Abstract
Background
The human β-defensins (hBDs) are a highly conserved family of cationic antimicrobial and immunomodulatory peptides expressed primarily by epithelial cells in response to invasion by bacteria, fungi and some viruses. To date, the most studied members of this family of peptides are hBD-1, -2, and -3. Expression of hBD-1 and -2 has been demonstrated previously in cultured microglia and astrocytes of both mouse and human brain. Unlike inducible hBD-2 and -3, hBD-1 is constitutively expressed and is not generally upregulated by proinflammatory factors. In this study, we investigated whether hBDs, as active components of the innate immune response, are affected by pathological events in the Alzheimer’s disease (AD) brain. We assessed the expression of hBD-1, -2, and -3 in tissue obtained at autopsy from AD and age-matched control brains.
Methods
Fixed and frozen choroid plexus and the CA1 region of the hippocampus were obtained at autopsy from individuals diagnosed with AD, or from age-matched control brains without diagnosed neurodegenerative disease. Histopathologically diagnosed AD brain tissue was obtained for our study. Immunocytochemical analysis was performed using affinity purified polyclonal antibodies directed against hBD-1, -2, or -3. TaqMan gene expression assays were used to quantify the mRNA of hBD-1, -2, and -3 in the choroid plexus and hippocampus. Immunocytochemical detection of iron deposits was achieved using a modified Perl’s stain for redox-active iron. In vitro experiments were performed on human primary oral epithelial cells to model the human choroid plexus epithelial response to ferric chloride. Cells were then exposed to ferric chloride added to selected wells at 0, 1, or 10 mM concentrations for 24 h at 37°C. Total mRNA was isolated to quantify hBD-1 mRNA expression by RTqPCR.
Results
hBD-1 peptide is apparent in astrocytes of the AD hippocampus and hippocampal neurons, notably within granulovacuolar degeneration structures (GVD). A higher level of hBD-1 was also seen in the choroid plexus of AD brain in comparison to age-matched control tissue. Increased expression of hBD-1 mRNA was observed only in the choroid plexus of the AD brain when compared to expression level in age-matched control brain. Redox-active iron was also elevated in the AD choroid plexus and in vitro addition of Fe+3Cl3 to cultured epithelial cells induced hBD-1 mRNA expression.
Conclusions
Our findings suggest interplay between hBD-1 and neuroimmunological responses in AD, marked by microglial and astrocytic activation, and increased expression of the peptide within the choroid plexus and accumulation within GVD. As a constitutively expressed component of the innate immune system, we propose that hBD-1 may be of considerable importance early in the disease process. We also demonstrate that increased iron deposition in AD may contribute to the elevated expression of hBD-1 within the choroid plexus. These findings represent a potentially important etiological aspect of Alzheimer’s disease neuropathology not previously reported.
Similar content being viewed by others
Background
Mammalian β-defensins are an evolutionarily conserved group of cationic, non-glycosylated antimicrobial and immunomodulatory peptides that are expressed in all mammals, including humans [1, 2]. Constitutively expressed human β-defensin-1 and inducible β-defensin-2 (hBD-1 and -2, respectively) are commonly expressed by epithelial cells, and are important contributors to innate host defense [3, 4]. These peptides have the potential to act as activators and modulators of adaptive immunity within astrocytes and microglia, brain cells critical to the cerebral neuroinflammatory response, as both cell types have been shown to express hBD-1 and -2 in vitro[5–9]. These findings were confirmed by Nakayama et al. who also reported constitutive expression of hBD-1 in the human choroid plexus (CP) [10]. The CP, composed principally of epithelium, endothelium and stroma, is strategically located between the peripheral circulation and cerebral ventricles, and actively participates in regulation of reciprocal immune functions between the brain and peripheral circulation. Direct involvement of the CP in Alzheimer’s disease (AD) neuropathology is well documented with overt epithelial cell atrophy and thickening of epithelial basement membrane with concomitant impairment of synthetic, secretory, and transporter functions, including reduced clearance of amyloid-β [11–14]. In addition to secretion of cerebrospinal fluid (CSF), which is reduced in AD [15], the CP acts as a primary immunosurveillance and defense mechanism against invading pathogens and systemic diseases [16]. The CP is a principal regulator of the intracerebral immune response and inflammation, as indicated by the presence of mast cells, macrophages, granulocytes, and immunocompetent cells within the tissue [17, 18]. Other immune alterations specific to AD, including pseudolinear deposits of IgG and deposits of C1q associated with IgM, suggestive of an autoimmune origin have been reported [19]. The cause or causes underlying pathological changes and dysfunction within the CP remain equivocal, although it is apparent that oxidative stress-induced modification of proteins, unrelated to presence of fibrillar amyloid-β, contributes to CP pathology in AD. These modifications include glycation end-products carboxymethyl-lysine and N-carboxyethyl-lysine within the CP of AD brain [20]. The studies cited above suggest CP dysfunction is a likely contributor to neuropathological and inflammatory sequelae in the AD brain.
