Abstract
Exercise-induced bronchoconstriction (EIB) is described by transient narrowing of the airways after exercise. It occurs in approximately 10% of the general population, while athletes may show a higher prevalence, especially in cold weather and ice rink athletes. Diagnosis of EIB is often made on the basis of self-reported symptoms without objective lung function tests, however, the presence of EIB can not be accurately determined on the basis of symptoms and may be under-, over-, or misdiagnosed. The goal of this review is to describe other clinical entities that mimic asthma or EIB symptoms and can be confused with EIB.
Similar content being viewed by others
Diagnosis of exercise-induced bronchoconstriction
Exercise-induced bronchoconstriction (EIB) is a common entity and is described by the transient narrowing of the airways during or most often after exercise [1–4]. It occurs in 10-15% of the general population [5, 6], while the prevalence of EIB in asthmatic patients is reported to be 80-90% [7–9]. Athletes generally show a high prevalence of EIB [10, 11], especially in the cold weather [12–15], and ice rink athletes demonstrate a much greater prevalence of EIB than their non-ice rink counterparts [16–19]. In varsity college or elite athletes, 21-50% demonstrate EIB, depending upon the specific sport demands [11, 20–23]
The diagnosis of EIB is often made on the basis of self-reported symptoms without objective lung function tests. However, the presence of EIB can not be accurately determined on the basis of symptoms [20, 23, 24]. Recent studies demonstrate a lack of sensitivity and specificity of the symptoms-based diagnosis [23]. In one study of elite athletes, 39% of athletes positive to exercise challenge reported two or more symptoms, while 41% of those negative reported 2 or more symptoms [24]. In fact, history is little more reliable than flipping a coin in making the diagnosis of EIB.
Accurate diagnosis of EIB is essential. EIB is effectively prevented by acute use of b2-agonists, leukotriene receptor antagonists, sodium cromoglycate, and nedocromil sodium and the chronic use of inhaled corticosteroids [7, 25, 26]. However, these medications are often needlessly prescribed for patients who do not have EIB; they are often used in combination when such patients do not respond to first-line therapy. Because of the high b2-agonist use among the elite athletes, the International Olympic Committee (IOC) requires objective evidence to demonstrate asthma or EIB as an indication for therapeutic use of b2-agonists during competition [27, 28].
Therefore, confirmation of the diagnosis of EIB through standardized testing utilizing spirometry should be performed. Current guidelines (ATS, ERS, IOC-MC) for a diagnosis of EIB require a 10% or greater decrease in forced expiratory flow in the first second of exhalation (FEV1) in response to exercise (Figure 1) or eucapnic voluntary hyperpnea (EVH).
Symptoms of EIB include dyspnea (sensation of discomfort when breathing), increased effort or work to breathe, chest tightness, shortness of breath, air hunger, wheezing, or cough [24, 29]. However, other clinical entities can produce similar symptoms [30]. Dyspnea, in particular, is associated with many disease processes [31, 32]. In fact, EIB is uncommon in subjects who complain of exercise-induced dyspnea. In patients who presented with exercise induced dyspnea, only 7-24% actually had EIB on cardiopulmonary testing [6, 33]. "Wheeze" or stridor can also be caused by airway abnormalities and may closely mimic EIB.
The goal of this review is to describe other clinical entities that mimic asthma or EIB symptoms and can be confused with it. More than one condition may coexist in a given patient.
Physiologic limitation and deconditioning
Increased ventilation is a normal physiologic response to exercise. However, the increase in respiratory drive and work may be interpreted as pathologic by subjects who find that it limits their ability to perform to their expectations or results in "normal" discomfort. In a study by Abu-hasan et al, physiologic limitation was the most common reason for exercise-induced dyspnea in pediatric patients who underwent cardiopulmonary exercise testing [34]. It occurred in 52% of referrals for EIB; of those, two thirds had normal or above normal cardiovascular conditioning. The dyspnea is likely related to the increase in ventilation that accompanies high intensity exercise which is necessary to meet increased metabolic demands. Minute ventilation and respiratory drive are further increased at or above the lactate or ventilatory threshold, the point in incremental exercise when lactate begins to accumulate in the serum; excess lactate buildup results in exercise-associated increases in ventilation and ultimately hypocapnia. Subjects perceive dyspnea and shortness of breath at these high exercise intensities as abnormal.
