are specific for neoplastic signalling, there is considerable overlap between neoplastic signalling and normal cellular signalling. In this regard, several putative interactions with radiation triggered signalling in normal issues exist and thus [3, 4] influences of targeted drugs on normal tissue reactions cannot be excluded [5–7].
The present article reviews the existing data on the toxicity profile and efficacy (if available) of targeted drugs when applied concurrently to radiotherapy.
Methods and materials
Using the following MESH headings and combinations of these terms, pubmed database was searched for randomized, prospective and retrospective trials as well as case reports (all sample sizes were considered):
-
1.
Radiotherapy AND cetuximab/trastuzumab/panitumumab/nimotuzumab
-
2.
Radiotherapy AND bevacizumab
-
3.
Radiotherapy AND sunitinib/sorafenib/lapatinib/gefitinib/erlotinib/sirolimus
-
4.
Radiotherapy AND thalidomide/lenalidomide.
-
5.
Radiotherapy AND erythropoietin
For citation crosscheck, the ISI web of science database was used employing the same search terms. A focus was put on prospective or phase I/II trials; if available, some smaller case studies or case reports were included if higher toxicities were reported.
In general, grade III + IV toxicities are reported. For cetuximab, focus was set on larger phase III trials and those reporting trials specifically reporting toxicities. In addition, key reviews focusing on the use of targeted drug in oncology were screened in order to identify clinically relevant drugs [8].
Results
Antibodies
Cetuximab
Cetuximab is a monoclonal chimeric antibody directed against the epidermal growth-factor receptor (EGF-R). It has first been approved for treatment of locally advanced or metastatic colorectal cancer (k-ras wildtype) refractory to irinotecan [9]. Regarding radiotherapy, it has been approved for head-and-neck cancer as an alternative to concomitant chemotherapy [10]; in the given phase III trial overall survival of patients who were treated by radiotherapy and cetuximab was improved compared to patients who underwent radiotherapy alone. Cetuximab also has a proven efficacy in locally advanced or metastatic head-and-neck cancer in combination with 5-FU/cisplatin [11].
Thus several pre-clinical and clinical studies have provided evidence for the efficacy of cetuximab in combination with radiotherapy [12–17]. Nevertheless, several reports are available pointing to increased skin toxicity after combining cetuximab with radiotherapy [18–27] (a complete overview is given in Table 1). The initial publication on the combined use by Bonner and colleagues reported an increased incidence of an acneiform rash [10]. However, in single cases more severe complications occurred [19]. A recent retrospective matched-pair evaluation of acute toxicity during cis-platinum-based radio-chemotherapy versus radiotherapy with simultaneous cetuximab treatment showed significantly higher grade 3 oral mucositis and dermatitis as well as a higher risk of weight loss (> 10%) and of enteral feeding requirement in the cetuximab-group. However, this may be outweighed by the higher risk of haematological toxicity by radio-chemotherapy. In keeping with this, higher compliance rate with less treatment interruptions in the cetuximab-treated group was described [26]. In trials on thoracic [28, 29] or pelvic radiotherapy with cetuximab increased rates of skin toxicity were not observed.
No other risks regarding additional or increased side effects concerning connective tissue, CNS [30–32] or peripheral nerves have been described so far in small early-phase clinical trials.
Panitumumab
Similar to cetuximab, panitumumab is a monoclonal antibody directed against EGF-R with a putatively higher affinity and less toxicity due to its non-chimeric design. It has been approved for stage IV colorectal cancer refractory to FOLFOX or FOLFIRI [33].
Only data from a single phase I study [34] and a single phase II trial described effects of a combination of panitumumab with a 5-FU/oxaliplatin-containing radio-chemotherapy for rectal cancer [35]. Pre-clinical data suggest a comparable efficacy to cetuximab [36]. Concerning toxicity, no additional toxicity was observed when combined with radiotherapy. The phase II trial reported one toxic death from diarrhea and a relatively high rate of grade III/IV diarrhea (39%) compared to the classical CAO/ARO/AIO-94 trial [37]. However, based on the design of the trial it is not possible to precisely attribute the side effects to any of the components of the given protocol.
Nimotuzumab
Nimotuzumab is another humanized therapeutic monoclonal antibody directed against EGF-R not yet been approved by the authorities in Europe. There are three small phase I trials testing radiotherapy and nimotuzumab in head-and-neck cancer as well as NSCLC patients; an increased rate of skin toxicity was observed [38–40]. The other larger phase II trial by Rodríguez and colleagues was prospectively randomized and 106 head-and-neck cancer patients were included [38]. No grade III or IV toxicity has been observed.
The data available suggest that the combination of cetuximab with radiation may lead to an increased rate of mucosal- and skin toxicity when applied together with radiation for the treatment of head-and-neck cancer. No such problems have been reported in other organ regions. It is unclear in how far this is an epitope-specific side effect-only limited data are available regarding similar effects after the combined use of panitumumab and nimotuzumab.
Anti -Her2/neu antibody trastuzumab
Trastuzumab is a humanized monoclonal antibody directed against the epidermal growth-factor-receptor Her-2/neu. It is approved for the treatment of metastatic her-2/neu-positive breast cancer as well as for the adjuvant treatment of her-2/neu-positive breast cancer in combination with chemotherapy [41–44].
Cardiac toxicity is a rare, but well described adverse effect of trastuzumab-especially with or after the treatment with anthracyclins [45–47]. As cardiac toxicity is also of concern in thoracic radiotherapy, the question of an increased toxicity has been raised. The largest trial focusing on side effects of the combined use of radiotherapy and trastuzumab is the phase III NLCCTG trial N9831 for adjuvant trastuzumab and radiotherapy including 1503 patients [48]. The trial did not reveal any significant differences in toxicity regarding skin, pneumonitis or cardiac events. Also, a French multicentric study [49] including 146 patients did not observe an increased cardiac toxicity. Another study retrospectively investigated the combinational approach of trastuzumab and radiotherapy including the internal mammary lymph nodes [50]. Again, no increased cardiac toxicity has been observed.
Thus, at present there are no strong indicators for an increased cardiac toxicity. However, follow-up periods are only sufficient for an estimation of early cardiac toxicity caused by trastuzumab, but not for an in-depth assessment of late radiation-induced cardiac effects.
Altogether, the current data suggest that the use of trastuzumab in a close time frame with radiotherapy may be safe. However, the reported studies might still reveal an increased cardiac toxicity, as minor vascular changes might lead to an increased mortality in long-term follow-up [51].
Bevacizumab
Bevacizumab is a humanized monoclonal antibody against the vascular endothelial growth-factor (VEGF). So far, bevacizumab has been approved for the treatment of metastatic colorectal carcinoma, in combination with standard chemotherapy (5-FU, irinotecan, oxaliplatin or capecitabine). Bevacizumab has been approved for the treatment of metastatic non-squamous-cell bronchial carcinoma, for the treatment of renal cell cancer and for the treatment of glioblastoma multiforme (US only). The FDA has withdrawn the approval for first line treatment of metastatic HER-2/neu-negative breast cancer-however, the drug still is approved in Europe.
The most common side effects of bevacizumab alone include impaired wound healing, hypertension, bleeding problems as well as an increased risk of thromboembolic events.
One of the first publications to describe an increased risk of combining bevacizumab with radiotherapy reported on patients with ischemic bowel complications after the administration of radiotherapy followed by bevacizumab [52].
A phase II study combining neoadjuvant bevacizumab, capecitabine and radiotherapy for locally advanced rectal cancer revealed an increased rate of wound complications such as delayed healing and wound dehiscence [53]. The data are in line with a number of similar reports and case studies, supporting the interpretation that the combined use of bevacizumab with neoadjuvant radiotherapy is associated with an increased risk of postoperative complications [54–57]. However, this interpretation is not homogenously supported by all available data [58–61]. In terms of tumour response, the rate of pathological complete responses seems to be enhanced [53].
The use of bevacizumab, capecitabine and radiotherapy in patients with locally advanced pancreatic cancer was associated with an increased rate GI-bleeding and ulcerations (12%) [62]. These complications preferentially occurred in patients with a mucosal infiltration of the tumour. In a consecutive study -after excluding patients with mucosa infiltration-no such side effects were reported [63]. A similar study reported the combination of radiotherapy with bevacizumab-partly in a neoadjuvant setting- as "feasible" [64].
The combination of radiotherapy with simultaneous administration of bevacizumab was also tested for lung cancer [65, 66]. In this setting, the occurrence of severe fistula leading to a discontinuation of both trials has been described [66].
In case of breast cancer the parallel combination of radiotherapy and bevacizumab had no significant side-effects in regard to lung and skin toxicity [65].