In this study, we evaluated the expression of hBDs within the AD brain and accumulation of redox-active iron within the CP epithelium. We show that the expression of hBD-1 mRNA is significantly elevated in the Alzheimer’s choroid plexus and the protein is apparent in neurons of the hippocampus. Further, granulovacuolar degeneration (GVD) is found to be a specific site of hBD-1 peptide localization. GVD are small cytoplasmic granules that are increased in neurons of the AD brain; proteins previously described in GVD represent the autophagic ubiquitin-proteasome system, unfolded protein response, endosomal and lysosomal proteins, and tau and amyloid processing events, as well as stress-activated and mitogen-activated protein induction [21–27]. The GVD may play a role similar to that of stress granules, structures that form following various stressors including heat shock, oxidative stress, and, of relevance to this study, infection [28]. The present study provides evidence for GVD formation reflecting a neuroprotective response and suggests that redox-active iron may contribute to alteration of the inflammatory process by upregulating expression of hBD-1, a principal component of the innate host defense system.
In AD, morphological changes and increased immunological responses in the CP contribute to decreased CSF synthesis and secretion, and increased oxidative stress [29]. Despite highly controlled homeostatic iron levels within the healthy brain [30], redox-active iron accumulates in the AD brain [31, 32] with a concentration appearing to correlate with the severity of cognitive deficit [33]. Our findings suggest that elevated iron levels in the AD CP, and perhaps also within hippocampal regions, contribute to the increase in hBD-1 expression and an altered innate immune response.
Methods
Materials
Brain tissue histopathologically diagnosed with AD using multiple criteria ('definite’ AD by CERAD criteria [34], Braak Stage V-VI [35], and 'high likelihood AD’ by NIA-Reagan criteria [36]), as well as age-matched control brain tissue, were used for our study. Specifically, fixed and frozen CP from AD patients (n = 8) and age-matched controls (n = 7) was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, USA. All tissue samples were obtained within 2 to 12 hours postmortem with similar ages between groups ranging from 66 to 89 years of age. Additionally, fixed and frozen samples of hippocampus were obtained from Case Western Reserve University and University Hospitals of Cleveland, according to approved Institutional Review Board protocols. Samples from control cases (n = 8, ages 74–91) and neuropathologically confirmed AD cases (n = 11, ages 72–94) were used for PCR experiments. For immunocytochemistry, AD cases (n = 9, ages 59–91, mean = 80.1 years) and control (n = 5, ages 53–101, mean = 72.2 years) were analyzed. Skin samples from 3 cases were also collected post-mortem for use as a positive control tissue. Fixed tissues were embedded in paraffin and used for immunocytochemical analysis. Antigen affinity purified polyclonal antibodies directed against hBD-1 (Cat # P25350), -2 (Cat # P161G50), and -3 (Cat # P24150) were purchased from PeproTech (Rocky Hill, NJ, USA). These primary antibodies were produced from rabbit (hBD-1 and -3) or goat sera (hBD-2) pre-immunized with >98% pure recombinant defensin peptide. Antibodies were subsequently purified by affinity chromatography using the respective immobilized defensin peptide matrix (PeproTech). TaqMan gene expression assays were purchased from Applied Biosystems (Life Technologies, Carlsbad, CA, USA).
RTqPCR
Total RNA was extracted from frozen brain tissue by homogenization in TRI reagent (Ambion, Life Technologies, Carlsbad, CA, USA) and further purified using an RNeasy Mini Kit from Qiagen (Valencia, CA, USA), as instructed by the manufacturer. The purified RNA was treated with Turbo DNase (Ambion) to remove trace DNA. RNA was converted to cDNA using the RETROScript RT-qPCR kit (Ambion), according to the manufacturer’s instructions. Briefly, the purified total RNA (100 ng) was reverse transcribed with cloned Moloney murine leukemia virus reverse transcriptase (5 U) by incubating at 44°C for 60 min, followed by heating at 92°C for 10 min. RT-qPCR using TaqMan gene specific assays with context sequence (GGCCTCAGGTGGTAACTTTCTCACA) was performed in triplicate on cDNA samples in 96-well optical plates on an ABI StepOne Plus Thermocycler (Applied Biosystems). For each 20 μL TaqMan reaction, 2 μL cDNA was mixed with 10 μL 2× TaqMan Fast Universal PCR Master Mix (Applied Biosystems) and 1 μL of 20× TaqMan Assay Mix. Standard fast PCR parameters were used.
Data was normalized to glyceraldehyde dehydrogenase (GAPDH) and shown as the relative RQ value. This stable reference gene was selected by testing AD and age-matched control brain samples on a TaqMan Human Endogenous Control Plate (Applied Biosystems® MicroAmp® Life Technologies, Carlsbad, CA, USA). The data indicated five stable genes that could be used for normalization. Using the geNORM (PrimerDesign) algorithm, the best choice for normalization of RT-qPCR data from AD vs. age-matched control brain samples was a combination of two genes, human acidic ribosomal protein (huPO) and human β2-microglobulin (huβ2m). Stability of huGAPDH was second only to that of huβ2m/huPO out of a total of 11 suggested reference genes.
Immunocytochemical analysis
Paraffin was removed from tissue sections with two changes of xylene; tissue sections were then hydrated through descending concentrations of ethanol to Tris buffered saline (TBS, 50 mM Tris, 150 mM NaCl, pH = 7.6). Endogenous peroxidase activity was ablated by 30 min incubation in 3% hydrogen peroxide in methanol. Antigen retrieval using citrate buffer (pH 6) in a pressure cooker (Biocare Medical, Concord, CA, USA) was performed. Non-specific antibody binding sites were blocked with a 30 minute incubation in 10% normal goat serum (NGS) in TBS, and sections incubated overnight in primary antibody diluted in 1% NGS in TBS at 4°C. Secondary antibodies were applied followed by PAP complex and staining developed with 3’-3’-diaminobenzidine (Dako North America, Carpenteria, CA, USA). Sections were dehydrated and mounted with Permount mounting medium.
