Abstract
Background
Torque teno virus (TTV) is a circular, single-stranded DNA virus that chronically infects healthy individuals of all ages worldwide. There is a lot of data on the prevalence and genetic heterogeneity of TTV in healthy populations and in patients with various diseases now available. However, little is known about TTV load among healthy human population. In this study we analyzed TTV load in the group of 512 Russian elite athletes, who are supposed to be, by some standards, the healthiest part of the human population.
Results
The prevalence rate of TTV among the Russian Olympic Reserve members was 94% (for test sensitivity about 1000 genome equivalents per 1 ml of blood). Quantities varied from 103 (which corresponded to detection limit) to 1010 copies per 1 ml of blood, with median at 2.7 × 106 copies.
Conclusion
About 94% of healthy individuals in Russian population have more than 1000 TTV genome copies per 1 ml of blood. This result exceeds the previously published data, and can be explained by either more sensitive PCR test system or by higher TTV distribution in Russian population or both. TTV viral load neither depends on gender, nor age.
Similar content being viewed by others
Background
Torque teno virus (TTV) was first discovered in 1997 in Japanese patients with non-A-G transfusion-acquired hepatitis [1]. TTV is a small, non-enveloped virus with a single-stranded, circular DNA genome of negative polarity, 3.4-3.9 Kb in length, containing two bigger (ORF1 and ORF2) and several smaller open reading frames [2]. TTV is currently classified to Circoviridae family [2]. Despite being a DNA virus, TTV demonstrates an extremely wide sequence divergence. At least 16 genotypes with evolutionary distance >0.30 has been described so far [3].
TTV is an ubiquitous virus revealed in more than 50% of the general human population throughout the world [4–6] and nearly 90% of pongid populations [7]. Co-infection of single individuals with TTV isolates belonging to one or several phylogenetic groups occurs frequently [8]. TTV was first characterized as a blood-born virus and thus referred to transfusion-transmitted (TT) group of viruses [1]. Recent studies suggested the existence of other ways of transmission including parenteral [3], sexual [9, 10], mother-to-child [11, 12] and others [13–15].
TTV has been suggested to be a causative agent of several diseases such as acute respiratory diseases [16], liver diseases [5, 6], AIDS [17] and cancer [18], however, without any convincing support. One of current hypothesis suggests a key role of TTV in development of autoimmune reactions [19].
Despite 10 years of investigation, the TTV distribution in humans is still a subject of discussion. Possibly, this is because of the variability of TTV genotypes and the inability to design a single set of PCR primers, corresponding to the vast majority (if not all) viral genotypes [20]. Also little is known about distribution of TTV in healthy adults [21, 22]. In this study we analyzed TTV viral load in blood of 512 Russian elite athletes who represents healthy Russian population.
Results
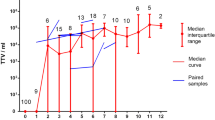
Using qPCR we demonstrated that 485 out of 512 (94%) healthy individuals have TTV viral load of more than 1000 copies per 1 ml of blood, which corresponds to 94.1% of males and 93.9% of females studied. Considerable part of the athletes (39.9%) had viral load about 106 copies per 1 ml of blood (median 2.7 × 106) with maximum about 1010 viral genomes per 1 ml (see Fig. 1).
We failed to detect any correlation between the viral load and the age of tested individuals (R = 0.020; p > 0.05). Also no difference was detected in viral load for men and women (independent t-test: t-value = -1.943; p = 0.052).
Discussion
Discovered frequency of TT virus presence in top rank Russian athletes exceeds the previously published data for healthy human populations (14-88%) [1–6, 15, 16, 21]. The difference may be explained by wider distribution of TTV in Russians and/or more sensitive PCR test system, which detects more TTV variants. The latter idea is supported by the fact that the frequency of TTV in populations detected in the recent studies is higher than in the early studies, which probably can be explained by the application of more accurate methods for TTV detection. In fact, the TTV genome has estimated sequence divergence of more than 30% [15], and the minority of methods used so far were able to detect all the TTV variants. Expansion of the TTV sequencing data enabled us to design primers corresponding to the most constant sites in the TTV genome.
Our PCR test system has calculated sensitivity about 1000 viral genomes per ml of blood. Thus, all the samples with the less viral load were considered as negative. Our data may suggest that the real frequency of TTV presence in human population tends to be close to 100%.
Conclusion
TTV viral load of more than 1000 copies per 1 ml of blood was detected for 94% of Russian elite athletes. Maximum viral load was about 1010, median was 2.7 × 106. There were no significant differences between men and women. Also viral load did not seem to be dependent on age (in the range of this study). In general, the presence of TTV in healthy human population was higher than it has been previously described in literature. This fact can be explained by the higher presence of TTV among Russian population and/or by more sensitive PCR test system used. The absence of correlation between the age and the viral load supports the previously published data.