The treatment of malignant tumours of the brain has been subject to a variety of studies combining radiotherapy with bevacizumab with or without temozolomide; regarding progression-free survival, these trials suggest a benefit of the combined use [67]. No intra-cerebral bleeding has been reported, however cases of wound dehiscence of the previous operation have been documented [68–70]. A collection of case reports points towards increased late toxicity such as optic neuropathy and a single case of Brown-Séquard syndrome after a combination of bevacizumab with radiotherapy [71]. (a complete overview is given in Table 2).
Altogether, the combined use of bevacizumab and radiotherapy seems to be associated with a considerable risk of side effects (wound dehiscence, bleeding, fistula or GI complications). However, in selected cases the combination was feasible and even favourable concerning overall survival (retrospective) [69] and progression-free survival [72].
Anti CD20 monoclonal antibody-rituximab
Rituximab is a monoclonal antibody directed against the CD20 antigen. It was initially developed and approved as a targeted agent for the treatment of CD20-positive non-Hodgkin lymphoma. In this setting, rituximab is mostly used in combination with chemotherapy (e. g. CHOP). Apart from the use of rituximab in oncology, its use has been extended to the treatment of refractory autoimmune diseases (e. g. rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus and idiopathic thrombocytopenic purpura among others).
The application of rituximab in combination with or shortly before/after radiotherapy of non-Hodgkin lymphoma has been prospectively studied [73–75]. So far no significant additional toxicities have been reported. All side effects seen in the trials have been attributed to the individual therapeutic modalities respectively [76]. Thus, at present the combination of rituximab with radiation does not seem to harbour any relevant risks.
Small molecules/tyrosine-kinase inhibitors (TKI's)
TKI's are small molecules able to pass the cell membrane and to inhibit intracellular tyrosine kinases of several growth factor receptors. Relevant examples are sunitinib, sorafenib, erlotinib or gefitinib. At present, TKI are used for diverse cancer entities and various clinical settings. Key indications are: Metastasized lung cancer/renal cell cancer/pancreatic cancer, locally advanced and metastatic breast cancer as well as hepatocellular carcinoma.
Up to now, no TKI has been approved for the simultaneous use with radiotherapy.
All toxicity data on combined toxicity are limited to case reports or studies with small numbers of patients. The clinical indications and the most common adverse effects of clinically used TKI's are summarized in Table 3.
When using sunitinib or sorafenib alone, mainly diarrhea, hypertension, fatigue, hand-foot syndrome, bleeding and hematotoxicity may occur as side effects. Concerning combined use with radiotherapy, one case report described a lethal small bowel perforation after 1x 8 Gy in a palliative setting, sorafenib had been stopped 2 days before and three days after radiotherapeutic treatment [77]. In another case, a lethal bronchial fistula occurred after radiation of the mediastinum [78]; as this phenomenon has been observed after sunitinib alone [79] no definite causality can be deduced. Furthermore, elevated bone-marrow toxicity was observed if large volumes of bones or liver were radiated; a phase I study concluded to avoid the combination with sunitinib when radiating volumes of more than 6 ccm of the liver. A dose reduction of sunitinib was advised for the following phase II study [80].
In patients with cerebral metastases increased intracerebral bleeding has been reported, this appears to happen with or without radiotherapy [81].
Concerning the simultaneous use of gefitinib/erlotinib and radiotherapy one case of fatal diarrhea after combining erlotinib with RT in the abdomen (2x8 Gy, q1w) has been reported [82]. And again, in patients with cerebral metastases increased intracerebral bleeding has been reported, however, this appears to happen with or without radiotherapy [83].
As long as no reliable data concerning the safety of the combination of TKI's and radiotherapy are available, such therapies should be used very carefully, especially if the above reported organs received relevant radiation doses. So far it is unclear if the increased intracerebral bleeding rates are induced by the combined treatment or by the drug alone. However, because of the severity of this adverse effect, special caution is warranted for combined treatment schedules. The same applies for tumours that tend to bleed outside of the brain. Radiotherapy in the abdomen or the pelvis together with TKI's might lead to an increased toxicity, including the occurrence of ulcera and bleeding.
mTOR inhibitors
According to pre-clinical data, an improvement of tumour growth by simultaneous administration of temsirolimus with radiotherapy seems possible [84–86]. However, the only study on long-term local tumor control revealed no beneficial effect regarding the combined treatment [84]. Preclinical data show an inhibition of vascular growth when combining everolimus with radiation, however a direct radio-sensitizing effect could not be consistently shown [85, 86]. A recent study [87] showed evidence for a suppressed dsDNA break repair by everolimus.
Concerning toxicity, there is one phase I study using temsirolimus with topotecan in recurrent gynaecological malignancies [88]. Dose-limiting toxicity of this combination was myelo-suppression. Although this toxicity cannot be attributed to temsirolimus, we advise caution when combining mTOR-inhibitors with concomitant or sequential radiotherapy, especially if large volumes of bone are in-field as the latter is already known to potentially cause myelo-suppression.
Nevertheless there are no sufficient clinical data to adequately judge the risks and potential benefits of a combined use of mTOR-inhibitors with radiotherapy. As long as this is the case, it can be assumed that-similar to anti-angiogenic substances-the combinational use may lead to wound healing deficits, increased bleeding and thrombosis.
Lenalidomide/thalidomide
Data on available studies combining radiotherapy and lenalidomide or thalidomide treatment are shown in Table 4. Thalidomide was initially used and approved as sedative drug until the early 1960s when it became clear that the intake of "Contergan" during pregnancy could lead to severe deformities. It was only in the late 1990s that thalidomide was rediscovered for its anti-angiogenic properties in cancer therapy [89]. Thalidomide is clinically used in the treatment of multiple myeloma; other areas of possible clinical use and ongoing clinical trials include leprosy, erythema nodosum leprosum and myelodysplastic syndrome.
The most common side effects of thalidomide-besides somnolence-are thromboembolic events as well as peripheral polyneuropathy.
In vitro studies with cells of squamous cell carcinoma and of multiple myeloma showed no evidence for any radio-sensitizing quality of thalidomide. However, a radio-sensitizing effect has been observed in normal hematopoietic bone marrow [90]. Experiments in mice showed thalidomide induced tumour re-oxygenation pointing to a possible radio-sensitizing effect in vivo [91]. Experiments in rats indicate that thalidomide might be protective against radiation-induced proctitis when given 7 days after a single-RT [92].
In humans, thalidomide has been tested in combination with radiotherapy in phase I-III studies. Most data exist for radiation of the CNS combined with the administration of thalidomide.
The largest study so far was conducted by Knisely and co-workers [93]. In this phase III study 183 patients with multiple cerebral metastases were randomized for palliative WBRT (37,5 Gy in 15 fx) vs. WBRT (same dose and number of fractions) with thalidomide. In this study, only the known side effects of thalidomide occurred in the usual frequency. Hints to a possible interaction with radiotherapy have not been reported. Nearly half of the patients discontinued the study in the thalidomide arm due to side effects. The major limiting side effect was somnolence [93].
In malignant glioma, thalidomide was used in combination with radiotherapy or radiotherapy plus temozolomide in primary or recurrent settings [94]. Intratumoural bleeding and thromboembolic complications have been reported. However, the rate of complications was not higher than the reported rates for thalidomide alone [95–97].
Other studies, combining radiotherapy of soft tissue/bone metastasis as well as pelvic tumours with thalidomide simultaneously or sequentially revealed no evidence for increased risks of acute or late side effects [99].
However, a single study using radiotherapy (66 Gy in 33 fx) combined with vinorelbine and thalidomide in NSCLC stage III was abrogated after 10 patients due to side effects (thromboembolic, 1 bradycardia II°) [100]. As in this study only known side effects of thalidomide occurred, it still remains unclear whether radiation including the lung or the heart leads to increased side effects when combined with thalidomide.
Altogether the combination of thalidomide with simultaneous or sequential RT does not seem to be critical. Only in cases when large volumes of the heart or the lung are exposed, a certain level of cautiousness should be advised.
Lenalidomide
Lenalidomide is a derivative of thalidomide. Thus, the anti-angiogenic effect and the adverse effects are to a large extent similar to thalidomide. However, it largely lacks the sedative side effect, making it better tolerable for patients. Leukopenia and thrombocytopenia have also been reported.
In Europe and the US it is only approved in combination with dexamethasone for the treatment of multiple myeloma as 2nd line therapy. There is only very limited data regarding the combination of lenalidomide and radiotherapy. A single phase I trial [101] in glioblastoma used lenalidomide with RT (60 Gy, 30 fx). Thromboembolic events, pneumonitis and elevation of transaminases have been reported. The maximal tolerable dose was reported to be 15 mg/m2, corresponding to the respective dose for monotherapy. Being chemically similar to thalidomide and having a similar profile of side effects, one can indirectly assume a similar pattern of interaction with radiation.