Deconditioned subjects have a lower lactate/ventilatory threshold and begin to accumulate lactate and increase minute ventilation with lesser amounts of exercise. Deconditioning is a common etiology for exercise-induced dyspnea [32]. In a study by Seear et al., 23% of patients were "unfit" [6]. In Abu-Hasan's study, roughly 17% had decreased cardiovascular conditioning [34]. An athlete who has become deconditioned during the "off season" may interpret an increase in respiratory drive with lesser amounts of exercise as pathologic or as "EIB" or asthma.
Exercise rehabilitation or training can improve aerobic fitness and endurance [8] and can shift the lactate/ventilatory threshold so more work is required before lactate accumulates and ventilation increases. Improved aerobic fitness through exercise training can thus decrease the hyperpnea and dyspnea associated with exercise [35–37].
Obesity
Exercise-induced dyspnea is very common in obese patients. In one epidemiological study, 80% of obese middle aged subjects reported dyspnea after climbing two flights of stairs [38]. In another study, 36.5% of obese adults with a body mass index (BMI) greater than 31 and 28% of "overweight" adults (BMI 27-31) reported dyspnea when walking up hill [39]. In formal cardiopulmonary exercise testing, 37% of healthy obese women had an elevated perception of breathlessness during exercise [40].
There are several reasons why obese subjects experience dyspnea during exercise. Obesity is associated with impairment of pulmonary mechanics; in mild obesity, there is a reduced expiratory reserve volume (ERV) which is likely due to the displacement of the diaphragm into the chest cavity by the fat stores within the abdomen. With increasing severity of obesity, there are decreases in total lung capacity (TLC), functional residual capacity (FRC), and maximal voluntary ventilation (MVV)[41–44]. Because of the lower lung volumes, there may be a decrease in airway caliber and increase in airway resistance. The chest wall and total respiratory system are less compliant, which increases the work and energy cost of breathing [45]. The decrease in end expiratory lung volume likely causes flow limitation during exercise.
Aerobic capacity and cardiopulmonary fitness may be decreased in obese patients. Cardiopulmonary fitness, reflected by maximal or peak oxygen consumption (VO2 max) is decreased when corrected for weight [46]. Obese subjects are very likely to be deconditioned and ventilatory threshold may be reduced. Cardiac performance in response to incremental work load may also be decreased [47].
A number of studies have demonstrated that obese subjects perceive a greater degree of dyspnea in response to stimuli such as exercise, methacholine challenges or asthma exacerbations [39, 48, 49]. In one study in obese women, the degree of exercise-induced dyspnea was directly correlated to increases in the oxygen cost of breathing [40]. Weight loss can improve the pulmonary mechanics and lung volumes in these patients [43, 50].
Vocal cord abnormalities
Obstruction of the upper airway can cause symptoms such as shortness of breath, increased inspiratory effort, stridor and wheeze. In many subjects, upper airway obstruction is dynamic and only presents during exercise.
Paradoxical vocal cord movement is the most common cause of upper airway obstruction during exercise [51]. Typically, during inspiration, the vocal cords abduct (open); however, in some subjects, they paradoxically adduct (close) during inspiration or early expiration which causes obstruction. The prevalence of vocal cord dysfunction (VCD) has been reported to range from 5 -15% in patients referred for exercise-induced dyspnea [34, 52, 53]. However, in one study, the incidence was as high as 27% [6].
Diagnosis may be suspected by history of inspiratory wheeze and throat tightness. VCD has been associated with gastroesophageal reflux [54] and "type A personalities" [Weiss, unpublished observation]. Prevalence of VCD appears to be gender related and is highest among young females [53]. In a study of 370 (174 female, 196 male) elite athletes by Rundell and Spiering, 30% (58 female, 53 male) tested positive for EIB and 5.1% demonstrated inspiratory stridor consistent with VCD; of those, 18 of 19 were females [53]. Ten of those demonstrating inspiratory stridor were positive for EIB. Eight of the 9 demonstrating stridor that were negative for EIB had a previous diagnosis of EIB and 7 of those were prescribed albuterol by their physician, with no resolution of stridor.
The diagnosis of VCD is suggested by flow-volume loops which may reveal variable blunting of the inspiratory loop. In one study, 60% of VCD-positive patients developed abnormal flow-volume loops after metacholine challenge [52]. Definitive diagnosis can be made by fiberoptic rhinolaryngoscopy, which reveals the paradoxical motion of the vocal cords. The typical findings from laryngoscopy are inspiratory vocal cord closure with posterior "chinking" (a small opening at the posterior aspect of the cords) or, less commonly, complete closure [55, 56].
