Abstract
Background
N-3 polyunsaturated fatty acids, such as docosahexaenoic acid (DHA; 22:6n-3), has clinical significance in the prevention and reversal of nonalcoholic steatohepatitis (NASH). However, the precious mechanism underlying remains unclear. The inflammasome, a multiprotein complex formed by NOD-like receptor (NLR) family members, has been recently shown to be activated in NASH and promote the cleavage of the pro-inflammatory cytokines to their maturation forms.
Methods
HepG2 cells were exposed to different dose of PA for 24 h with or without the preincubation of 50 μM DHA for another 24 h and then lipid deposition was assessed with Oil red O staining and intracellular triglyceride (TG) determination. Secretory levels of inflammatory cytokines and Caspase-1 activity were determined by ELISA assays. Gene expression and protein levels were determined by quantitative RCR and western blotting, respectively.
Results
Palmitate (PA) dose-dependently increased lipid accumulation, TG content and induced the secretion of interleukin-1β (IL-1β), IL-18, TNF-α and MCP-1 from HepG2 cells. Preincubation with DHA significantly alleviated PA-induced lipid accumulation and inflammatory agents. DHA was also found to attenuate PA-induced NOD-like receptor protein 4 (NLRC4) mRNA expression. Furthermore, PA induced caspase-1 activation in a dose-dependent manner, resulting in exacerbating of procaspase-1 and pro-IL-1β processing. Knockdown of NLRC4 partially abrogated PA-induced caspase-1 activation and IL-1β maturation and completely abolished these events in the presence of DHA.
Conclusions
Our findings indicate DHA attenuates PA-induced lipid accumulation and inflammation through suppressing NLRC4 inflammasome activation, caspase-1 activation and IL-1β cleavage.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is a worldwide common liver disease that has an increasing prevalence not only in adults with metabolic syndrome but also in obese children and adolescents [1–3]. Non-alcoholic steatohepatitis (NASH) is one of pathologic stages of NAFLD and the main histopathological changes are steatosis, inflammation and hepatocyte injury with or without fibrosis [4]. Although the precise mechanisms need to be elucidated, the two-hit hypothesis of NASH has been widely accepted [5]. The 1st hit involves fat accumulation in the liver as a result of excessive delivery of free fatty acids (FFAs) from the adipose tissue, and imbalance of lipid synthesis and export in hepatocyte. The consequent injures such as oxidative stress and inflammation are regarded as the 2nd hit culminating in fibrosis of liver.
Despite abundant evidences accumulated for a role of inflammatory mediators in NASH, less is known about how the inflammatory mediators are generated. Recent studies implicated that the inflammasomes have emerged as pivotal sensors of infection and stress in intracellular compartments and appear to regulate the production of the proinflammatory cytokines IL-1β and IL-18 [6, 7]. It has been demonstrated that one of the NOD-like receptor (NLR) family members, NOD-like receptor protein 3 (NLRP3) inflammasome, was involved in caspase-1 activation and caspase-1-mediated IL-1β and IL-18 release [8]. However, the role of another inflammasome NOD-like receptor protein 4 (NLRC4) on the generation of inflammatory mediators in NASH has not been elucidated.
Many studies have suggested that FFAs composition and abnormal fatty acid metabolism have been implicated in the pathogenesis of NASH [9–12]. Diets with a high intake of fat, especially saturated fatty acids, may promote the development of NASH [13, 14]. Conversely, polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA), have been found to decrease liver fat content in children with NAFLD and possess anti-inflammatory effects [15, 16]. It has been reported that PA induced the activation of the NLRP3 inflammasome which then senses obesity-associated danger signals and contributes to obesity-induced inflammation [17, 18]. However, the effects of DHA on NLRC4 inflammasome in PA-induced inflammatory responses remain unknown. In the present study, our findings demonstrated that NLRC4 inflammasome is activated in PA-induced lipid accumulation and inflammation; and that DHA alleviates these events by suppressing the activity of caspase-1 and the cleavage of procaspase-1 and pro-IL-1β through inhibiting NLRC4 inflammasome activation.