Imatinib
Imatinib is a tyrosine-kinase inhibitor (TKI) of bcr-abl, PDGFR alpha/beta and c-kit. The first successful clinical application of imatinib was in chronic myeloid leukaemia as the bcr-abl-fusion gene plays a crucial role in this disease. As GIS-tumours display a high number of c-kit-mutations, they are currently also treated with imatinib. Imatinib alone is usually well tolerated. Known adverse effects are diarrhea, nausea, vomiting, erythema, edema or the increase of transaminases; leukopenia or thrombopenia usually occur only in leukemic diseases. Grade III-IV toxicity is reported in fewer than 10% of the patients.
Several in vitro experiments showed a putative radio-sensitizing effect of imatinib [102]. Additionally it has been shown, that the proliferation of fibroblasts can be slowed down in vitro by imatinib [103]. This leads to speculations about a potential protective effect of imatinib with regard to radiation-induced fibrosis. Three in vivo experiments support this hypothesis [104–106].
Regarding the clinical use of radiotherapy and imatinib only limited data is available. Imatinib has been used in recurrent glioma after radiotherapy (one 112-patient-trial with imatinib alone after radiotherapy and three 30-40-patient trials in combination with hydroxyurea). Unexpected adverse effects pointing to an increased toxic profile for the sequential use have not been reported [107–110]. In another trial, 27 patients have been treated with imatinib after radiotherapy in prostate cancer without unexpected side effects [111].
There is only one clinical phase I study regarding the simultaneous application of imatinib to radiotherapy (55.8 Gy in 21 fx) in children with brainstem-tumours. Retrospectively compared to a similar collective, subclinical bleeding seemed increased, but no other unexpected toxicities have been reported [112]. Additionally, there are two case reports for the combinational approach [113, 114]. Again, in both cases no unexpected side effects have been reported.
Altogether, sequential application of imatinib with radiotherapy might not bear an increased risk for adverse effects. For the simultaneous application the limited amount of data does not allow a valid judgement about potentially increased side effects.
Discussion
Radiotherapy combined with molecular targeted agents may be associated with unforeseen yet specific toxicities. Based on putative interactions of radiotherapy and the given agent with the targeted signalling cascade, any interactions may not only interfere with any anti-tumour efficacy but may also increase side effects. On the other hand, also radio-protective effects for the tumour are possible if new combined treatment schedules are used. Examples are cetuximab in multimodal radio-chemotherapy regimens for rectal cancer [115–119] or erythropoietin, which was thought to increase the haemoglobin level in head-and-neck cancer patients, but decreased survival most likely due to EPO-receptors on the cancer cells which were not known as a proliferative factor for tumours before [120, 121].
However, there are still clinical situations where patients may benefit from the application of a targeted drug in combination with radiotherapy outside approved treatment schedules or clinical trials. The best example is a palliative systemic treatment for disseminated metastases and at the same time an indication for palliative or symptomatic radiotherapy of a single region. In this case, interruption of the systemic treatment may lead to systemic progression under radiotherapy. The present work aims to provide a helpful tool for clinical treatment decisions in such situations.
At present, only limited data is available on the interactions of targeted agents and radiotherapy. Data on toxicity are mostly derived from small case series, retrospective analyses or at best cohort and few randomized studies. For most substances, mild complications are reported-however, rarely exceptional fatal complications have been documented.
Overall, for any of the drugs mentioned here indications for a combination with radiotherapy have to be made cautiously (is a sequential treatment possible?). Furthermore, patients have to be questioned very specifically regarding the intake of targeted drugs. Frequently patients have been advised that these drugs are not "classical cytostatic drugs". Thus patients often do not self-report intake of targeted drugs when counselled for radiotherapy.
Simultaneous applications of targeted drugs during radiotherapy in non-established schedules should be an exception and reserved for those patients where the systemic tumour situation mandates rapid treatment. Whenever possible, large volume radiotherapy plus targeted drugs should be avoided. These remarks are especially important for hypo-fractionated regimens where high toxicities have been observed (in part with fatal consequences).
In conclusion, molecular targeted agents should only very cautiously in combination with radiotherapy. A meticulous and careful balancing of benefits and risks of increased toxicity is advised.
Abbreviations
- CNS:
-
central nervous system
- CT:
-
chemotherapy
- DFS:
-
disease-free survival
- EBRT:
-
external beam radiotherapy
- EGFRi:
-
epidermal growth factor receptor inhibitor
- IGRT:
-
image-guided radiotherapy
- KPS:
-
Karnofsky Performance Status
- mo:
-
months
- mPFS:
-
median progression-free survival
- MST:
-
median survival time
- MTD:
-
maximum tolerated dose
- mTOR:
-
mammalian target of rapamycin.
- mTTP:
-
median time to progression
- NTD:
-
normalized total dose
- OS:
-
overall survival
- PFS-6/-12:
-
progression-free survival rate at 6/12 months
- pt(s):
-
patient(s)
- PTEN:
-
phosphatase and tensin homolog deleted on Chromosome 10
- QOL:
-
quality of life
- RCHT:
-
radio-chemotherapy
- RT:
-
radiotherapy
- VEGF-R:
-
vascular endothelial growth factor receptor
- WBRT:
-
whole brain radiotherapy
- wk:
-
week.
References
Streffer C: Strong association between cancer and genomic instability. Radiat Environ Biophys. 2010, 49 (2): 125-131.
Brahme A, Lind BK: A systems biology approach to radiation therapy optimization. Radiat Environ Biophys. 2010, 49 (2): 111-124.
Hille A, Gruger S, Christiansen H, Wolff HA, Volkmer B, Lehmann J, Dorr W, Rave-Frank M: Effect of tumour-cell-derived or recombinant keratinocyte growth factor (KGF) on proliferation and radioresponse of human epithelial tumour cells (HNSCC) and normal keratinocytes in vitro. Radiat Environ Biophys. 2010, 49 (2): 261-270.
Jacob P, Ron E: Late health effects of ionizing radiation: bridging the experimental and epidemiological divide. Radiat Environ Biophys. 2010, 49 (2): 109-110.
Miller AC, Cohen S, Stewart M, Rivas R, Lison P: Radioprotection by the histone deacetylase inhibitor phenylbutyrate. Radiat Environ Biophys. 2011, 50 (4): 585-596.
Soucy KG, Attarzadeh DO, Ramachandran R, Soucy PA, Romer LH, Shoukas AA, Berkowitz DE: Single exposure to radiation produces early anti-angiogenic effects in mouse aorta. Radiat Environ Biophys. 2010, 49 (3): 397-404.
Wolff HA, Rolke D, Rave-Frank M, Schirmer M, Eicheler W, Doerfler A, Hille A, Hess CF, Matthias C, Rodel RM, et al: Analysis of chemokine and chemokine receptor expression in squamous cell carcinoma of the head and neck (SCCHN) cell lines. Radiat Environ Biophys. 2011, 50 (1): 145-154.
Amir E, Seruga B, Martinez-Lopez J, Kwong R, Pandiella A, Tannock IF, Ocana A: Oncogenic targets, magnitude of benefit, and market pricing of antineoplastic drugs. J Clin Oncol. 2011, 29 (18): 2543-2549.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004, 351 (4): 337-345.
Bonner Ja, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2006, 354: 567-578.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, et al: Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008, 359 (11): 1116-1127.
Harari PM, Huang SM: Modulation of molecular targets to enhance radiation. Clin Cancer Res. 2000, 6 (2): 323-325.
Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP: Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005, 280 (35): 31182-31189.
Bonner Ja, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer Sa, Zhu J: Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. The Lancet Oncology. 2010, 11: 21-28.
Gurtner K, Deuse Y, Butof R, Schaal K, Eicheler W, Oertel R, Grenman R, Thames H, Yaromina A, Baumann M, et al: Diverse effects of combined radiotherapy and EGFR inhibition with antibodies or TK inhibitors on local tumour control and correlation with EGFR gene expression. Radiother Oncol. 2011, 99 (3): 323-330.
Krause M, Gurtner K, Deuse Y, Baumann M: Heterogeneity of tumour response to combined radiotherapy and EGFR inhibitors: differences between antibodies and TK inhibitors. Int J Radiat Biol. 2009, 85 (11): 943-954.
Toulany M, Dittmann K, Kruger M, Baumann M, Rodemann HP: Radioresistance of K-Ras mutated human tumor cells is mediated through EGFR-dependent activation of PI3K-AKT pathway. Radiother Oncol. 2005, 76 (2): 143-150.
Budach W, Bolke E, Homey B: Severe cutaneous reaction during radiation therapy with concurrent cetuximab. N Engl J Med. 2007, 357 (5): 514-515.
Berger B, Belka C: Severe skin reaction secondary to concomitant radiotherapy plus cetuximab. Radiation oncology (London, England). 2008, 3: 5-
Bölke E, Gerber PA, Lammering G, Peiper M, Müller-Homey A, Pape H, Giro C, Matuschek C, Bruch-Gerharz D, Hoffmann TK, et al: Development and management of severe cutaneous side effects in head-and-neck cancer patients during concurrent radiotherapy and cetuximab. Strahlentherapie und Onkologie: Organ der Deutschen Röntgengesellschaft [et al]. 2008, 184: 105-110.