VCD may respond to breathing retraining diaphragmatic breathing - relaxation of larynx with conscious activation of the diaphragm [57, 58]. Speech pathologists are often an invaluable resource in providing subjects with instruction on breathing training exercises.
Laryngomalacia is less common cause of exercise-induced stridor. It primarily affects female competitive athletes who abruptly develop stridor at near peak exercise [59]. It is differentiated from vocal cord dysfunction by fiberoptic rhinolaryngoscopy. It is characterized by collapse of the arytenoid area; vocal cord motion is normal. The larynx in females may be predisposed to collapse, because it is shorter and narrower than in males. One reported patient had a history of laryngomalacia as an infant [60]. Laryngomalacia has been successfully treated with laser supraglottoplasty [61, 62].
Anxiety and Hyperventilation Syndrome
Anxiety may produce a heightened sense of breathlessness and dyspnea during exercise. Hyperventilation is a common physiologic response to both exercise and anxiety but may be interpreted as a primary problem that could be associated with chest tightness and shortness of breath [63]. In severe cases, it may be associated with carpopedal spasm, tetany and seizures [64]. In fact, in the past it was suggested that patients with panic and anxiety disorders actually had inherent respiratory and autonomic abnormalities. More recently, the entity of primary hyperventilation syndrome has been deemed a "chimera" [65]; that it is "no longer tenable." It is more likely that hyperventilation is a result of the panic attacks and associated anxiety [65–67].
The emotional state of subjects may impact their perception of dyspnea. In subjects with high levels of anxiety and multiple somatic complaints, there is an exaggerated perception of the intensity of dyspnea when hyperventilation is evoked by breathing 5% CO2 enriched air [68, 69]. In asthmatic patients stress, negative emotions and fear or anticipation increase subjective reports of dyspnea [70–72].
In subjects with a high level of anxiety, who have an exaggerated sense of dyspnea during exercise, it would be worthwhile to perform cardiopulmonary exercise testing and document the absence of EIB. In many cases, reassurance that the response to exercise is normal may allay anxiety and improve symptoms. The power of positive suggestion plays an important role in the relief of dyspnea and pain perception by decreasing anxiety [73]. Breathing retraining exercises may be helpful to decrease hyperventilation and self-hypnosis has been effective in reducing dyspnea in pediatric subjects [74]. In severe cases, pharmacologic therapy for anxiety may be indicated.
Cardiac abnormalities
In previously healthy persons, cardiac abnormalities are a rare cause of exercise-induced dyspnea. In older patients with cardiovascular diseases, particularly congestive heart failure, exercise performance is limited because of decreases in cardiac and pulmonary reserve. Patients may hyperventilate and experience dyspnea at lower work loads because of earlier onset of metabolic acidosis, decreased lung compliance and increased airways resistance because of pulmonary edema and increased dead space ventilation [75]. The ventilatory response to exercise can be improved by treatment of the underlying heart failure [76].
Pulmonary vascular diseases, such as pulmonary hypertension, can be associated with dyspnea, cardiac limitation and abnormal ventilatory responses to exercise [77]. Pulmonary hypertension may be associated with lower airways obstruction and increased airways hyperreactivity [78, 79]. In rare cases, it may present as refractory asthma because of extrinsic proximal airway obstruction by dilated pulmonary arteries [80]. Diagnosis is usually made on the basis of cardiac echocardiography and catheterization.
Hypertrophic cardiomyopathy (HCM), a feared cause of sudden death in athletes, can be associated with exercise-induced dyspnea and progressive heart failure [81–84]. It would be unlikely, but not impossible for it to present as "exercise-induced asthma." Those at highest risk of sudden death are subjects with a history of cardiac arrest or ventricular tachycardia, family history of HCM-related death, syncope, or left ventricular hypertrophy [82]. All athletes who are screened by a pre-sports participation physical should be asked about risk factors.
Cardiac dysrythmias are a rare cause of exercise-induced dyspnea. Atrial fibrillation and other supraventricular tachyarrhythmias are uncommon in elite athletes and similar to that observed in the general population (< 1%) [85]. Tachyarrythmias are often associated with palpitations or, rarely, syncope. Abu-Hassan et al reported one teenager who developed supraventricular tachycardia as a cause of his exercise-induced dyspnea; of note, he did not complain of palpitations [34]. Atrioventricular block could potentially cause exertional dyspnea [86–88]. Patients may present with exercise intolerance and AV block from Lyme disease [89]; we have documented one pediatric patient with exercise intolerance attributed to a complete AV block from Lyme disease [Weiss, unpublished observation].