Methods
Materials
DHA (Sigma, St. Louis, MO) was dissolved in ethanol (stock solution 100 mM). Stock solutions were kept at −20°C before the experiments. PA (Sigma, St. Louis, MO) was conjugated with FFA-free bovine serum albumin (BSA) at a 5:1 molar ratio before treatment as previously described [19]. Solutions and reagents used for cell cultures were from GIBCO Life Technologies Ltd. (Grand Island, NY, USA). Antibodies used are from Cell Signaling Technology Inc. (Danvers, MA, USA). All other reagents and kits were purchased from Sigma Aldrich and Invitrogen (Carlsbad, CA, USA) unless otherwise noted.
Cell culture and stimulation
HepG2 cells (ATCC, Manassas, VA, USA) were routinely cultured with Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum. 2 × 104 cells were seeded into 24-well plates 24 h prior to treatments at approximately 60% confluence. After the starvation for 24 h, cells were exposed to different dose of PA for 24 h with or without the preincubation of 50 μM DHA for another 24 h. BSA and ethanol were used as controls. Cell viability after FFA treatments was monitored by trypan blue exclusion. No change in viability was observed with the concentrations used in this study. To knock down NLRC4 expression, HepG2 cells were transfected with 20 pmol NLRC4 siRNA, a commercially available small interfering RNAs (siRNAs) duplex components against this molecule (sc-60328A, sc-60328B and sc-60328 C; Santa Cruz, CA, USA) or a negative control siRNA (sc-37007; Santa Cruz, CA, USA), a RNA duplex with no known sequence homology, in a 24-well format using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Observation of lipid accumulation
The lipid accumulation in HepG2 cells was evaluated by Oil Red O staining and the measurement of triglyceride (TG) content. Briefly, samples were fixed with 4% paraformaldehyde then stained with Oil Red O for 15 min. Then, the samples were counterstained with hematoxylin for 5 min. Results were examined by light microscopy. Intracellular TG content was evaluated after lysis of the cells with 1 × cell lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM phenylmethylsulfonylfluoride, 0.2 mM sodium orthovanadate, and 0.5% NP-40) (Promega, Madison, WI, USA). TG concentration was determined by the EnzyChromTM triglyceride assay kit (Bioassay Systems) according to the protocal provided by manufacturer and normalized by protein content.
Measurement of inflammatory cytokines by ELISA assay
The cell culture media were centrifuged at 10,000 g for 10 min at 4°C and the supernatants were stored at −20°C before analysis. Secretory levels of inflammatory cytokines, including TNF-α, IL-1β, IL-18 and MCP-1 proteins, in cell-free culture supernatants were determined by Quantikine ELISA kit (R&D Systems Inc, Mineapolis, USA). The color generated was determined by measuring the OD value at 450 nm of spectrophotometric microtiter plate reader (Molecular Devices Corp., Sunnyvale, CA, USA). A standard curve was run on each assay plate using recombinant proteins in serial dilutions. The limits of sensitivity of the kits are 0.5, 7.0, 3.9 and 5.5 pg/mL for TNF-α, IL-1β, IL-18 and MCP-1, respectively.
Real-time PCR assay of NLRC4 mRNA in HepG2 cells
Total RNA in cells was isolated using TRIzol reagent and reversely transcribed to cDNA using Super Script II Rnase H Reverse Transcriptase (Invitrogen, Carlsbad, Calif) according to the manufacturer’s instructions. The cDNA samples were stored at −20°C for real-time PCR. The PCR primers were designed by Premier Primer 5.0 software as the following: 5’-CCAGTCCCCTCACCATAGAAG-3’ (forward) 5’-ACCCAAGCTGTCAGTCAGACC-3’ (reverse) for NLRC4; 5’-CCTGGCACC- CAGCACAAT-3’ (forward) and 5’-GCCGATCCACACGGAGTACT-3’ (reverse) for β-actin. Real-time PCR was done with SYBR Green PCR Master Mix (Invitrogen, Carlsbad, Calif) using a 7500 Real-Time PCR System (Applied Biosystems). Melt curve analysis was included to assure a single PCR product was formed. Values were corrected using human β-actin gene. The relative NLRC4 mRNA levels were presented as percentage of that in control cells.