Billan S, Abdah-Bortnyak R, Kuten A: Severe desquamation with skin necrosis: a distinct pattern of skin toxicity secondary to head and neck irradiation with concomitant cetuximab. Isr Med Assoc J. 2008, 10 (3): 247-
Pryor DI, Porceddu SV, Burmeister BH, Guminski A, Thomson DB, Shepherdson K, Poulsen M: Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009, 90: 172-176.
Giro C, Berger B, Bölke E, Ciernik IF, Duprez F, Locati L, Maillard S, Ozsahin M, Pfeffer R, Robertson aG, et al: High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009, 90: 166-171.
Koutcher LD, Wolden S, Lee N: Severe Radiation Dermatitis in Patients With Locally Advanced Head and Neck Cancer Treated With Concurrent Radiation and Cetuximab. American journal of clinical oncology. 2009, 32: 472-476.
Studer G, Brown M, Salgueiro EB, Schmückle H, Romancuk N, Winkler G, Lee SJ, Sträuli A, Kissling B, Dummer R, et al: Grade 3/4 Dermatitis in Head and Neck Cancer Patients Treated with Concurrent Cetuximab and IMRT. International journal of radiation oncology, biology, physics. 2011, 81 (1): 110-117.
Walsh L, Gillham C, Dunne M, Fraser I, Hollywood D, Armstrong J, Thirion P: Toxicity of cetuximab versus cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer (LAHNSCC). Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011, 98: 38-41.
Kanakamedala MR, Packianathan S, Vijayakumar S: Lack of Cetuximab induced skin toxicity in a previously irradiated field: case report and review of the literature. Radiat Oncol. 2010, 5: 38-
Hallqvist a, Wagenius G, Rylander H, Brodin O, Holmberg E, Lödén B, Ewers S-B, Bergström S, Wichardt-Johansson G, Nilsson K, et al: Concurrent cetuximab and radiotherapy after docetaxel-cisplatin induction chemotherapy in stage III NSCLC: satellite--a phase II study from the Swedish Lung Cancer Study Group. Lung cancer (Amsterdam, Netherlands). 2010, 71: 166-172.
Hughes S, Liong J, Miah A, Ahmad S, Leslie M, Harper P, Prendiville J, Shamash J, Subramaniam R, Gaya A, et al: A brief report on the safety study of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III non-small cell lung cancer (NSCLC): SCRATCH study. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008, 3: 648-651.
Horisberger K, Treschl A, Mai S, Barreto-Miranda M, Kienle P, Ströbel P, Erben P, Woernle C, Dinter D, Kähler G, et al: Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. International journal of radiation oncology, biology, physics. 2009, 74: 1487-1493.
Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, Plette A, Evans D, Wanebo H, Akerman P, et al: Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. International journal of radiation oncology, biology, physics. 2008, 70: 391-395.
Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, Broholm H, Stockhausen MT, Poulsen HS: Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 12 (5): 508-516.
Giusti RM, Shastri K, Pilaro AM, Fuchs C, Cordoba-Rodriguez R, Koti K, Rothmann M, Men AY, Zhao H, Hughes M, et al: U.S. Food and Drug Administration approval: panitumumab for epidermal growth factor receptor-expressing metastatic colorectal carcinoma with progression following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens. Clin Cancer Res. 2008, 14 (5): 1296-1302.
Wirth LJ, Allen aM, Posner MR, Haddad RI, Li Y, Clark JR, Busse PM, Chan aW, Goguen La, Norris CM, et al: Phase I dose-finding study of paclitaxel with panitumumab, carboplatin and intensity-modulated radiotherapy in patients with locally advanced squamous cell cancer of the head and neck. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010, 21: 342-347.
Pinto C, Di Fabio F, Maiello E, Pini S, Latiano T, Aschele C, Garufi C, Bochicchio A, Rosati G, Aprile G, et al: Phase II study of panitumumab, oxaliplatin, 5-fluorouracil, and concurrent radiotherapy as preoperative treatment in high-risk locally advanced rectal cancer patients (StarPan/STAR-02 Study). Ann Oncol. 2011, 22 (11): 2424-2430.
Kruser TJ, Armstrong EA, Ghia AJ, Huang S, Wheeler DL, Radinsky R, Freeman DJ, Harari PM: Augmentation of radiation response by panitumumab in models of upper aerodigestive tract cancer. Int J Radiat Oncol Biol Phys. 2008, 72 (2): 534-542.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004, 351 (17): 1731-1740.
Rodríguez MO, Rivero TC, del Castillo Bahi R, Muchuli CR, Bilbao MA, Vinageras EN, Alert J, Galainena JJ, Rodríguez E, Gracias E, et al: Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer biology & therapy. 2010, 9: 343-349.
Bebb G, Smith C, Rorke S, Boland W, Nicacio L, Sukhoo R, Brade A: Phase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapy. Cancer chemotherapy and pharmacology. 2011, 67 (4): 837-45.
Choi HJ, Sohn JH, Lee CG, Shim HS, Lee I-J, Yang WI, Kwon JE, Kim SK, Park M-S, Lee JH, et al: A phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung cancer (Amsterdam, Netherlands). 2010, 71: 55-59.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001, 344 (11): 783-792.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005, 353 (16): 1659-1672.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010, 376 (9742): 687-697.
Bourgier C, Ozsahin M, Azria D: Multidisciplinary approach of early breast cancer: the biology applied to radiation oncology. Radiat Oncol. 2010, 5: 2-
Chien AJ, Rugo HS: The cardiac safety of trastuzumab in the treatment of breast cancer. Expert Opin Drug Saf. 2010, 9 (2): 335-346.
de Azambuja E, Bedard PL, Suter T, Piccart-Gebhart M: Cardiac toxicity with anti-HER-2 therapies: what have we learned so far?. Target Oncol. 2009, 4 (2): 77-88.
Ewer MS, Ewer SM: Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010, 7 (10): 564-575.
Halyard MY, Pisansky TM, Dueck AC, Suman V, Pierce L, Solin L, Marks L, Davidson N, Martino S, Kaufman P, et al: Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009, 27 (16): 2638-2644.
Belkacémi Y, Gligorov J, Ozsahin M, Marsiglia H, De Lafontan B, Laharie-Mineur H, Aimard L, Antoine E-C, Cutuli B, Namer M, et al: Concurrent trastuzumab with adjuvant radiotherapy in HER2-positive breast cancer patients: acute toxicity analyses from the French multicentric study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2008, 19: 1110-1116.
Shaffer R, Tyldesley S, Rolles M, Chia S, Mohamed I: Acute cardiotoxicity with concurrent trastuzumab and radiotherapy including internal mammary chain nodes: a retrospective single-institution study. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009, 90: 122-126.
Magné N, Védrine L, Chargari C: Impact on cardiac toxicity with trastuzumab and radiotherapy: the question is still ongoing. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009, 27: e239-author reply e240-231
Lordick F, Geinitz H, Theisen J, Sendler A, Sarbia M: Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. International journal of radiation oncology, biology, physics. 2006, 64: 1295-1298.
Crane CH, Eng C, Feig BW, Das P, Skibber JM, Chang GJ, Wolff Ra, Krishnan S, Hamilton S, Janjan Na, et al: Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. International journal of radiation oncology, biology, physics. 2010, 76: 824-830.
Velenik V, Oblak I, Anderluh F: Long-term results from a randomized phase II trial of neoadjuvant combined-modality therapy for locally advanced rectal cancer. Radiat Oncol. 2010, 5: 88-
Dipetrillo T, Pricolo V, Lagares-Garcia J, Vrees M, Klipfel A, Cataldo T, Sikov W, McNulty B, Shipley J, Anderson E, et al: Neoadjuvant Bevacizumab, Oxaliplatin, 5-Fluorouracil, and Radiation for Rectal Cancer. International journal of radiation oncology, biology, physics. 2012, 82 (1): 124-129.
Bege T, Lelong B, Viret F, Turrini O, Guiramand J, Topart D, Moureau-Zabotto L, Giovannini M, Goncalves A, Delpero JR: Bevacizumab-related surgical site complication despite primary tumor resection in colorectal cancer patients. Ann Surg Oncol. 2009, 16 (4): 856-860.
Velenik V, Ocvirk J, Music M, Bracko M, Anderluh F, Oblak I, Edhemovic I, Brecelj E, Kropivnik M, Omejc M: Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study. Radiat Oncol. 2011, 6: 105-
Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY, et al: Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009, 27: 3020-3026.