Vascular anomalies of the thoracic aorta such as a double aortic arch or right aortic arch with persistent ligamentum arteriosum or aberrant left subclavian artery have been associated with dyspnea on exertion [90, 91]. The mechanisms for the symptoms include associated tracheomalacia and extrinsic compression of the airways which may worsen during exercise because of aortic arch dilatation. Surgical correction may be necessary.
Pulmonary arteriovenous malformations
Pulmonary arteriovenous malformations (AVM) can be associated with exercise-intolerance and arterial hypoxemia. Most pulmonary AVMs are associated with an autosomal dominant disorder, hereditary hemorrhagic telangiectasia (HHT) or Osler-Weber Rendu [92]. The incidence of HHT is estimated to be greater than one in 10,000 [93]; approximately 35% of patients with HHT have pulmonary AVMs [94]. The complications of pulmonary AVMs are related to the intrapulmonary right-to-left shunt. Paradoxical emboli can result in cerebral abscesses, cerebrovascular accidents and transient ischemic attacks [93, 95]. Most pulmonary AVMs are located at the lung bases. Some patients demonstrate platypnea or improvement in breathing on reclining [96]. Arterial hypoxemia which is worse in the upright position or with exercise is common [97]. Spirometry is usually normal, however, diffusing lung capacity for carbon monoxide (DLCO) may be decreased [97–100].
The gold-standards for diagnosis of AVMs are pulmonary angiography and chest computed tomography [101, 102]. Chest radiography, arterial oxygen measurements, cardiopulmonary exercise testing, radionuclide lung scanning, contrast-enhanced MR angiography and transthoracic contrast echocardiography (TTCE) have been used as screening methods [103–108]. Transcatheter embolization is the therapy of choice and has been shown to decrease the right to left-shunt and improve arterial hypoxemia and exercise tolerance [97–100].
Pulmonary abnormalities
Other pulmonary abnormalities can present with exercise-induced dyspnea. Chest wall or other musculoskeletal abnormalities can impair pulmonary mechanics. In the series of Abu-Hasan et al, 11% of patients had restrictive physiology due to mild scoliosis or pectus abnormality as the cause of their exercise-induced dyspnea [34]. Pectus excavatum has been associated with exercise intolerance and dyspnea; improvement after surgical correction has been documented [109–111]. Mild scoliosis in adolescents has been associated with abnormal ventilatory response to exercise [112]. In contrast, in adults with moderate kyphoscoliosis, dyspnea has been attributed to deconditioning rather than disordered pulmonary mechanics [113].
Tracheobronchomalacia, dynamic collapse of the central airways, may produce airflow limitation during exercise and has been associated with exercise intolerance [114]. The incidence of malacia has been estimated to be in 1: 2,100 children [115]. The symptoms overlap with those of asthma and it is often unsuspected until documented by bronchoscopy.
Interstitial lung disease is associated with exercise-induced dyspnea. Mechanisms for dyspnea and exercise limitation are expiratory flow limitation, hypoxemia and altered pulmonary mechanics [116–118]. Diagnosis may be made on the basis of pulmonary function tests revealing restrictive physiology, decreases in diffusing lung capacity for carbon monoxide (DLCO), chest CT, serum serology, bronchoscopy and/or lung biopsy.
The sequelae of moderate and severe chronic obstructive pulmonary disease (COPD) on exercise-induced dyspnea are well recognized. However, mild COPD can also be associated with increased dyspnea with exertion which reflects abnormal ventilatory mechanics including airway obstruction, increases in end-expiratory lung volumes and deconditioning [119–122].
Myopathy
Dyspnea can be associated with diseases of skeletal muscles (myopathies) [123]. In muscular dystrophies, there is a progressive loss of muscle fibers which results in increasing muscle weakness. In disorders of muscle energy metabolism, there is an imbalance in muscle energy production and utilization during exercise which can result in exertional muscle pain, cramping, weakness, or fatigue. Mitochondrial myopathy is an often unrecognized cause of exertional dyspnea and exercise intolerance [124, 125]. Flaherty et al, described 28 patients with biopsy proven myopathy and found that exercise dyspnea was associated with decreased respiratory muscle function [126]. Cardiopulmonary exercise testing revealed mechanical ventilatory limitation and an exaggerated increase in respiratory frequency and tachycardia in response to exercise. Many patients had respiratory muscle weakness.