Western blotting
Cellular protein was extracted with 1 × cell lysis buffer. Protein concentration was determined using the BCATM assay kit from Thermo Fisher Scientific Inc. (Huntsville, AL) according to the user manual. Protein (40 μg) from each sample was separated by 10-12% SDS-PAGE and electrotransferred to polyvinyl denedifluoride (PVDF) membranes. The membranes were blocked with 5% BSA in TBS for 1 h at room temperature and incubated overnight at 4°C using the following primary antibodies: 1:1000 rabbit anti β-actin, 1:500 NLRC4 (Santa Cruz, CA, USA), 1:1000 caspase-1 and 1:500 IL-1β, followed by 1:2000 dilution of goat anti-rabbit horseradish peroxidase-labeled antibody. The bands were visualized using the ECL system, and the band density was determined by Image J software (NIH, USA).
Caspase-1 activity assay
HepG2 cells were homogenized in 1 × cell lysis buffer and caspase-1 activity was determined using colorimetric assay based on the cleavage of the substrate Aacetyl-Tyr-Val-Ala-Asp p-nitroanilide (Ac-YVAD-p NA) (R&D Systems, Minneapolis, MN, USA). 1 × 104 cells were seeded into 96-well plates and treated in the same way as the method mentioned above. Then, cell-free culture media were analyzed with caspase-1 activity commercial kit according to the protocols provided by the manufacturer. Briefly, 90 μL standards, samples or control was added to the appropriate microtiter wells, and then 10 μL Ac-YVAD-p NA was pipetted into each well, and incubated for 2 h at 37°C. and the absorbance of each well at 405 nm was recorded after calibrating the plate reader against the chromogen blank. Cleavage of the substrate by caspase-1 releases p NA that can subsequently be quantified by the absorbance difference.
Statistical analyses
All of the experiments were performed in triplicate and repeated at least three times. Data are expressed as mean ± SE. The significance of differences was determined by One-way ANOVA using SPSS13.0 software (SPSS, Chicago, IL). A value of P < 0.05 was considered to be statistically significant.
Results
DHA suppresses PA-induced lipids accumulation in HepG2 cells
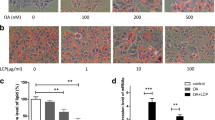
We first analyzed the lipid accumulation qualitatively by Oil red O staining. As shown in Figure 1A, HepG2 cells treated for 24 h with PA exhibited significant lipid droplet accumulation dose-dependently compared with untreated cells. Preincubation with DHA significantly prevented 0.4 mM PA-induced lipid deposition and the most effective inhibition of lipid accumulation occurred at a dose of 50 μM. DHA alone or vehicle (ethanol) did not affect basal levels of lipid deposition (data not shown). To evaluate the effect of PA and DHA on lipids accumulation quantitatively, we next measured TG concentration in cells lysates. Consistently, treatment with PA resulted in a obvious increase in TG content compared to control cells, which was attenuated significantly by pretreatment with 50 μM DHA (Figure 1B). The lipid deposition and TG content in BSA carrier control cells were comparable with untreated cells (data not shown).
DHA suppresses PA-induced lipids accumulation in HepG2 cells. (A) Representative Oil Red O staining of cells with different treatments is shown. Cells were examined by light microscopy at a magnification of 400×; (B) Intracellular TG content was measured by an ELISA assay. TG concentration was normalized by protein content. Data are shown as the mean ± S.E.; n = 4. *P < 0.05, **P < 0.01 compared with untreated cells; #P < 0.01 versus 0.4 mM PA-treated cells.
DHA attenuates PA-induced inflammatory cytokines in HepG2 cells
We examined the effects of PA on the productions of IL-1β, IL-18, TNF-α and MCP-1 in HepG2 cells. Figure 2 showed that PA exposure significantly increased the productions of all cytokines in a dose-dependent manner compared with the control cells. Pretreatment with 50 μM DHA for 24 h significantly alleviated PA-induced overproduction of all these inflammatory cytokines.
DHA attenuates PA-induced inflammatory cytokines overproduction in HepG2 cells. Cells were treated accordingly and then cell-free culture supernatants were assayed for IL-1β (A), IL-18 (B), TNF-α (C) and MCP-1 (D) by commercial ELISA kits. The data represent a mean of 4 experiments. *P < 0.05, **P < 0.01 compared with untreated cells; #P < 0.01 versus 0.4 mM PA-treated cells.