Koukourakis MI, Giatromanolaki A, Sheldon H, Buffa FM, Kouklakis G, Ragoussis I, Sivridis E, Harris AL: Phase I/II trial of bevacizumab and radiotherapy for locally advanced inoperable colorectal cancer: vasculature-independent radiosensitizing effect of bevacizumab. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009, 15: 7069-7076.
Koukourakis MI, Tsoutsou PG, Karpouzis A, Tsiarkatsi M, Karapantzos I, Daniilidis V, Kouskoukis C: Radiochemotherapy with cetuximab, cisplatin, and amifostine for locally advanced head and neck cancer: a feasibility study. International journal of radiation oncology, biology, physics. 2010, 77: 9-15.
Czito BG, Bendell JC, Willett CG, Morse Ma, Blobe GC, Tyler DS, Thomas J, Ludwig Ka, Mantyh CR, Ashton J, et al: Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. International journal of radiation oncology, biology, physics. 2007, 68: 472-478.
Crane CH, Ellis LM, Abbruzzese JL, Amos C, Xiong HQ, Ho L, Evans DB, Tamm EP, Ng C, Pisters PWT, et al: Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006, 24: 1145-1151.
Crane CH, Winter K, Regine WF, Safran H, Rich Ta, Curran W, Wolff Ra, Willett CG: Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009, 27: 4096-4102.
Small W, Mulcahy MF, Rademaker A, Bentrem DJ, Benson AB, Weitner BB, Talamonti MS: Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010, 80 (2): 476-482.
Goyal S, Rao MS, Khan A, Huzzy L, Green C, Haffty BG: Evaluation of Acute Locoregional Toxicity in Patients with Breast Cancer Treated with Adjuvant Radiotherapy in Combination with Bevacizumab. International journal of radiation oncology, biology, physics. 2010, 79: 408-413.
Spigel DR, Hainsworth JD, Yardley Da, Raefsky E, Patton J, Peacock N, Farley C, Burris Ha, Greco FA: Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010, 28: 43-48.
Beal K, Abrey LE, Gutin PH: Antiangiogenic agents in the treatment of recurrent or newly diagnosed glioblastoma: analysis of single-agent and combined modality approaches. Radiat Oncol. 2011, 6: 2-
Gutin PH, Iwamoto FM, Beal K, Mohile Na, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE: Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. International journal of radiation oncology, biology, physics. 2009, 75: 156-163.
Niyazi M, Ganswindt U, Schwarz SB, Kreth F-W, Tonn J-C, Geisler J, la Fougère C, Ertl L, Linn J, Siefert A, et al: Irradiation and Bevacizumab in High-Grade Glioma Retreatment Settings. International journal of radiation oncology, biology, physics. 2010, 1-10.
Lai A, Filka E, McGibbon B, Nghiemphu PL, Graham C, Yong WH, Mischel P, Liau LM, Bergsneider M, Pope W, et al: Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008, 71 (5): 1372-1380.
Kelly PJ, Dinkin MJ, Drappatz J, O'Regan KN, Weiss SE: Unexpected late radiation neurotoxicity following bevacizumab use: a case series. J Neurooncol. 2010, 102 (3): 485-490.
Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al: Phase II Study of Bevacizumab Plus Temozolomide During and After Radiation Therapy for Patients With Newly Diagnosed Glioblastoma Multiforme. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010, 29:
Wirth A: The rationale and role of radiation therapy in the treatment of patients with diffuse large B-cell lymphoma in the Rituximab era. Leuk Lymphoma. 2007, 48 (11): 2121-2136.
Yahalom J: Radiation therapy after R-CHOP for diffuse large B-cell lymphoma: the gain remains. J Clin Oncol. 2010, 28 (27): 4105-4107.
Phan J, Mazloom A, Jeffrey Medeiros L, Zreik TG, Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V, et al: Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010, 28: 4170-4176.
Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, et al: CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7 (5): 379-391.
Peters NaJB, Richel DJ, Verhoeff JJC, Stalpers LJa: Bowel perforation after radiotherapy in a patient receiving sorafenib. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008, 26: 2405-2406.
Basille D, Andrejak M, Bentayeb H, Kanaan M, Fournier C, Lecuyer E, Boutemy M, Garidi R, Douadi Y, Dayen C: Bronchial fistula associated with sunitinib in a patient previously treated with radiation therapy. Ann Pharmacother. 44 (2): 383-386.
Hur H, Park AR, Jee SB, Jung SE, Kim W, Jeon HM: Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol. 2008, 14 (39): 6096-6099.
Kao J, Packer S, Vu HL, Schwartz ME, Sung MW, Stock RG, Lo Y-C, Huang D, Chen S-H, Cesaretti Ja: Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: acute toxicity and preliminary response. Cancer. 2009, 115: 3571-3580.
Pouessel D, Culine S: High frequency of intracerebral hemorrhage in metastatic renal carcinoma patients with brain metastases treated with tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor. Eur Urol. 2008, 53 (2): 376-381.
Silvano G, Lazzari G, Lovecchio M, Palazzo C: Acute and fatal diarrhoea after erlotinib plus abdominal palliative hypofractionated radiotherapy in a metastatic non-small cell lung cancer patient: a case report. Lung cancer (Amsterdam, Netherlands). 2008, 61: 270-273.
Yan DF, Yan SX, Yang JS, Wang YX, Sun XL, Liao XB, Liu JQ: Hemorrhage of brain metastasis from non-small cell lung cancer post gefitinib therapy: two case reports and review of the literature. BMC cancer. 2010, 10: 49-
Weppler SA, Krause M, Zyromska A, Lambin P, Baumann M, Wouters BG: Response of U87 glioma xenografts treated with concurrent rapamycin and fractionated radiotherapy: possible role for thrombosis. Radiother Oncol. 2007, 82 (1): 96-104.
Shinohara E, Cao C, Niermann K, Mu Y, Zeng F, Hallahan D, Lu B: mTOR Inhibitors Are Safe and Effective Radiosensitizers in Glioblastoma Multiforme Pre-Clinical Models. International Journal of Radiation Oncology Biology Physics. 2005, 63: 172-
Manegold C: New options for integrating antiangiogenic therapy and platinum-based first-line chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer. 2008, 9 (Suppl 3): S100-108.
Chen H, Ma Z, Vanderwaal RP, Feng Z, Gonzalez-Suarez I, Wang S, Zhang J, Roti Roti JL, Gonzalo S: The mTOR inhibitor rapamycin suppresses DNA double-strand break repair. Radiat Res. 2010, 175 (2): 214-224.
Temkin SM, Yamada SD, Fleming GF: A phase I study of weekly temsirolimus and topotecan in the treatment of advanced and/or recurrent gynecologic malignancies. Gynecologic oncology. 2010, 117: 473-476.
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, et al: Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999, 341 (21): 1565-1571.
Epperly MW, Greenberger EE, Franicola D, Jacobs S, Greenberger JS: Thalidomide radiosensitization of normal murine hematopoietic but not squamous cell carcinoma or multiple myeloma tumor cell lines. In Vivo. 2006, 20 (3): 333-339.
Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Grégoire V, Feron O, Gallez B: Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005, 11: 743-750.
Kim KT, Chae HS, Kim JS, Kim HK, Cho YS, Choi W, Choi KY, Rho SY, Kang SJ: Thalidomide effect in endothelial cell of acute radiation proctitis. World J Gastroenterol. 2008, 14 (30): 4779-4783.
Knisely JPS, Berkey B, Chakravarti A, Yung AWK, Curran WJ, Robins HI, Movsas B, Brachman DG, Henderson RH, Mehta MP: A phase III study of conventional radiation therapy plus thalidomide versus conventional radiation therapy for multiple brain metastases (RTOG 0118). International journal of radiation oncology, biology, physics. 2008, 71: 79-86.
Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth F-W, Tonn J-C, Belka C: Therapeutic options for recurrent malignant glioma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011, 98: 1-14.
Chang SM, Lamborn KR, Malec M, Larson D, Wara W, Sneed P, Rabbitt J, Page M, Nicholas MK, Prados MD: Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. International journal of radiation oncology, biology, physics. 2004, 60: 353-357.
Groves MD, Puduvalli VK, Chang SM, Conrad CA, Gilbert MR, Tremont-Lukats IW, Liu TJ, Peterson P, Schiff D, Cloughesy TF, et al: A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007, 81 (3): 271-277.
Turner CD, Chi S, Marcus KJ, MacDonald T, Packer RJ, Poussaint TY, Vajapeyam S, Ullrich N, Goumnerova LC, Scott RM, et al: Phase II study of thalidomide and radiation in children with newly diagnosed brain stem gliomas and glioblastoma multiforme. Journal of neuro-oncology. 2007, 82: 95-101.
Kim SY, Hong YS, Kim DY, Kim TW, Kim JH, Im SA, Lee KS, Yun T, Jeong S-Y, Choi HS, et al: Preoperative Chemoradiation with Cetuximab, Irinotecan, and Capecitabine in Patients with Locally Advanced Resectable Rectal Cancer: A Multicenter Phase II Study. International journal of radiation oncology, biology, physics. 2011, 81 (3): 677-683.