To verify the presence of hBD-1 peptide in glial cells, double label immunocytochemistry was performed on hippocampal sections from either AD or non-AD brain using rabbit polyclonal antibody to hBD-1 (PeproTech) detected using the PAP method with brown reaction product DAB and with mouse monoclonal antibody to glial fibrillary acidic protein (GFAP, clone GA5, Millipore), detected with the alkaline phosphatase anti-alkaline phosphatase method using Fast Blue as chromogen. Stained sections were mounted with Crystalmount, coverslipped and imaged using Zeiss Axiocam mounted on a Zeiss Axiophot (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). Epidermal sections of human skin known to express hBD-1 were also subjected to hBD-1 antibody as a positive control.
Immunocytochemical detection of iron deposits within the CP and hippocampus was achieved by a modified Perl’s stain for redox-active iron using 7% potassium ferrocyanide in 3% HCl [30, 31]. The CP epithelial cell layer was identified using an affinity purified rabbit polyclonal antibody (ABBiotec, San Diago, CA, USA) to transthyretin, a marker for CP epithelial cells.
Cell culture of human primary epithelial cells and exposure to redox-active iron
Primary oral epithelial cells were used to model the response of CP epithelial cells to redox-active iron (Fe+3). Cells were obtained from human donors according to the policies described by the Institutional Review Board at Case Western Reserve University. After informed consent by selected patients oral epithelial cells were extracted from healthy oral tissue overlying impacted third molars, as described by Oda and Watson [37]. In brief, cells were cultured in EpiLife growth medium (Cascade Biologists, Portland, OR, USA) and maintained under 5% CO2 at 37°C. Primary cells were grown as a monolayer under serum-free conditions. Upon reaching confluence, cells from at least three donors were trypsinized, detached, and pooled before reseeding in EpiLife growth medium at 4 × 104 cells/well in 6-well culture plates. Before challenging with dilutions of ferric chloride, cells were incubated under 5% CO2 at 37°C, and cultured until ~80–95% confluence (~3 × 105 cells/well). Ferric chloride at 1 and 10 mM was added to selected wells and cells were incubated for an additional 24 h. Experiments were terminated by aspiration of the incubation medium and addition of TRIzol reagent (Life Technologies, Grand Island, NY, USA) to each well for subsequent quantitation of hBD-1 mRNA by RT-qPCR.
Statistical analysis
Data are presented as the Mean ± S.E. Statistical analysis was performed using exact probabilities for the non-parametric Mann Whitney 'U’ test where N1 = 7, N2 = 8 (CP), or N1 = 8, N2 = 11 (hippocampus).
Results
Beta defensin-1 peptide is increased in hippocampus and CP of the AD brain
Examination of hippocampal sections revealed the apparent accumulation of hBD-1 peptide in the cytoplasm of pyramidal neurons in only 1 out of 5 age-matched control cases (Figure 1A,B) versus 8 out of 9 AD cases (Figure 1C,D). The hBD-1 peptide was also tentatively identified in astrocytes of the AD brain (Figure 1C, inset), an expected finding since hBD-1 expression has been reported in cultured human astrocytes. Most striking was the strong and specific staining of GVD within pyramidal neurons of AD hippocampus (Figure 1D arrows, and inset).
Immunocytochemical demonstration of hBD-1 in the hippocampus. Age-matched controls (A,B) vs. AD (C,D) shows an elevated protein expression in the cytoplasm of pyramidal neurons in AD compared to non-AD control brain. The expected localization of hBD-1 in glial cells was also demonstrated in AD cases (C, inset). In AD cases, GVD is also specifically stained (D, arrows). In D, inset shows a higher magnification image of the GVD-containing neuron marked with an asterisk (*). Scale bar A–D = 50 μm; Insets C,D = 20 μm.
The CP epithelial cell layer from age-matched control brain was clearly delineated by the presence of transthyretin (Figure 2A). Within the CP, the hBD-1 peptide was detected only in epithelial cells, consistent with presence of intracellular hBD-1. We noted weak expression of the peptide in non-AD age-matched CP (Figure 2B), but a strong, distinct signal in the CP epithelium from AD brain (Figure 2C). We could not detect hBD-2 or -3 peptide in either CP or hippocampal tissue sections derived from either AD or control brain (data not shown).
Transthyretin is a marker protein for epithelial cells. Epithelial cells of the choroid plexus (CP) are demonstrated in an age-matched control brain using antibody to transthyretin (A). While hBD-1 is only weakly expressed in control CP epithelium (B), the peptide is strongly expressed by these cells in the AD CP (C).
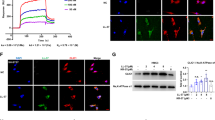
In Figure 3A we show the presence of hBD-1 peptide in glial cells expressing GFAP, a glial cell marker. Human skin used as a positive control for the presence of hBD-1 shows high expression of the peptide in the upper epidermal layer (Figure 3B).
hBD-1 is apparent in astroglial cells of the hippocampus. The expected localization of hBD-1 brown reaction product in glial cells was demonstrated in AD cases by colocalization with an antibody to GFAP (blue) (A, inset). Arrows mark the numerous glial cells containing both GFAP and hBD-1, while arrowheads denote neurons containing only the brown hBD-1 reaction product. Specificity of the antibody and method used for detection of hBD-1 was tested on tissue sections of human epidermal skin cells, known to express hBD-1, as a positive control (B). Scale bar (inset) = 50 μm.