Methods
Samples and DNA purification
We analyzed the venous blood of 205 men and 232 women of Russian elite athletes aged between 12 and 36. All samples were stored less than three hours at +4°C before analysis. DNA was extracted from blood samples by standard phenol-chloroform extraction [23]. To prevent PCR contamination by blood samples or DNA samples, DNA isolation was performed in a separate DNA-extraction room (Zone 1). To prevent samples cross-contamination, all the procedures were carried out with sterile disposable tubes and aerosol-resistant tips in UV-equipped PCR-box.
Primer and probe design and sequences
The primers and the probe for PCR were designed using the Oligo Primer Analysis software v6.31 (Molecular Biology Insights Inc., USA) and mfold v3.1 [24] based on alignment of different TTV genome sequences (GenBank, [25]). The set of primers used in this study amplify all known TTV variants (for primer and probe sequences see Table 1).
qPCR
qPCR was used for detection and quantification of TTV load. For each PCR sample contained 1-μl-blood equivalent, we may estimate test sensitivity as 1000 viral copies per 1 ml of blood (with PCR sensitivity of 1 target per PCR tube).
PCR was carried out in reaction volumes of 35 μL with 80 mM Tris-HCl pH 8.6, 20 mM (NH4)2SO4, 3 mM MgCl2, 200 μM of each dNTP, 300 nM of each primer, 75 nM of probe and 2.0 U of Taq-polymerase. Real-Time PCR was performed using DT-96 Real-Time PCR Cycler (DNA-Technology, Russia) with the first denaturation step of 60 s at 94°C, followed by 50 cycles at 94°C for 10 s, 64°C for 20 s, 72°C for 10 s, with fluorescence reading at 64°C. The delta-TF method was used for curve normalization [26] and ΔΔCp for quantification. Human MTHFR gene was used for DNA amount standardization.
To prevent PCR contamination by previous reactions nor biological samples, all the reactions were combined using aerosol-resistant tips in UV-equipped PCR-box in a separate PCR-preparation room (Zone 2). Also, no electrophoresis (or other procedures with PCR-tube opening) was done with TTV PCR primers in this building. All the negative controls and surface washings were negative.
Data analysis
PCR data was analyzed using DT-96 Real-Time PCR Cycler Software v.7.2 (DNA-Technology, Russia). Statistica 8.0 (StatSoft, USA) was used for statistical analysis.
References
Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M: A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun 1997, 241: 92-97. 10.1006/bbrc.1997.7765
Bendinelli M, Pistello M, Maggi F, Fornai C: Molecular Properties, Biology, and Clinical Implications of TT Virus, a Recently Identified Widespread Infectious Agent of Humans. Clin Microbiol Rev 2001, 14: 98-113. 10.1128/CMR.14.1.98-113.2001
Nishizawa T, Okamoto H, Tsuda F, Aikawa T, Sugai Y, Konishi K, Akahane Y, Ukita M, Tanaka T, Miyakawa Y, Mayumi M: Quasispecies of TT Virus (TTV) with Sequence Divergence in Hypervariable Regions of the Capsid Protein in Chronic TTV Infection. J Virol 1999, 73: 9604-9608.
Gallian P, Berland Y, Olmer M, Raccah D, de Micco P, Biagini P, Simon S, Bouchouareb D, Mourey C, Roubicek C, Touinssi M, Cantaloube J-F, Dussol B, de Lamballerie X: TT Virus Infection in French Hemodialysis Patients: Study of Prevalence and Risk Factors. J Clin Microbiol 1999, 37: 2538-2542.
Hsieh S-Y, Wu Y-H, Ho Y-P, Tsao K-C, Yeh C-T, Liaw Y-F: High Prevalence of TT Virus Infection in Healthy Children and Adults and in Patients with Liver Disease in Taiwan. J Clin Microbiol 1999, 37: 1829-1831.