Kerst JM, Bex A, Mallo H, Dewit L, Haanen JB, Boogerd W, Teertstra HJ, de Gast GC: Prolonged low dose IL-2 and thalidomide in progressive metastatic renal cell carcinoma with concurrent radiotherapy to bone and/or soft tissue metastasis: a phase II study. Cancer Immunol Immunother. 2005, 54 (9): 926-931.
Anscher MS, Garst J, Marks LB, Larrier N, Dunphy F, Herndon JE, Clough R, Marino C, Vujaskovic Z, Zhou S, et al: Assessing the ability of the antiangiogenic and anticytokine agent thalidomide to modulate radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2006, 66 (2): 477-482.
Drappatz J, Wong ET, Schiff D, Kesari S, Batchelor TT, Doherty L, Lafrankie DC, Ramakrishna N, Weiss S, Smith ST, et al: A pilot safety study of lenalidomide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. International journal of radiation oncology, biology, physics. 2009, 73: 222-227.
Topaly J, Fruehauf S, Ho AD, Zeller WJ: Rationale for combination therapy of chronic myelogenous leukaemia with imatinib and irradiation or alkylating agents: implications for pretransplant conditioning. Br J Cancer. 2002, 86 (9): 1487-1493.
Li M, Ping G, Plathow C, Trinh T, Lipson KE, Hauser K, Krempien R, Debus J, Abdollahi A, Huber PE: Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC cancer. 2006, 6: 79-
Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, et al: Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005, 201 (6): 925-935.
Thomas DM, Fox J, Haston CK: Imatinib therapy reduces radiation-induced pulmonary mast cell influx and delays lung disease in the mouse. Int J Radiat Biol. 86 (6): 436-444.
Li M, Abdollahi A, Grone HJ, Lipson KE, Belka C, Huber PE: Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat Oncol. 2009, 4: 66-
Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PM, Frenay M, Rampling R, Stupp R, Kros JM, et al: Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008, 26 (28): 4659-4665.
Reardon DA, Egorin MJ, Quinn JA, Rich JN, Gururangan S, Vredenburgh JJ, Desjardins A, Sathornsumetee S, Provenzale JM, Herndon JE, et al: Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005, 23 (36): 9359-9368.
Dresemann G: Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol. 2005, 16 (10): 1702-1708.
Desjardins A, Quinn JA, Vredenburgh JJ, Sathornsumetee S, Friedman AH, Herndon JE, McLendon RE, Provenzale JM, Rich JN, Sampson JH, et al: Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007, 83 (1): 53-60.
Bajaj GK, Zhang Z, Garrett-Mayer E, Drew R, Sinibaldi V, Pili R, Denmeade SR, Carducci MA, Eisenberger MA, DeWeese TL: Phase II study of imatinib mesylate in patients with prostate cancer with evidence of biochemical relapse after definitive radical retropubic prostatectomy or radiotherapy. Urology. 2007, 69 (3): 526-531.
Pollack IF, Jakacki RI, Blaney SM, Hancock ML, Kieran MW, Phillips P, Kun LE, Friedman H, Packer R, Banerjee A, et al: Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007, 9 (2): 145-160.
Ciresa M, D'Angelillo RM, Ramella S, Cellini F, Gaudino D, Stimato G, Fiore M, Greco C, Nudo R, Trodella L: Molecularly targeted therapy and radiotherapy in the management of localized gastrointestinal stromal tumor (GIST) of the rectum: a case report. Tumori. 2009, 95 (2): 236-239.
Boruban C, Sencan O, Akmansu M, Atik ET, Ozbek S: Metastatic gastrointestinal stromal tumor with long-term response after treatment with concomitant radiotherapy and imatinib mesylate. Anti-cancer drugs. 2007, 18: 969-972.
Rödel C, Arnold D, Hipp M, Liersch T, Dellas K, Iesalnieks I, Hermann RM, Lordick F, Hinke A, Hohenberger W, et al: Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. International journal of radiation oncology, biology, physics. 2008, 70: 1081-1086.
Weiss C, Arnold D, Dellas K, Liersch T, Hipp M, Fietkau R, Sauer R, Hinke A, Rodel C: Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: A pooled analysis of three prospective phase I-II trials. Int J Radiat Oncol Biol Phys. 2010, 78 (2): 472-478.
Bertolini F, Chiara S, Bengala C, Antognoni P, Dealis C, Zironi S, Malavasi N, Scolaro T, Depenni R, Jovic G, et al: Neoadjuvant treatment with single-agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy: a phase II study in locally advanced rectal cancer. International journal of radiation oncology, biology, physics. 2009, 73: 466-472.
Velenik V, Ocvirk J, Oblak I, Anderluh F: A phase II study of cetuximab, capecitabine and radiotherapy in neoadjuvant treatment of patients with locally advanced resectable rectal cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010, 36: 244-250.
Machiels J-P, Sempoux C, Scalliet P, Coche J-C, Humblet Y, Van Cutsem E, Kerger J, Canon J-L, Peeters M, Aydin S, et al: Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2007, 18: 738-744.
Henke M, Laszig R, Rübe C, Schäfer U, Haase K-D, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, et al: Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003, 362: 1255-1260.
Henke M, Mattern D, Pepe M, Bézay C, Weissenberger C, Werner M, Pajonk F: Do erythropoietin receptors on cancer cells explain unexpected clinical findings?. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006, 24: 4708-4713.
Jensen AD, Bergmann ZP, Garcia-Huttenlocher H, Freier K, Debus J, Münter MW: Cetuximab and radiation for primary and recurrent squamous cell carcinoma of the head and neck (SCCHN) in the elderly and multi-morbid patient: a single-centre experience. Head & neck oncology. 2010, 2: 34-
Garcia-Huttenlocher HI, Timke C, Dinkel J, Huber PE, Debus J, Muenter MW: Acute Toxicity of Skin and Mucosa in Patients with Head and Neck Cancer Receiving Radiotherapy Alone or in Combination with Chemotherapy or Cetuximab. International Journal of Radiation OncologyBiologyPhysics. 2009, 75: S385-S386.
Jatoi a, Schild SE, Foster N, Henning GT, Dornfeld KJ, Flynn PJ, Fitch TR, Dakhil SR, Rowland KM, Stella PJ, et al: A phase II study of cetuximab and radiation in elderly and/or poor performance status patients with locally advanced non-small-cell lung cancer (N0422). Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010, 21: 2040-2044.
Koutcher L, Sherman E, Fury M, Wolden S, Zhang Z, Mo Q, Stewart L, Schupak K, Gelblum D, Wong R, et al: Concurrent Cisplatin and Radiation Versus Cetuximab and Radiation for Locally Advanced Head-and-Neck Cancer. International journal of radiation oncology, biology, physics. 2011, 81 (4): 915-922.
Buiret G, Combe C, Favrel V, Pommier P, Martin L, Ecochard R, Fayette J, Tartas S, Ramade A, Céruse P: A retrospective, multicenter study of the tolerance of induction chemotherapy with docetaxel, Cisplatin, and 5-Fluorouracil followed by radiotherapy with concomitant cetuximab in 46 cases of squamous cell carcinoma of the head and neck. International journal of radiation oncology, biology, physics. 2010, 77: 430-437.
Garcia-Huttenlocher H, Stoiber E, Timke C, Debus J, Münter M: Skin Toxicity under Combined Radio-Immune-Therapy with Cetuximab in Head and Neck Cancer. International Journal of Radiation Oncology • Biology • Physics. 2008, 72: S417-S418.
Merlano M, Russi E, Benasso M, Corvò R, Colantonio I, Vigna-Taglianti R, Vigo V, Bacigalupo a, Numico G, Crosetto N, et al: Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010, 1-6.
Suntharalingam M, Kwok Y, Goloubeva O, Parekh A, Taylor R, Wolf J, Zimrin A, Strome S, Ord R, Cullen KJ: Phase II Study Evaluating the Addition of Cetuximab to the Concurrent Delivery of Weekly Carboplatin, Paclitaxel, and Daily Radiotherapy for Patients With Locally Advanced Squamous Cell Carcinomas of the Head and Neck. Int J Radiat Oncol Biol Phys.
De Vita F, Orditura M, Martinelli E, Vecchione L, Innocenti R, Sileni VC, Pinto C, Di Maio M, Farella A, Troiani T, et al: A multicenter phase II study of induction chemotherapy with FOLFOX-4 and cetuximab followed by radiation and cetuximab in locally advanced oesophageal cancer. British journal of cancer. 2011, 104: 427-432.
Argiris A, Heron DE, Smith RP, Kim S, Gibson MK, Lai SY, Branstetter BF, Posluszny DM, Wang L, Seethala RR, et al: Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010, 28: 5294-5300.