DHA inhibits PA-induced NLRC4 mRNA expression
Since only the processed cytokines are biologically active, we hypothesized that the inflammasomes which are essential in regulating the proteolytic maturation of pro-IL-1β and other proinflammatory cytokines would involved in PA-induced inflammation. As shown in Figure 3, PA elicited the expression of NLRC4 mRNA in a dose-dependent manner. DHA pretreatment significantly decreased PA-induced NLRC4 mRNA expression.
DHA inhibits PA-induced NLRC4 mRNA expression. Cells were treated accordingly and then NLRC4 mRNA was measured by real-time PCR The data are normalized using β-actin as control and expressed as fold over untreated cells. The experiment was repeated 4 times. *P < 0.05, **P < 0.01 compared with untreated cells; #P < 0.01 versus 0.4 mM PA-treated cells.
DHA reverses PA-induced lipid accumulation and inflammation through repressing NLRC4 expression
To test whether NLRC4 might modulate PA-induced steatohepatitis, cells were transfected with NLRC4 siRNA (NLRC4+/− cells). As shown in Figure 4A, the protein level of NLRC4 decreased dramatically by 91.2% by the transient RNA interference technology. Then, NLRC4+/− cells were exposed to PA with or without DHA preincubation. Compared to the wild-type cells, the lipid deposition and TG content were significantly ameliorated in PA-treated NLRC4+/− cells and were completely abolished by DHA preincubation (Figure 4B and C). Consistently, DHA almost abrogated PA-elicited production of IL-1β, IL-18, TNF-α and MCP-1 in NLRC4+/− cells (Figure 5). No significant difference was observed in cells treated with the control siRNA compared with untreated cells (Additional file 1: Figure S1 and Table S1).
DHA reverses PA-induced lipid accumulation through repressing NLRC4 expression. (A) Generation of a HepG2 cell model with specific NLRC4 knockdown (NLRC4+/−). NLRC4 and β-actin protein levels were evaluated by immunoblotting. (B) Representative Oil Red O staining of wild-type (WT) and NLRC4+/− HepG2 cells were treated with different dose of PA with or without DHA preincubation wild-type (WT) and NLRC4+/− cells are shown. Cells were examined by light microscopy at a magnification of 400×; (C) Intracellular TG content was measured by an ELISA assay. TG concentration was normalized by protein content. Data are shown as the mean ± S.E.; n = 4. *P < 0.05, **P < 0.01 compared with untreated cells; #P < 0.01 versus 0.4 mM PA-treated cells.
DHA reverses PA-induced inflammation through repressing NLRC4 expression. WT and NLRC4+/− HepG2 cells were treated with different dose of PA with or without DHA preincubation and then cell-free culture supernatants were assayed for IL-1β (A), IL-18 (B), TNF-α (C) and MCP-1 (D) by commercial ELISA kits. The data represent a mean of 4 experiments. *P < 0.05, **P < 0.01 compared with untreated cells; #P < 0.01 versus 0.4 mM PA-treated cells.
DHA inhibits caspase-1 activity and IL-1β maturation
Conversion of the inactive pro-IL-1β to its mature form IL-1β requires the proteolytic action of IL-1β-converting enzyme (ICE), which is also termed as caspase-1. The enzyme is activated by inflammsomes, such as NLRP3 and NLRC4 [17]. We tested the effect of DHA on caspase-1 activity by an ELISA assay. In the presence of different dose of PA, caspase-1 activity increased accordingly whereas partially reversed by DHA preincubation. Compared to PA-treated wild-type cells, the knockdown of NLRC4 led to a more depressed caspase-1 activity in each experimental treatment. Furthermore, DHA preincubation completely reversed PA-induced caspase-1 activation in NLRC4+/− HepG2 cells (Figure 6A).