Hafez MM, Shaarawy SM, Hassan AA, Salim RF, El Salam FMA, Ali AE: Prevalence of transfusion transmitted virus (TTV) genotypes among HCC patients in Qalupbia governorate. Virol J 2007, 4: 135. 10.1186/1743-422X-4-135
Abe K, Inami T, Ishikawa K, Nakamura S, Goto S: TT Virus Infection in Nonhuman Primates and Characterization of the Viral Genome: Identification of Simian TT Virus Isolates. J Virol 2000, 74: 1549-1553. 10.1128/JVI.74.3.1549-1553.2000
Maggi F, Andreoli E, Lanini L, Fornai C, Vatterloni M, Pistello M, Presciuttini S, Bendinelli M: Relationships between Total Plasma Load of Torquetenovirus (TTV) and TTV Genogroups Carried. J Clin Microbiol 2005, 43: 4807-4810. 10.1128/JCM.43.9.4807-4810.2005
Krekulova L, Rehak V, Killoran P, Madrigal N, Riley LW: Genotypic distribution of TT virus (TTV) in a Czech population: evidence for sexual transmission of the virus. J Clin Virol 2001, 23: 31-41. 10.1016/S1386-6532(01)00185-8
MacDonald DM, Scott GR, Clutterbruck D, Simmonds P: Infrequent detection of TT virus infection in intravenous drug users, prostitutes and homosexual men. J Infect Dis 1999, 179: 686-689. 10.1086/314642
Gerner P, Oettinger R, Gerner W, Falbrede J, Wirth S: Mother-to-infant transmission of TT virus: prevalence, extent and mechanism of vertical transmission. Pediatr Infect Dis J 2000, 19: 1074-1077. 10.1097/00006454-200011000-00009
Iso K, Suzuki Y, Takayama M: Mother-to-infant transmission of TT virus in Japan. Int J Gynaecol Obstet 2001, 75: 11-19. 10.1016/S0020-7292(01)00450-7
Griffin JS, Plummer JD, Long SC: Torque teno virus: an improved indicator for viral pathogens in drinking waters. Virol J 2008, 5: 112.
Haramoto E, Katayama H, Oguma K, Ohgaki S: Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa river in Japan. Appl Environ Microbiol 2005, 71: 2403-2411. 10.1128/AEM.71.5.2403-2411.2005
Irshad M, Joshi YK, Sharma Y, Dhar I: Transfusion transmitted virus: A review on its molecular characteristics and role in medicine. World J Gastroenterol 2006, 12: 5122-5134.
Maggi F, Pifferi M, Tempestini E, Fornai C, Lanini L, Andreoli E, Vatterloni M, Presciuttini S, Pietrobelli A, Boner A, Pistello M, Bendinelli M: TT virus load and lymphocyte subpopulations in children with acute respiratory diseases. J Virol 2003, 77: 9081-9083. 10.1128/JVI.77.16.9081-9083.2003
Shibayama T, Masuda G, Ajisawa A, Takahashi M, Nishizawa T, Tsuda F, Okamoto H: Inverse relationshio between the titre of TT virus DNA and the CD4 cell count in patients infected with HIV. AIDS 2001, 15: 563-570. 10.1097/00002030-200103300-00004
McLaughlin-Dubin ME, Munger K: Viruses associated with human cancer. Biochim Biophys Acta 2008, 1782: 127-150.
Blasek A, Sillo P, Ishii N, Gergely P, Poor G, Preisz K, Hashimoto T, Medvecz M, Karpati S: Searching for foreign antigens as possible triggering factors of autoimmunity: Torque Teno virus DNA prevalence is elevated in sera of patients with bullous pemphigoid. Experimental Dermatology 2008, 17: 446-454. 10.1111/j.1600-0625.2007.00663.x
Devalle S, Niel C: A multiplex PCR assay able to simultaneously detect Torque teno virus isolates from phylogenetic groups 1 to 5. Brasilian J Med Biol Research 2005, 38: 853-860.
Kato T, Misashi M, Mukaide M, Orito E, Ohno T, Nakano T, Tanaka Y, Kato H, Sugauchi F, Ueda R, Hirashima N, Shimamatsu K, Kage M, Kojiro M: Development of a TT virus DNA quantification system using Real-Time Detection PCR. J Clin Microbiol 2000, 38: 94-98.
Saláková M, Nemeček V, König J, Tachezy R: Age-specific prevalence, transmission and phylogeny of TT virus in the Czech Republic. BMC Infec Dis 2004, 4: 56. 10.1186/1471-2334-4-56
Sambrook J, Fitsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Press; 1989.
Zuker M: Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003, 31: 3406-3415. 10.1093/nar/gkg595
NCBI GenBank[http://www.ncbi.nlm.nih.gov/Genbank/]
Trofimov DY, Rebrikov DV, Samatov GA, Semenov PA, Baluev AB, Goncharova EV, Alexeev LP, Khaitov RM: The Delta-TF Method for Real-Time PCR Data Standardization. Dokl Biol Sci 2008, 419: 118-121. 10.1134/S0012496608020142
Acknowledgements
This work was partly supported by grant FP6 (project no. 037212) and the grant of Federal Health and Innovation Agency (project no. 02.522.12.2001 and 02.512.11.2339).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EVV performed all the experimental procedures and interpreted the data. DYT designed the primers. AGT arranged the sample collections and drafted the manuscript. VVI interpreted the data and drafted the manuscript. DOK interpreted the data. DVR designed the study and wrote the manuscript. All authors read and approved the final version of manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vasilyev, E.V., Trofimov, D.Y., Tonevitsky, A.G. et al. Torque Teno Virus (TTV) distribution in healthy Russian population. Virol J 6, 134 (2009). https://doi.org/10.1186/1743-422X-6-134
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-422X-6-134