Heron DE, Rwigema J-CM, Gibson MK, Burton SA, Quinn AE, Ferris RL: Concurrent Cetuximab With Stereotactic Body Radiotherapy for Recurrent Squamous Cell Carcinoma of the Head and Neck: A Single Institution Matched Case-Control Study. American journal of clinical oncology. 2011, 34 (2): 165-72.
Birnbaum A, Dipetrillo T, Rathore R, Anderson E, Wanebo H, Puthwala Y, Joyce D, Safran H, Henderson D, Kennedy T, et al: Cetuximab, paclitaxel, carboplatin, and radiation for head and neck cancer: a toxicity analysis. American journal of clinical oncology. 2010, 33: 144-147.
Jensen AD, Münter MW, Bischoff HG, Haselmann R, Haberkorn U, Huber PE, Thomas M, Debus J, Herfarth KK: Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: The NEAR trial. Cancer. 2011, 1-9.
Ruhstaller T, Pless M, Dietrich D, Kranzbuehler H, von Moos R, Moosmann P, Montemurro M, Schneider PM, Rauch D, Gautschi O, et al: Cetuximab in Combination With Chemoradiotherapy Before Surgery in Patients With Resectable, Locally Advanced Esophageal Carcinoma: A Prospective, Multicenter Phase IB/II Trial (SAKK 75/06). Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011, 1-6.
Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, Zahalsky AJ, Lake S, Needle MN, Shaha AR, et al: Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006, 24: 1072-1078.
Hofheinz R-D, Horisberger K, Woernle C, Wenz F, Kraus-Tiefenbacher U, Kähler G, Dinter D, Grobholz R, Heeger S, Post S, et al: Phase I trial of cetuximab in combination with capecitabine, weekly irinotecan, and radiotherapy as neoadjuvant therapy for rectal cancer. International journal of radiation oncology, biology, physics. 2006, 66: 1384-1390.
Kuhnt T, Sandner a, Wendt T, Engenhart-Cabillic R, Lammering G, Flentje M, Grabenbauer G, Schreiber a, Pirnasch a, Dunst J: Phase I trial of dose-escalated cisplatin with concomitant cetuximab and hyperfractionated-accelerated radiotherapy in locally advanced squamous cell carcinoma of the head and neck. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010, 21: 2284-2289.
Zwicker F, Roeder F, Thieke C, Timke C, Münter MW, Huber PE, Debus J: IMRT Reirradiation with Concurrent Cetuximab Immunotherapy in Recurrent Head and Neck Cancer. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2011, 32-38.
Jensen AD, Krauss J, Weichert W, Debus J, Münter MW: RadioImmunotherapy for adenoid cystic carcinoma: a single-institution series of combined treatment with cetuximab. Radiation oncology (London, England). 2010, 5: 102-
Balermpas P, Hambek M, Seitz O, Rödel C, Weiss C: Combined cetuximab and reirradiation for locoregional recurrent and inoperable squamous cell carcinoma of the head and neck. Strahlentherapie und Onkologie: Organ der Deutschen Röntgengesellschaft [et al]. 2009, 185: 775-781.
Caussa L, Kirova YM, Gault N, Pierga J-Y, Savignoni A, Campana F, Dendale R, Fourquet A, Bollet Ma: The acute skin and heart toxicity of a concurrent association of trastuzumab and locoregional breast radiotherapy including internal mammary chain: a single-institution study. European journal of cancer (Oxford, England: 1990). 2011, 47: 65-73.
Anderson PR, Freedman G, Li T, Nicolaou N, Denlinger C: Concurrent Trastuzumab and Breast Radiotherapy in the Adjuvant Setting: Analysis of Acute Toxicity. International Journal of Radiation OncologyBiologyPhysics. 2009, 75: S202-S203.
Chargari C, Idrissi HR, Pierga J-Y, Bollet Ma, Diéras V, Campana F, Cottu P, Fourquet A, Kirova YM: Preliminary Results of Whole Brain Radiotherapy with Concurrent Trastuzumab for Treatment of Brain Metastases in Breast Cancer Patients. International journal of radiation oncology, biology, physics. 2011, 81 (3): 631-636.
Horton JK, Halle J, Ferraro M, Carey L, Moore DT, Ollila D, Sartor CI: Radiosensitization of chemotherapy-refractory, locally advanced or locally recurrent breast cancer with trastuzumab: a phase II trial. International journal of radiation oncology, biology, physics. 2010, 76: 998-1004.
Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J, Renginfo E, et al: Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004, 22: 1646-1654.
Vredenburgh JJ, Desjardins A, Kirkpatrick JP, Reardon Da, Peters KB, Herndon JE, Marcello J, Bailey L, Threatt S, Sampson J, et al: Addition of Bevacizumab to Standard Radiation Therapy and Daily Temozolomide Is Associated with Minimal Toxicity in Newly Diagnosed Glioblastoma Multiforme. International journal of radiation oncology, biology, physics. 2012, 82 (1): 58-66.
Seiwert TY, Haraf DJ, Cohen EEW, Stenson K, Witt ME, Dekker A, Kocherginsky M, Weichselbaum RR, Chen HX, Vokes EE: Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008, 26: 1732-1741.
Spigel DR, Hainsworth JD, Yardley DA, Raefsky E, Patton J, Peacock N, Farley C, Burris HA, Greco FA: Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2009, 28 (1): 43-48.
Koukourakis MI, Giatromanolaki A, Tsoutsou P, Lyratzopoulos N, Pitiakoudis M, Kouklakis G, Chloropoulou PA, Manolas K, Sivridis E: Bevacizumab, capecitabine, amifostine, and preoperative hypofractionated accelerated radiotherapy (HypoArc) for rectal cancer: a Phase II study. Int J Radiat Oncol Biol Phys. 2010, 80 (2): 492-498.
Resch G, De Vries A, Ofner D, Eisterer W, Rabl H, Jagoditsch M, Gnant M, Thaler J: Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer-A two stage phase II clinical trial. Radiother Oncol.
Vargo JA, Snelling BM, Ghareeb ER, John K, Frame JN, Schmidt JH, Peters KB: Dural venous sinus thrombosis in anaplastic astrocytoma following concurrent temozolomide and focal brain radiotherapy plus bevacizumab. J Neurooncol. 2011, 104 (2): 595-598.
Chi K-H, Liao C-S, Chang C-C, Ko H-L, Tsang Y-W, Yang K-C, Mehta MP: Angiogenic blockade and radiotherapy in hepatocellular carcinoma. International journal of radiation oncology, biology, physics. 2010, 78: 188-193.
Staehler M, Haseke N, Stadler T, Nuhn P, Roosen A, Stief CG, Wilkowski R: Feasibility and effects of high-dose hypofractionated radiation therapy and simultaneous multi-kinase inhibition with sunitinib in progressive metastatic renal cell cancer. Urologic oncology.
Hui EP, Lui VW, Wong CS, Ma BB, Lau CP, Cheung CS, Ho K, Cheng SH, Ng MH, Chan AT: Preclinical evaluation of sunitinib as single agent or in combination with chemotherapy in nasopharyngeal carcinoma. Invest New Drugs. 2010, 29 (6): 1123-1131.
Harrington KJ, El-Hariry Ia, Holford CS, Lusinchi A, Nutting CM, Rosine D, Tanay M, Deutsch E, Matthews J, D'Ambrosio C, et al: Phase I study of lapatinib in combination with chemoradiation in patients with locally advanced squamous cell carcinoma of the head and neck. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009, 27: 1100-1107.
Cohen EEW, Haraf DJ, Kunnavakkam R, Stenson KM, Blair Ea, Brockstein B, Lester EP, Salama JK, Dekker A, Williams R, et al: Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010, 28: 3336-3343.
Pollack IF, Stewart CF, Kocak M, Poussaint TY, Broniscer A, Banerjee A, Douglas JG, Kun LE, Boyett JM, Geyer JR: A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: A report from the Pediatric Brain Tumor Consortium. Neuro-oncology. 2011, 13 (3): 290-297.
Valentini V, De Paoli A, Gambacorta MA, Mantini G, Ratto C, Vecchio FM, Barbaro B, Innocente R, Rossi C, Boz G, et al: Infusional 5-fluorouracil and ZD1839 (Gefitinib-Iressa) in combination with preoperative radiotherapy in patients with locally advanced rectal cancer: a phase I and II Trial (1839IL/0092). International journal of radiation oncology, biology, physics. 2008, 72: 644-649.
Wang J, Xia TY, Wang YJ, Li HQ, Li P, Wang JD, Chang DS, Liu LY, Di YP, Wang X, et al: Prospective Study of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors Concurrent With Individualized Radiotherapy for Patients With Locally Advanced or Metastatic Non-Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2011, 81 (3): e59-e65.