DHA inhibits caspase-1 activity and IL-1β maturation. WT and NLRC4+/− HepG2 cells were treated with different dose of PA with or without DHA preincubation and cells were homogenized in lysis buffer. (A) Caspase-1 activity was determined using colorimetric assay based on the cleavage of substrate Ac-YVAD-pNA. The results were expressed as fold over untreated WT cells. *P < 0.05, **P < 0.01 compared with untreated cells; #P < 0.01 versus 0.4 mM PA-treated cells. (B) Immunoblot analysis showing caspase-1 cleavage and active IL-1β accumulation. This gel is the representative image of three independent experiments (upper panel). Quantified data of protein levels indicates in lower panel. The values of density of proteins were all justified with β-actin. The relative density ratios of untreated WT cells were set at a value of 1.0. The values represent the mean ± S.E (*P < 0.05, **P < 0.01 compared with WT cells treated with 0.4 mm PA for the ratio of caspase-1/procaspase-1; #P < 0.01 versus 0.4 mM PA-treated WT cells for the ratio of IL-1β/pro-IL-1β).
To determine the effect of DHA on IL-1β maturation, we measured the procaspase-1 and pro-IL-1β processing by western blotting assay. As presented in Figure 6B, PA elicited caspase-1 and IL-1β release dose-dependently compared with control cells. DHA reduced PA-induced procaspase-1 and pro-IL-1β processing significantly. NLRC4 siRNA repressed both caspase-1 and IL-1β release induced by 0.4 mM PA, and these events were potentiated by the preincubation of DHA.
Discussion
NAFLD encompasses four histopathology stages: simple steatosis, steatosis with inflammation termed as NASH, cirrhosis and hepatocellular carcinoma [20]. The steatosis and inflammation in NASH often result in the following more severe liver injuries [21]. Hence, elucidation of the possible mechanisms responsible for NASH and impeding the progress of NASH are of emerging importance. DHA possesses hepatoprotective effects and DHA supplementation decreases liver fat content in children with NAFLD [16, 22]. In the present study, we reported that DHA ameliorates PA-induced lipid accumulation, inflammatory cytokines production in HepG2 cells. Further experiments indicated that DHA down regulates NLRC4 mRNA expression and caspase-1 activity, resulting in the reduction of IL-1β release. These data suggested that DHA exerts protective effects on NASH not only at the 1st hit but also at the 2ed one.
The development of NASH has been initially linked to increased lipids deposition and plasma concentrations of FFAs are elevated in patients with NASH [23, 24]. Previous study has suggested that FFAs induce steatohepatitis by participating in lipotoxic responses and chronic inflammatory responses [25, 26]. It has also been suggested that saturated FFAs such as laurate and PA increases lipid accumulation and induces a pro-inflammatory response [19, 27]. In this study, we chose a molar ratio 5:1 of PA to BSA to mimic the pathophysiological states in which unbound FFA concentrations are high [28]. Our data demonstrates that exposure of HepG2 cells to PA results in lipid accumulation and the overproduction of inflammatory cytokines in a dose-dependent manner. This is in agreement with a previous study that the serum concentrations of FFAs were increased in patients with NAFLD and correlated with the development of more severe liver disease [29]. Importantly, recent evidence showed that saturated fatty (palmitic) acid activated the inflammasome and triggered release of danger signals from hepatocytes in a caspase-1-dependent manner [30], suggesting that the activation of inflammasomes also may contribute to the development of NASH. In the present study, we demonstrated that PA activated NLRC4 inflammasome function, indicated by cleavage of procaspase-1 and increased IL-1 production, along with the increased expression of NLRC4 mRNA. This novel observation differs from a previous report that NLRC4 had no effect on PA-induced IL-1β or IL-18 production in macrophages [17]. The discrepancy may represent cell-specific differences in response to PA between hepatic cells (HepG2) and macrophages or pathways by which pro-IL-1β was cleavaged [31]. Interestingly, the knockdown of NLRC4 has not completely inhibited PA-induced IL-1β production. This observation complements a previous report that NALP3, another inflammasome, is involved in PA-induced IL-1β overproduction in hepatocytes [30].