Expression of hBD-1 mRNA is elevated in CP epithelium, but not in hippocampal cells of the AD brain
Using RT-qPCR we assessed the expression of hBD-1, -2, and -3 mRNAs in both the hippocampus and CP from AD brains and age-matched controls. Expression of hBD-1 mRNA is significantly elevated in the AD CP in comparison to age-matched controls (*P = 0.02, Figure 4A). However, the levels of hBD-1 mRNA were similar in both AD and control hippocampus (Figure 4B). Expression of mRNA for hBD-2 and -3 was not detected in either CP or hippocampus from AD or control brains (data not shown).
hBD-1 expression is increased in the AD CP epithelium. hBD-1 mRNA expression in the CP epithelium from AD brain (n = 8) shows a statistically significant (*P = 0.02) elevation in peptide expression, compared to age-matched control tissue (n = 7) (A). Results are expressed as the mean ± S.E. No statistically significant difference in hBD-1 mRNA expression was found between the hippocampus from AD brain (n = 11) compared to age-matched controls (n = 8) (B).
Redox-active iron deposition occurs in CP epithelial cells of the AD brain
Using a modification of the Perl’s stain to specifically detect Fe+3, a redox-active form of non-heme iron, we visualized a strong signal within the CP epithelium of the AD cases, but not in epithelium of the control CP (Figure 5).
Immunocytochemical demonstration of redox-active iron deposits within the CP epithelium of the AD brain. Redox-active iron deposits are not detectable in the CP of age-matched control brain. Redox-active iron was detected using a modified Perl’s stain [30].
Redox-active iron upregulates in vitro expression of hBD-1 in human epithelial cells
The 24 h exposure of cultured human primary epithelial cells to either 1 or 10 mM Fe+3Cl3 induced a detectable dose-dependent upregulation of hBD-1 mRNA (n = 3, U = 0, P = 0.014) relative to untreated control cells (Figure 6).
Redox-active iron increases hBD-1 expression in epithelial cells in vitro . hBD-1 mRNA expression in human epithelium grown in culture subjected to sham-exposure or exposed to 1 or 10 mM Fe+3Cl3 during a 24 h incubation at 37°C. Results are expressed as the mean ± S.D. of 3 independent experiments (*P = 0.014).
Discussion
The hBD gene cluster is located in chromosome 8p22-23, a region containing multiple genes related to innate immunity and the nervous system [38]. With the exception of constitutively expressed hBD-1, inducible hBD-2 and -3 are generally upregulated by an inflammatory environment [39, 40]. Thus, surprisingly, we detected the upregulated expression of hBD-1, which is generally unresponsive to inflammation. Conversely, we failed to detect expression of hBD-2 or hBD-3, despite the reported presence of neuroinflammation in affected regions of the AD brain, including the hippocampus and CP [41, 42]. The hBD-1 gene (DEFB1) is a single copy gene with several SNPs that have been associated with the pathogenesis of some chronic inflammatory diseases, including asthma and chronic obstructive pulmonary disease [43, 44]. Genomic variations in DEFB1 also contribute to the clinical course of severe sepsis and inflammation with presence of specific haplotypes associated with either increased susceptibility to, or protection from, severe infection and fatal outcome [45]. This latter study underscores a possible role for hBD-1 in modulating inflammation within the CNS and suggests that the observed elevation in hBD-1 expression within the AD brain is a protective response to an inflammatory environment. One obvious question is whether susceptibility to inflammation within the CNS is influenced by specific genomic variants of DEFB1, and whether individuals with AD express a gene variant that is less effective in modulating inflammation.
hBD-1 is the only known hBD that is constitutively expressed and is rarely upregulated [46–48]. The peptide is not only immunomodulatory through chemotaxis of memory T lymphocytes and stimulation to maturity of immature myeloid dendritic cells [49, 50], but responsive and cytotoxic to gram-negative bacteria and adenovirus. Expression of hBD-1 is not significantly affected by exposure to LPS, Salmonella Dublin, or Escherichia coli[51]. This study is the first to report the effect of a neurodegenerative disease on expression of a well characterized antimicrobial and immunomodulatory peptide. Here, we show significant elevation of the hBD-1 peptide and mRNA level in CP epithelium, and increased peptide in hippocampal neurons of the AD brain relative to age-matched control brain tissues.
The underlying cause(s) of increased hBD-1 expression in the AD brain is not apparent. It is also unclear whether the hBD-1 peptide could be functioning as an antimicrobial, immunomodulator or both, and whether functionality of the peptide affects clinical symptoms of AD. We thus propose several mechanisms that might explain our results based upon our own recent findings, and the published studies of others.
The presence of hBD-1 within the CP, an interface between brain parenchyma and the systemic circulation, suggests that increased expression in the AD brain CP could be a response to microbial-derived stimuli impinging from the systemic side of the blood-CSF barrier. Influenza, Sendai and Herpes simplex viruses are known to modulate hBD-1 expression [52], and Herpes simplex DNA has been shown to localize quite specifically to the amyloid plaques of the AD brain [53]. To date though, identification of pathogenic determinants in AD, including bacteria, viruses and fungi has been an inconsistent finding, and no single pathogenic agent has been categorically identified as a contributor to AD neuropathology or cognitive decline [54]. Nonetheless, the functional role that hBD-1 plays within the CP could be influenced by pathogenic stimuli, such as non-self-pattern recognition associated with AD.