Zhang G, Xie L, Xu X, Chen J, Fu X, Jiang G, Fan M: Thoracic Radiotherapy and Concurrent Gefitinib in Patients with IIIB/IV Non-small Cell Lung Cancer (NSCLC): Phase I Study. International Journal of Radiation OncologyBiologyPhysics. 2009, 75: S455-S455.
Chen C, Kane M, Song J, Campana J, Raben A, Hu K, Harrison L, Quon H, Dancey J, Baron A, et al: Phase I trial of gefitinib in combination with radiation or chemoradiation for patients with locally advanced squamous cell head and neck cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007, 25: 4880-4886.
Maurel J, Martin-Richard M, Conill C, Sánchez M, Petriz L, Ginès A, Miquel R, Gallego R, Cajal R, Ayuso C, et al: Phase I trial of gefitinib with concurrent radiotherapy and fixed 2-h gemcitabine infusion, in locally advanced pancreatic cancer. International journal of radiation oncology, biology, physics. 2006, 66: 1391-1398.
Czito BG, Willett CG, Bendell JC, Morse Ma, Tyler DS, Fernando NH, Mantyh CR, Blobe GC, Honeycutt W, Yu D, et al: Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006, 24: 656-662.
Center B, Petty WJ, Ayala D, Hinson WH, Lovato J, Capellari J, Oaks T, Miller Aa, Blackstock AW: A phase I study of gefitinib with concurrent dose-escalated weekly docetaxel and conformal three-dimensional thoracic radiation followed by consolidative docetaxel and maintenance gefitinib for patients with stage III non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010, 5: 69-74.
Schwer AL, Damek DM, Kavanagh BD, Gaspar LE, Lillehei K, Stuhr K, Chen C: A phase I dose-escalation study of fractionated stereotactic radiosurgery in combination with gefitinib in patients with recurrent malignant gliomas. International journal of radiation oncology, biology, physics. 2008, 70: 993-1001.
Olsen CC, Schefter TE, Chen H, Kane M, Leong S, McCarter MD, Chen Y, Mack P, Eckhardt SG, Stiegmann G, et al: Results of a phase I trial of 12 patients with locally advanced pancreatic carcinoma combining gefitinib, paclitaxel, and 3-dimensional conformal radiation: report of toxicity and evaluation of circulating K-ras as a potential biomarker of response to the. American journal of clinical oncology. 2009, 32: 115-121.
Brown PD, Krishnan S, Sarkaria JN, Wu W, Jaeckle Ka, Uhm JH, Geoffroy FJ, Arusell R, Kitange G, Jenkins RB, et al: Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008, 26: 5603-5609.
Prados MD, Chang SM, Butowski N, DeBoer R, Parvataneni R, Carliner H, Kabuubi P, Ayers-Ringler J, Rabbitt J, Page M, et al: Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009, 27: 579-584.
Herchenhorn D, Dias FL, Viegas CMP, Federico MH, Araújo CMM, Small I, Bezerra M, Fontão K, Knust RE, Ferreira CG, et al: Phase I/II study of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck. International journal of radiation oncology, biology, physics. 2010, 78: 696-702.
Choong NW, Mauer AM, Haraf DJ, Lester E, Hoffman PC, Kozloff M, Lin S, Dancey JE, Szeto L, Grushko T, et al: Phase I trial of erlotinib-based multimodality therapy for inoperable stage III non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008, 3: 1003-1011.
Peereboom DM, Shepard DR, Ahluwalia MS, Brewer CJ, Agarwal N, Stevens GHJ, Suh JH, Toms Sa, Vogelbaum Ma, Weil RJ, et al: Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. Journal of neuro-oncology. 2010, 98: 93-99.
Chang C-C, Chi K-H, Kao S-J, Hsu P-S, Tsang Y-W, Chang H-J, Yeh Y-W, Hsieh Y-S, Jiang J-S: Upfront gefitinib/erlotinib treatment followed by concomitant radiotherapy for advanced lung cancer: A mono-institutional experience. Lung cancer (Amsterdam, Netherlands). 2011, 73 (2): 189-194.
Li G, Hu W, Wang J, Deng X, Zhang P, Zhang X, Xie C, Wu S: Phase II Study of Concurrent Chemoradiation in Combination with Erlotinib for Locally Advanced Esophageal Carcinoma. International journal of radiation oncology, biology, physics. 2010, 78: 1407-1412.
Broniscer A, Baker SJ, Stewart CF, Merchant TE, Laningham FH, Schaiquevich P, Kocak M, Morris EB, Endersby R, Ellison DW, et al: Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009, 15: 701-707.
Robertson J, Ballouz S, Jaiyesimi I, Jury R, Margolis J: A Phase I Study of Dose Escalating Conformal Radiation Therapy with Concurrent Full-dose Gemcitabine and Erlotinib for Unresected Pancreas Cancer. International Journal of Radiation OncologyBiologyPhysics. 2009, 75: S270-S270.
Duffy a, Kortmansky J, Schwartz GK, Capanu M, Puleio S, Minsky B, Saltz L, Kelsen DP, O'Reilly EM: A phase I study of erlotinib in combination with gemcitabine and radiation in locally advanced, non-operable pancreatic adenocarcinoma. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2008, 19: 86-91.
Krishnan S, Brown PD, Ballman KV, Fiveash JB, Uhm JH, Giannini C, Jaeckle Ka, Geoffroy FJ, Nabors LB, Buckner JC: Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. International journal of radiation oncology, biology, physics. 2006, 65: 1192-1199.
Iannitti D, Dipetrillo T, Akerman P, Barnett JM, Maia-Acuna C, Cruff D, Miner T, Martel D, Cioffi W, Remis M, et al: Erlotinib and Chemoradiation Followed by Maintenance Erlotinib for Locally Advanced Pancreatic Cancer. American Journal of Clinical Oncology. 2005, 28: 570-575.
Nogueira-Rodrigues A, do Carmo CC, Viegas C, Erlich F, Camisão C, Fontão K, Lima R, Herchenhorn D, Martins RG, Moralez GM, et al: Phase I trial of erlotinib combined with cisplatin and radiotherapy for patients with locally advanced cervical squamous cell cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008, 14: 6324-6329.
Arias de la Vega F, Contreras J, de Las Heras M, de la Torre A, Arrazubi V, Herruzo I, Prieto I, Garcia-Saenz JA, Romero J, Calvo FA: Erlotinib and chemoradiation in patients with surgically resected locally advanced squamous cell carcinoma of the head and neck: a GICOR phase I trial. Ann Oncol. 2011,
Lind JSW, Lagerwaard FJ, Smit EF, Senan S: Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2009, 74: 1391-1396.
Dobelbower MC, Russo SM, Raisch KP, Seay LL, Clemons LK, Suter S, Posey J, Bonner Ja: Epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib, and concurrent 5-fluorouracil, cisplatin and radiotherapy for patients with esophageal cancer: a phase I study. Anti-cancer drugs. 2006, 17: 95-102.
Huang Y-J, Liu S-F, Wang C-J, Huang M-Y: Exacerbated radiodermatitis and bilateral subdural hemorrhage after whole brain irradiation combined with epidermal growth factor receptor tyrosine kinase inhibitors for brain metastases in lung cancer. Lung cancer (Amsterdam, Netherlands). 2008, 59: 407-410.
Sarkaria JN, Schwingler P, Schild SE, Grogan PT, Mladek AC, Mandrekar SJ, Tan AD, Kobayashi T, Marks RS, Kita H, et al: Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2007, 2: 751-757.
Bourgier C, Massard C, Moldovan C, Soria JC, Deutsch E: Total recall of radiotherapy with mTOR inhibitors: a novel and potentially frequent side-effect?. Ann Oncol. 2011, 22 (2): 485-486.
Chang SM, Lamborn KR, Malec M, Larson D, Wara W, Sneed P, Rabbitt J, Page M, Nicholas MK, Prados MD: Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004, 60 (2): 353-357.
Atkins MB, Sosman Ja, Agarwala S, Logan T, Clark JI, Ernstoff MS, Lawson D, Dutcher JP, Weiss G, Curti B, et al: Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008, 113: 2139-2145.
Ch'ang H-J, Hsu C, Chen C-H, Chang Y-H, Chang JS, Chen L-T: Phase II Study of Concomitant Thalidomide During Radiotherapy for Hepatocellular Carcinoma. International journal of radiation oncology, biology, physics. 2011,
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MN and CM performed the literature search and wrote the manuscript. MK, CMR and WB performed critical revision. CB participated in the conception as well as the preparation of the manuscript. All authors read and approved the final manuscript.
Maximilian Niyazi, Cornelius Maihoefer contributed equally to this work.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Niyazi, M., Maihoefer, C., Krause, M. et al. Radiotherapy and "new" drugs-new side effects?. Radiat Oncol 6, 177 (2011). https://doi.org/10.1186/1748-717X-6-177
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-717X-6-177