Our study clearly demonstrates that preincubation with DHA significantly attenuates the lipid accumulation and inflammatory cytokines overproduction in cells exposed to excess PA. It was consistent with a previous study that DHA alleviates lipid deposition and inflammation and the supplementation of n-3 PUFAs ameliorates NAFLD in Japanese men and women [32]. However, the exact mechanisms of DHA in preventing NASH are not yet clear. Recent studies demonstrated that DHA inhibits lipid accumulation by coordinately suppressing lipid synthesis in the liver by impairing the proteolytic release of SREBP-1c and/or by suppressing SREBP-1c gene expression and upregulating fatty acid oxidation by serving as an in vivo activator of peroxisome proliferator-activated receptor alpha (PPAR-α) [33]. DHA was also found to exert anti-inflammatory effects by decreasing the production of inflammatory eicosanoids [34], reactive oxygen species production [35] and the expression of adhesion molecules [36, 37] or giving rise to a family of anti-inflammatory mediators termed resolvins [38]. Our data suggested that DHA preincubation inhibited PA-induced NLRC4 inflammasome activation and decreased the cleavage of procaspase-1 and IL-1 production. Further, knockdown of NLRC4 significantly inhibited PA-induced lipid accumulation and almost completely abolished that in the presence of DHA. To our best knowledge, we show here for the first time that DHA ameliorates NASH through suppressing the NLRC4 inflammasome activation.
Our current study shows that PA triggered caspase-1 activity in a dose-dependent manner whereas DHA inhibited PA-induced caspase-1 activation, leading to the decreased cleavage of pro-IL-1β and IL-1β production. IL-1β has been implicated in the pathogenesis of steatohepatitis [39]. Unlike other secreted proteins, IL-1β is synthesized as a precursor protein pro-IL-1β, whose multiple extracellular activities in response to various proinflammatory stimuli are attributed to receptor binding of IL-1β itself [31, 40]. It is generally accepted that cleavage of pro-IL-1β is mediated by inflammasomes [41] and conversion of the inactive pro-IL-1β to IL-1β requires the proteolytic action of caspase-1 [42]. It was suggested that the specific inhibition of caspase-1 by the caspase inhibitor blocked the maturation of IL-1β [43] and no significant IL-1β produced in PA-treated macrophages derived from caspase-1−/− mice [17]. We showed an inhibitory effect of DHA on caspase-1 activity and IL-1β maturation in HepG2 cells, an effect which, to our best knowledge, has not been reported previously.
Inflammation is a response to lipid accumulation and can accelerate the injury. Treatment of mice with IL-1β decreased fatty acid metabolism, resulting in hepatic triglyceride storage [44]. In the present study, we found that knockdown of NLRC4 inhibited PA-induced inflammation, thereby further attenuating lipid accumulation in cells. This is agreement with previous studies that IL-1β stimulation exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice [45] and enhanced liver injury induced by inflammatory stress was observed in NASH [46]. Thus, exposure of DHA is effective for preventing and reversing lipid accumulation and the inflammatory response which may accelerate the lipid metabolism disorder and/or more severe liver injuries.
In summary, our present findings clearly show that PA triggers lipid accumulation and inflammatory cytokines overgeneration in HepG2 cells, which is ameliorated by DHA through attenuating NLRC4 inflammasome activation, caspase-1 activation and IL-1β cleavage. Taken together, these data suggests that DHA may server as a potential nutrient antagonist to NLRC4 and our findings offer new insights into the potential mechanisms of action of DHA in mediating the beneficial hepatoprotective effects in NASH.
Abbreviations
- DHA:
-
Docosahexaenoic acid
- PUFAs:
-
Polyunsaturated fatty acids
- NASH:
-
Nonalcoholic steatohepatitis
- IL-1β:
-
Interleukin-1β
- TG:
-
Triglyceride
- PA:
-
Palmitate
- NAFLD:
-
Non-alcoholic fatty liver disease
- FFAs:
-
Free fatty acids
- Ac-YVAD-pNA:
-
Aacetyl-Tyr-Val-Ala-Asp p-nitroanilide.
References
Williams R: Global challenges in liver disease. Hepatology. 2006, 44: 521-526. 10.1002/hep.21347.
Loomba R, Sirlin CB, Schwimmer JB, Lavine JE: Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009, 50: 1282-1293. 10.1002/hep.23119.
Fan JG, Farrell GC: Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009, 50: 204-210. 10.1016/j.jhep.2008.10.010.
McCullough AJ: Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006, 40 (Suppl 1): S17-S29.
Day CP, James OF: Steatohepatitis: a tale of two "hits"?. Gastroenterology. 1998, 114: 842-845. 10.1016/S0016-5085(98)70599-2.
Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM: Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010, 207: 1745-1755. 10.1084/jem.20100257.