Another intriguing mechanism that could underlie changes in hBD-1 expression within the CNS is a non-inflammatory upregulation of the peptide by altered biological clock control of peptide expression through c-myc. Sherman et al. [55] have shown that albumin can upregulate expression of hBD-1 in HCT-116 cells concomitant with elevation of c-myc, a response that was inhibited by addition of a c-myc inhibitor. Additional studies showed that the 5 kbp promoter region of the defensin peptide contains putative binding sites (E-box-like sequences) for c-myc that are binding sites for the dimerized transcription factors CLOCK and BMAL1 [55, 56]; noteworthy is that components of the innate immune system are regulated by the biological clock [57, 58]. Thus, hBD-1 expression level may conform to influence from the biological clock and binding of c-myc to the defensin promoter region [51]. This mechanism may be quite relevant to hBD expression in AD in view of the altered circadian rhythm of the master clock cells in the superchiasmatic nucleus of the AD brain [59]. Although alterations in biological clock function occur in both aging and AD, the circadian disturbance appears to be more pronounced in the AD brain [60].
It is possible that the observed increase in hBD-1 within the CP and hippocampus occurs by different mechanisms. We believe that the increased presence of hBD-1 within neurons may represent an acute neuroprotective response of the brain to extra- or intra-cerebral factors. Presence of hBD-1 within GVD, structures which can function as autophagosomes [61] but also show both morphological and protein-based similarities to stress granules [28], may signal a neuronal response to induction of cellular stress by a chronic, proinflammatory environment. Since the majority of neurons containing GVD are not yet undergoing neurodegeneration, i.e., they lack hyperphosphorylated tau, neurofibrillary pathology, and are not apoptotic, the apparent elevation of hBD-1 in these structures could help identify the cellular response to stress. Additional study of mild cognitive impairment, including cases with mild AD, is necessary to clarify the role of glial activation known to occur early on in the disease process [62].
An increase in hBD-1 peptide in the hippocampus of the AD brain without a respective increase in mRNA would not be without precedent. In fact, attempts to correlate protein levels with similar changes in mRNA expression have met with mixed results [63]. The increase in hBD-1 peptide in the AD hippocampus may reflect upregulation of a post-translational pathway such as that described by Acquaviva et al. [64] to explain their observed increase in the frataxin protein without an associated increase in expressed mRNA. The lack of an increase in mRNA expression, corresponding to an increase in protein level is not an infrequent occurrence as is demonstrated by a study on new molecular targets for the anticancer drug, rapamycin [65]. In this study, out of 56 yeast proteins increased in abundance following exposure to rapamycin, 26 did so without a detectable change in mRNA expression. It is also possible that accumulation of the hBD-1 peptide in the GVD may occur over years and derive from glial cell death and release of hBD-1 peptide that subsequently is sequestered via the hypothesized protective function of neuronal GVD. Borenstein et al. [66] proposed a similar hypothesis to explain the presence of defensin peptides (NP-1, -2, and -5) in infected rabbit testis. These defensins are expressed by macrophages and neutrophils, but both these cell-types were absent from the tissue samples in which the defensins were detected. Similarly, Sahasrabudhe et al. [67] noted increased expression of hBD-1 in inflamed human salivary gland ducts, but without a corresponding increase in hBD-1 mRNA. In their study, detectable levels of the peptide were demonstrated by immunoblot. Unfortunately, we could not verify the presence of this small (3.9 kDa) peptide by Western blot of hippocampal tissue. We attribute this to a peptide concentration in the brain that is below the level of detectability for our antibody. It cannot be completely rules out that our antibody is cross-reacting with another protein; however, we believe this to be unlikely, given that there is no cross-reactivity between our antibody to hBD-1 and other beta-defensins having close homology to hBD-1 (PeproTech, personal communication). Nevertheless, without quantitative verification that our antibody is, in fact, detecting hBD-1 in the hippocampus we make the tentative assertion that hBD-1 is present and its expression upregulated in the AD hippocampus.
Redox-active iron insidiously accumulates within the ageing brain, but the rate of accumulation is accelerated in several neurodegenerative diseases, including AD [68, 69]. Accumulation occurs primarily in regions of the brain that show susceptibility to AD neuropathology [70]. Of particular interest is the underlying cause of hBD-1 upregulation within the epithelium of the AD CP where we also observed increased sequestering of redox-active iron. We are unaware of any histological verification of abnormal iron accumulation specifically within the CP epithelium. Iron levels within the CNS are largely regulated by the blood-CSF and blood–brain barriers [71, 72]. Dysregulation of iron homeostasis with resulting increase of redox-active iron is a probable contributor to AD neuropathology and inflammation through the promotion of oxidative conditions [73]. We propose that the elevated level of redox-active iron within the CP epithelium underlies our observed increase in hBD-1. This proposal is supported by the demonstrated role of iron as a regulator of immune function and our findings from the present study showing that iron can induce the upregulation of hBD-1 in cultured epithelium (Figure 6). Furthermore, Lee et al. [74] have shown that both cellular iron deficiency and overload can modulate elements of the innate and adaptive immune response, including the upregulated expression of the chemotactic cytokine MIP3α/CCL20. As a constitutively expressed antimicrobial/immunomodulatory peptide, hBD-1 expression would be expected to respond to signals impinging upon the epithelium indicative of pathogen infection. Our demonstrated ability to upregulate hBD-1 mRNA expression in vitro through exposure of epithelial cells to redox-active ferric iron (Fe+3Cl3) but not ferrous iron (data not shown), suggests that redox-active iron underlies upregulated expression of hBD-1, perhaps through activation of cellular pathways and innate immune defenses normally upregulated by intracellular invasion of iron-containing pathogens [75]. In fact, both iron depletion and iron overload have been shown to alter patterns of cellular gene expression [76]. As an interface between the systemic circulation and CSF, the CP epithelium undoubtedly plays a critical role in regulating iron metabolism and therefore iron availability to pathogens attempting to invade the CNS [77]. We suggest that the increased presence of intracellular iron within the CP, regardless of the underlying cause, contributes to the upregulation of the hBD-1 peptide and possibly other components of the innate immune response by the CP epithelium.