Davis BK, Wen H, Ting JP: The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011, 29: 707-735. 10.1146/annurev-immunol-031210-101405.
Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ: Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009, 119: 305-314.
Te Sligte K, Bourass I, Sels JP, Driessen A, Stockbrugger RW, Koek GH: Non-alcoholic steatohepatitis: review of a growing medical problem. Eur J Intern Med. 2004, 15: 10-21. 10.1016/j.ejim.2003.12.008.
Musso G, Gambino R, Pacini G, De Michieli F, Cassader M: Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr. 2009, 89: 558-567. 10.3945/ajcn.2008.26720.
Tomita K, Teratani T, Yokoyama H, Suzuki T, Irie R, Ebinuma H, Saito H, Hokari R, Miura S, Hibi T: Plasma free myristic acid proportion is a predictor of nonalcoholic steatohepatitis. Dig Dis Sci. 2011, 56: 3045-3052. 10.1007/s10620-011-1712-0.
Lomonaco R, Ortiz-Lopez C, Orsak B, Finch J, Webb A, Bril F, Louden C, Tio F, Cusi K: Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology. 2011, 54: 837-845.
Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Faga E, Silli B, Pagano G: Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003, 37: 909-916. 10.1053/jhep.2003.50132.
Gentile CL, Pagliassotti MJ: The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008, 19: 567-576. 10.1016/j.jnutbio.2007.10.001.
Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z: EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005, 67: 867-874. 10.1111/j.1523-1755.2005.00151.x.
Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, Agostoni C: Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011, 96: 350-353. 10.1136/adc.2010.192401.
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP: Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011, 12: 408-415. 10.1038/ni.2022.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD: The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011, 17: 179-188. 10.1038/nm.2279.
Igoillo-Esteve M, Marselli L, Cunha DA, Ladriere L, Ortis F, Grieco FA, Dotta F, Weir GC, Marchetti P, Eizirik DL, Cnop M: Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010, 53: 1395-1405. 10.1007/s00125-010-1707-y.
Tiniakos DG, Vos MB, Brunt EM: Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010, 5: 145-171. 10.1146/annurev-pathol-121808-102132.
Takayama F, Nakamoto K, Totani N, Yamanushi T, Kabuto H, Kaneyuki T, Mankura M: Effects of docosahexaenoic acid in an experimental rat model of nonalcoholic steatohepatitis. J Oleo Sci. 2010, 59: 407-414. 10.5650/jos.59.407.
Chen WY, Lin SY, Pan HC, Liao SL, Chuang YH, Yen YJ, Lin SY, Chen CJ: Beneficial effect of docosahexaenoic acid on cholestatic liver injury in rats. J Nutr Biochem. 2011, Epub ahead of print
de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME: Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002, 21: 219-223. 10.1054/clnu.2001.0529.
Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, Sanyal AJ: The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009, 50: 1827-1838. 10.1002/hep.23229.
Brookheart RT, Michel CI, Schaffer JE: As a matter of fat. Cell Metab. 2009, 10: 9-12. 10.1016/j.cmet.2009.03.011.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006, 116: 3015-3025. 10.1172/JCI28898.
Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P: Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009, 24: 830-840. 10.1111/j.1440-1746.2008.05733.x.
Nakamura T, Azuma A, Kuribayashi T, Sugihara H, Okuda S, Nakagawa M: Serum fatty acid levels, dietary style and coronary heart disease in three neighbouring areas in Japan: the Kumihama study. Br J Nutr. 2003, 89: 267-272. 10.1079/BJN2002747.
Nehra V, Angulo P, Buchman AL, Lindor KD: Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001, 46: 2347-2352. 10.1023/A:1012338828418.
Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G: Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011, 54: 133-144. 10.1002/hep.24341.
Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R: Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008, 205: 1967-1973. 10.1084/jem.20071632.
Oya J, Nakagami T, Sasaki S, Jimba S, Murakami K, Kasahara T, Wasada T, Sekiguchi H, Hasegawa M, Endo Y, Iwamoto Y: Intake of n-3 polyunsaturated fatty acids and non-alcoholic fatty liver disease: a cross-sectional study in Japanese men and women. Eur J Clin Nutr. 2010, 64: 1179-1185. 10.1038/ejcn.2010.139.
Sun C, Wei ZW, Li Y: DHA regulates lipogenesis and lipolysis genes in mice adipose and liver. Mol Biol Rep. 2011, 38: 731-737. 10.1007/s11033-010-0160-9.
Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, Yu R, Chandra RK, Mackey BE: Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999, 34: 317-324. 10.1007/s11745-999-0369-5.
Richard D, Wolf C, Barbe U, Kefi K, Bausero P, Visioli F: Docosahexaenoic acid down-regulates endothelial Nox 4 through a sPLA2 signalling pathway. Biochem Biophys Res Commun. 2009, 389: 516-522. 10.1016/j.bbrc.2009.09.013.
Chen W, Esselman WJ, Jump DB, Busik JV: Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2005, 46: 4342-4347. 10.1167/iovs.05-0601.
Yates CM, Tull SP, Madden J, Calder PC, Grimble RF, Nash GB, Rainger GE: Docosahexaenoic acid inhibits the adhesion of flowing neutrophils to cytokine stimulated human umbilical vein endothelial cells. J Nutr. 2011, 141: 1331-1334. 10.3945/jn.111.139287.
Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J: Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009, 23: 1946-1957. 10.1096/fj.08-125674.
Zhao L, Chen Y, Tang R, Chen Y, Li Q, Gong J, Huang A, Varghese Z, Moorhead JF, Ruan XZ: Inflammatory stress exacerbates hepatic cholesterol accumulation via increasing cholesterol uptake and de novo synthesis. J Gastroenterol Hepatol. 2011, 26: 875-883. 10.1111/j.1440-1746.2010.06560.x.
Wilson KP, Black JA, Thomson JA, Kim EE, Griffith JP, Navia MA, Murcko MA, Chambers SP, Aldape RA, Raybuck SA: Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994, 370: 270-275. 10.1038/370270a0.
Martinon F, Tschopp J: Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007, 14: 10-22. 10.1038/sj.cdd.4402038.
Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA: Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992, 256: 97-100. 10.1126/science.1373520.
Colomar A, Marty V, Medina C, Combe C, Parnet P, Amedee T: Maturation and release of interleukin-1beta by lipopolysaccharide-primed mouse Schwann cells require the stimulation of P2X7 receptors. J Biol Chem. 2003, 278: 30732-30740. 10.1074/jbc.M304534200.
Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Muller M: Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010, 51: 511-522. 10.1002/hep.23337.
Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z: Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008, 48: 770-781. 10.1002/hep.22423.
Szabo G, Velayudham A, Romics L, Mandrekar P: Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005, 29: 140S-145S. 10.1097/01.alc.0000189287.83544.33.
Acknowledgements
This work has been funded by grant from the Key Project of National Natural Science Foundation of China (81130052) and National Basic Research Program (973 Program, 2012CB517506).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XQL and WHL contributed to design the study, analysis and interpretation of data, and drafting of the manuscript. YY, MX, YJX and TBZ assisted with interpretation of the result and critical revision of the manuscript. TRS and XLT carried out the ELISA studies. All authors have given their final approval of the submitted version of the manuscript.
Electronic supplementary material
12986_2012_441_MOESM1_ESM.doc
Additional file 1: Figure S1. Representative Oil Red O staining of cells with different treatments is shown. Cells were transiently transfected with 20 pmoL control siRNA or NLRC4 siRNA for 48 h and examined by light microscopy at a magnification of 400×. The images are representative of typical staining. Table S1. Effects of siRNA transfection on the production of cytokines (mean ± SE; pg/mL) in HepG2 cells. Cells were transiently transfected with 20 pmoL control siRNA or NLRC4 siRNA for 48 h. Then the cell-free culture supernatants were centrifuged at 10,000 g for 10 min at 4°C and the supernatants were stored at -20°C before assaying IL-1β, IL-18, TNF-α and MCP-1 by commercial ELISA kits. The data represent a mean of 4 experiments. (DOC 299 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Luo, X., Yang, Y., Shen, T. et al. Docosahexaenoic acid ameliorates palmitate-induced lipid accumulation and inflammation through repressing NLRC4 inflammasome activation in HepG2 cells. Nutr Metab (Lond) 9, 34 (2012). https://doi.org/10.1186/1743-7075-9-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-7075-9-34