Modulation of the innate and adaptive immune response in the CNS is an intricate process likely involving the hBD-1 peptide. Recently, Cribbs et al. [78] showed extensive upregulation of innate immune system pathways in post mortem hippocampus and cortex from aged and AD-afflicted individuals relative to these same regions from young (20 to 59 years) cases. Our observed increase in hBD-1, a component of the innate immune system, within the AD brain is therefore consistent with this observed activation of innate immune pathways and underscores the need for further study of hBD-1 function and possible contribution of genomic variation in the DEFB1 gene to neuroinflammation in AD. Absence of detectable hBD-2 or -3 in either AD or age-matched control brains suggests hBD-1 may have a unique influence on innate immune response within the brain. However, future studies will require additional comparisons to young adult brain in order to accurately assess hBD function.
The possibility that hBD-1 may function as an antimicrobial or immuno-modulating element in neuroinflammation is supported by the literature cited above and by the novel findings presented in this study. The role or roles that specific antimicrobial peptides, such as hBD-1, may play in neurodegenerative diseases, including AD, requires further investigation in order to clarify their contributions to the overall host immune response in these diseases.
Conclusions
Our study results suggest the expression of the human antimicrobial peptide hBD-1 is elevated in the CP and hippocampus of the AD brain, relative to hBD-1 levels in the brain of non-demented, age-matched controls. This increased expression localized to the CP, an interface between the brain and systemic circulation, and the apparent increase in the neuronal cell bodies and glia within the hippocampus, suggest an active role for hBD-1 as a potential modulator of the host innate immune response within the CNS.
Abbreviations
- CP:
-
Choroid plexus
- CSF:
-
Cerebrospinal fluid
- DEFB1:
-
Human beta-defensin 1 gene
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GVD:
-
Granulovacuolar degeneration
- hBDs:
-
Human beta-defensins
- huβ2m:
-
Human β2-microglobulin
- huPO:
-
Human acidic ribosomal protein.
References
De Smet K, Contreras R: Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 2005, 27:1337–1347.
Taylor K, Barran PE, Dorin R: Structure-activity relationships in β-defensin peptides. Peptide Sci 2008, 90:1–7.
Beisswenger C, Bals R: Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci 2005, 6:255–264.
Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Ganz T Jr: Human beta- defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 1998, 101:1633–1642.
Hao HN, Zhao J, Lotoczky G, Grever WE, Lyman WD: Induction of human beta- defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J Neurochem 2001, 77:1027–1035.
Harder J, Bartels J, Christophers E, Schröder JM: A peptide antibiotic from human skin. Nature 1997, 387:861.
Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schröder JM: Mucoid Pseudomonas aeruginosa, TNF-α, and IL-1beta, but not IL-6, induce human beta- defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol 2000, 22:714–721.
Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, Tack BF, McCray PB Jr: Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun 1999, 67:2740–2745.
Zhao C, Wang I, Lehrer RI: Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 1996, 396:319–322.
Nakayama K, Okamura N, Arai H, Sekizawa K, Sasaki H: Expression of human beta- defensin-1 in the choroid plexus. Ann Neurol 1999, 45:685.
Johanson C, McMillan P, Tavares R, Spangenberger A, Duncan J, Silverberg G, Stopa E: Homeostatic capabilities of the choroid plexus epithelium in Alzheimer’s disease. Cerebrospinal Fluid Res 2004, 1:1–16.
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ: Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 2010, 330:1774.
Serot J-M, Zmudka J, Jouanny P: A possible role for CSF turnover and choroid plexus in the pathogenesis of late onset Alzheimer’s disease. J Alzheimers Dis 2012, 28:1–10.
Serot J-M, Béné M-C, Foliguet B, Faure GC: Morphological alterations of the choroid plexus in late onset Alzheimer’s disease. Acta Neuropathol 2000, 99:105–108.
Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, Saul TA: The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurol 2001, 57:1763–1766.
Wolburg K, Gerhardt H, Schulz M, Wolburg H, Engelhardt B: Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res 1999, 296:259–269.
Engelhardt B: Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ 2006, 43:259–280.
Wolburg H, Paulus W: Choroid plexus: biology and pathology. Acta Neuropathol 2010, 119:75–88.
Serot J-M, Béné M-C, Faure GC: Comparative immunohistochemical characteristics of human choroid plexus in vascular and Alzheimer’s dementia. Human Pathol 1994, 25:1185–1190.
Perez-Garcia E, Blanco R, Carmona M, Carro E, Ferrer I: Oxidative stress damage and oxidative stress responses in the choroid plexus. Acta Neuropathol 2009, 118:497–504.
Funk KE, Mrak RE, Kuret J: Granulovacuolar degeneration (GVD) bodies of Alzheimer’s disease (AD) resemble late-stage autophagic organelles. Neuropathol Appl Neurobiol 2011, 37:295–306.
Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W: The unfolding protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 2009, 174:1241–1251.
Kannanayakai TJ, Tao H, Vandre DD, Kuret J: Casein kinase-1 isoforms differentially associate with neurofibrillary and granulovacuolar degeneration lesions. Acta Neuropathol 2006, 111:413–421.
Lagalwar S, Berry RW, Binder LI: Relation of hippocampal phosphor-SAPK/JNK granules in Alzheimer’s disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol 2007, 113:63–73.
Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X: Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer’s disease links oxidative stress to abnormal phosphorylation. Neuroreport 1999, 10:2411–2415.
Yamazaki Y, Takahashi T, Kurashige T, Izumi Y, Yamawaki T, Matsumoto M: Immunopositivity for ESCRT-III subunit CHMP2B in granulovacuolar degeneration of neurons in the Alzheimer’s disease hippocampus. Neurosci Lett 2010, 477:86–90.
Zhu X, Castellani RJ, Moreira PI, Aliev G, Shenk JC, Harris PL, Fujioka H, Sayre LM, Szweda PA, Szweda LI, Smith MA, Perry G: Hydroxynonenal-generated crosslinking fluorophore accumulation in Alzheimer disease reveals a dichotomy of protein turnover. Free Radi Biol Med 2012, 52:699–704.
Castellani RJ, Gupta Y, Sheng B, Siedlak SL, Harris PL, Coller JM, Perry G, Lee HG, Tabaton M, Smith MA, Wang X, Zhu X: A novel origin for granulovacuolar degeneration in aging and Alzheimer’s disease: parallels to stress granules. Lab Invest 2011, 91:1777–1786.
Serot JM, Bene MC, Faure GC: Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci 2003, 8:s515–521.
Bishop GM, Smith MA, LaManna JC, Wilson AC, Perry G, Atwood CS: Iron homeostasis is maintained in the brain, but not the liver, following mild hypoxia. Redox Rep 2007,12(6):257–266.
Smith MA, Harris PLR, Sayre LM, Perry G: Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA 1997, 94:9866–9868.
Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G: Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis 2010,19(1):363–372.
Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T: Quantitative MR phase- corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology 2009,253(2):497–504.
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41:479–486.
The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease: Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging 1997,18(4 Suppl):S1-S2.
Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991, 82:239–259.
Oda A, Watson E: Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell Dev Biol 1990,26(6):589–595.
Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheimer JJ: Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol 2004, 22:181–215.
Lee SH, Kim JE, Lim HH, Lee HM, Choi JO: Antimicrobial defensin peptides of the human nasal mucosa. Ann Otol Rhinol Laryngol 2002,111(2):135–141.
Ramasundara M, Leach ST, Lemberg DA, Day AS: Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol 2009, 24:202–208.
Amor S, Puentes F, Baker D, van der Valk P: Inflammation in neurodegenerative diseases. Immunol 2010, 129:154–169.
Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, Comi C: Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis 2013,2(2):89–107.
Levy H, Raby BA, Lake S, Tantisira KG, Kwiatkowski D, Lazarus R, Silverman EK, Richter B, Klimecki WT, Vercelli D, Martinez FD, Weiss ST: Association of defensin beta-1 gene polymorphisms with asthma. J Allergy Clin Immunol 2005, 115:252–258.
Matsushita I, Hasegawa K, Nakata K, Yasuda K, Tokunaga K, Keicho N: Genetic variants of human beta-defensin-1 and chronic obstructive pulmonary disease. Biochem Biophys Res Commun 2002, 291:17–22.
Chen QX, Lv C, Huang LX, Cheng BL, Xie GH, Wu SJ, Feg XM: Genomic variations within DEFB1 are associated with the susceptibility to and the fatal outcome of severe sepsis in Chinese Han population. Genes Immun 2007, 8:439–443.
Lehrer RI, Ganz T: Defensins of vertebrate animals. Curr Opin Immunol 2002, 14:96–102.
Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ: Defensin participation in innate and adaptive immunity. Curr Pharm Des 2007, 13:3131–3138.
Zhu BD, Feng Y, Huang N, Wu Q, Wang BY: Mycobacterium bovis bacille Calmette- Guerin (BCG) enhances human beta-defensin-1 gene transcription in human pulmonary gland epithelial cells. Acta Pharmacol Sin 2003, 24:907–912.
Pazgier M, Prahl A, Hoover DM, Lubkowski J: Studies of the biological properties of human β-defensin 1. J Biol Chem 2007, 282:1819–1829.
Yang D, Chertov O, Bykovskaia N, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, Oppenheim JJ: β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999, 286:525–528.
Sherman H, Froy O: Expression of human β-defensin 1 is regulated via c-myc and the biological clock. Molec Immunol 2008, 45:3163–3167.
Ryan LK, Dai J, Yin Z, Megjugorac N, Uhlhorn V, Yim S, Schwartz KD, Abrahams JM, Diamond G, Fitzgerald-Bocarsly P: Modulation of human beta- defensin-1 (hBD-1) in plasmacytoid dendritic cells (PDC), monocytes, and epithelial cells by influenza virus, herpes simplex virus, and Sendai virus and its possible role in innate immunity. J Leuk Biol 2011, 90:343–356.
Itzhaki RF, Wozniak MA: Correspondence: Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement 2010, 6:83–84.
Honjo K, van Reekum R, Verhoeff NPLG: Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement 2009, 5:348–360.
Sherman H, Chapnik N, Froy O: Albumin and amino acids upregulate the expression of human beta-defensin 1. Mol Immunol 2006, 43:1617–1623.
Reppert SM, Weaver DR: Coordination of circadian timing in mammals. Nature 2002, 418:935–941.
Froy O: Regulation of mammalian defensin expression by toll-like receptor- dependent and independent signaling pathways. Curr Microbiol 2005, 7:1387–1397.
Froy O, Chapnik N: Circadian oscillation of innate immunity components in mouse small intestine. Mol Immunol 2007, 44:1964–1970.
de Haan W, Stam CJ, Jones BF, Zuiderwijk IM, van Dijk BW, Scheltens P: Resting-state oscillatory brain dynamics in Alzheimer’s disease. J Clin Neurophysiol 2008, 25:187–193.
Wu YH, Swaab DF: Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med 2007, 8:623–636.
Okamoto K, Hirai S, Lisuka T, Yanagisawa T, Watanabe M: Reexamination of granulovacuolar degeneration. Acta Neuropathol 1991, 82:340–345.
Lautner R, Mattsson N, Schöll M, Augutis K, Blennow K, Olsson B: Biomarkers for microglial activation in Alzheimer’s disease. Int J Alzheimer’s Dis 2011, 2011:1–5.
Greenbaum D, Colangelo C, Williams K, Gerstein M: Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 2003,4(9):117–117.8.
Acquaviva F, Castaldo I, Filla A, Giacchetti M, Marmolino D, Monticelli A, Pinelli M, Saccà F, Cocozza S: Recombinant human erythropoietin increases frataxin protein expression without increasing mRNA expression. Cerebellum 2008,7(3):360–365.
Fournier ML, Paulson A, Pavelka N, Mosley AL, Gaudenz K, Bradford WD: Delayed correlation of mRNA and protein expression in rapamycin-treated cells and a role for Ggc-1 in cellular sensitivity to rapamycin. Molec Cell Proteomics 2010, 9:271–284.
Borenstein LA, Ganz T, Sell S, Lehrer RI, Miller JN: Contribution of rabbit leukocyte defensins to the host response in experimental syphilis. Infect Immun 1991,59(4):1368–1377.
Sahasrabudhe KS, Kimball JR, Morton TH, Weinberg A, Dale BA: Expression of the antimicrobial peptide, human β-defensin 1, in duct cells of minor salivary glands and detection in saliva. J Dent Res 2000,79(9):1669–1674.
Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL: Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann NY Acad Sci 2004, 1012:224–236.
Zecca H, Youdim MB, Riederer P, Connor JA: Iron, brain aging and neurodegenerative disorders. Nat Rev Neurosci 2004, 5:863–873.
Castellani RJ, Moreira PI, Liu G, Dobson J, Perry G, Smith MA, Zhu X: Iron: The redox-active center of oxidative stress in Alzheimer disease. Neurochem Res 2007, 32:1640–1645.
Moos T, Rosengren Nielsen T, Skjorringe T, Morgtan E: Iron trafficking inside the brain. J Neurochem 2007, 103:1730–1740.
Rouault TA, Zhang DL, Jeong SY: Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 2009, 24:673–684.
Ong W-Y, Farooqui AA: Iron, neuroinflammation, and Alzheimer’s disease. J Alzheimers Dis 2005, 8:183–200.
Lee H-J, Choi S-C, Choi E-Y, Lee M-H, Seo G-S, Kim E-C, Yang B-J, Lee M-S, Shin Y-II, Park K-I Jun C-D: Iron chelator induces MIP-3α/CCL20 in human intestinal epithelial cells: implication for triggering mucosal adaptive immunity. Exper Molec Med 2005,37(4):297–310.
Wang L, Cherayil BJ: Ironing out the wrinkles in host defense: interactions between iron homeostasis and innate immunity. J Innate Immun 2009, 1:455–464.
Templeton DM, Liu Y: Genetic regulation of cell function in response to iron overload or chelation. Biochim Biophys Acta 2003, 1619:113–124.
Marques F, Falcao AM, Sousa JC, Coppola G, Geschwind D, Sousa GN, Correia-Neves M, Palha JA: Altered iron metabolism is part of the choroid plexus response to peripheral inflammation. Neuroendocrinol 2009,150(6):2822–2828.
Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW: Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation 2012, 9:179.
Acknowledgments
This work was supported by a grant from the University Center on Aging and Health through the generous support of the McGregor Foundation and the President’s Discretionary Fund, Case Western Reserve University. Additional support from NIH grant AG024028 to XZ. Support was also provided by a grant from the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health, and by the Semmes Foundation. Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, USA, and Case Western Reserve University and University Hospitals of Cleveland. The role of the NICHD Brain and Tissue Bank is to distribute tissue, and, therefore, cannot endorse the studies performed or the interpretation of results.
Special thanks to Aaron Weinberg, D.M.D., Ph.D., for material support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
WW proposed the original hypothesis, wrote the manuscript, performed RNA extractions, and data analyses; ST performed RTqPCR, and contributed to writing the manuscript; RC obtained and contributed post-mortem tissues for analysis; SS performed immunocytochemical analyses, and contributed to writing the manuscript; MS, GP, and XZ contributed their considerable expertise to directing the course of the study. All authors read and approved the final version of the manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Williams, W.M., Torres, S., Siedlak, S.L. et al. Antimicrobial peptide β-defensin-1 expression is upregulated in Alzheimer’s brain. J Neuroinflammation 10, 898 (2013). https://doi.org/10.1186/1742-2094-10-127
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-2094-10-127