Summary
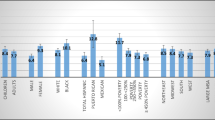
In summary, reported symptoms and history without objective lung function tests are not adequate to make a definitive diagnosis of EIB. Approximately half of those athletes reporting symptoms of EIB have normal airway function and about half of those who report no symptoms will demonstrate bronchoconstriction after exercise or other indirect challenge [20, 23, 24, 127]. It is therefore important to confirm a diagnosis of EIB through objective measures of lung function using standardized procedures. Indirect challenges such as exercise, eucapnic voluntary hyperpnea (EVH) or inhaled powdered mannitol are more specific to EIB than direct challenges such as histamine or methacholine [128, 129]. Figure 2 provides an algorithm for differential diagnosis of EIB. Differential diagnosis of EIB should include normal physiologic limitation and deconditioning, obesity, upper airway obstruction such as vocal cord dysfunction or laryngomalacia, anxiety-associated dyspnea and hyperventilation, exercise-induced supraventricular tachycardia, as well as other cardiac and pulmonary abnormalities.
References
Anderson SD, Silverman M, Walker SR: Metabolic and ventilatory changes in asthmatic patients during and after exercise. Thorax. 1972, 27: 718-25. 10.1136/thx.27.6.718.
Anderson SD, McEvoy JD, Bianco S: Changes in lung volumes and airway resistance after exercise in asthmatic subjects. Am Rev Respir Dis. 1972, 106: 30-7.
Godfrey S, Anderson SD, Silverman M: Physiologic aspects of exercise-induced asthma. Chest. 1973, 63 (Suppl): 36S-7S. 10.1378/chest.63.4_Supplement.36S.
McFadden ER, Nelson JA, Skowronski ME, Lenner KA: Thermally induced asthma and airway drying. Am J Respir Crit Care Med. 1999, 160: 221-6.
Busquets RM, Anto JM, Sunyer J, Sancho N, Vall O: Prevalence of asthma-related symptoms and bronchial responsiveness to exercise in children aged 13-14 yrs in Barcelona, Spain. Eur Respir J. 1996, 9: 2094-8. 10.1183/09031936.96.09102094.
Seear M, Wensley D, West N: How accurate is the diagnosis of exercise induced asthma among Vancouver schoolchildren?. Arch Dis Child. 2005, 90: 898-902. 10.1136/adc.2004.063974.
McFadden ER, Gilbert IA: Exercise-induced asthma. N Engl J Med. 1994, 330: 1362-7. 10.1056/NEJM199405123301907.
Sidiropoulou M, Tsimaras V, Fotiadou E, Aggelopoulou-Sakadami N: [Exercised-induced asthma in soccer players ages from 8 to 13 years]. Pneumologie. 2005, 59: 238-43. 10.1055/s-2004-830211.
Cabral AL, Conceicao GM, Fonseca-Guedes CH, Martins MA: Exercise-induced bronchospasm in children: effects of asthma severity. Am J Respir Crit Care Med. 1999, 159: 1819-23.
Schoene RB, Giboney K, Schimmel C, Hagen J, Robinson J, Schoene RB: Spirometry and airway reactivity in elite track and field athletes. Clin J Sport Med. 1997, 7: 257-61. 10.1097/00042752-199710000-00003.
Feinstein RA, LaRussa J, Wang-Dohlman A, Bartolucci AA: Screening adolescent athletes for exercise-induced asthma. Clin J Sport Med. 1996, 6: 119-23.
Helenius IJ, Tikkanen HO, Haahtela T: Occurrence of exercise induced bronchospasm in elite runners: dependence on atopy and exposure to cold air and pollen. Br J Sports Med. 1998, 32: 125-9. 10.1136/bjsm.32.2.125.
Wilber RL, Rundell KW, Szmedra L, Jenkinson DM, Im J, Drake SD: Incidence of exercise-induced bronchospasm in Olympic winter sport athletes. Med Sci Sports Exerc. 2000, 32: 732-7. 10.1097/00005768-200004000-00003.
Heir T, Oseid S: Self-reported asthma and exercise-induced asthma symptoms in high-level competitive cross-country skiers. Scand J Med Sci Sports. 1994, 4: 128-33.
Helenius IJ, Tikkanan HO, Haahtela T: Exercise-induced bronchospasm at low temperature in elite runners. Thorax. 1996, 51: 628-9. 10.1136/thx.51.6.628.
Rundell KW, Spiering BA, Evans TM, Baumann JM: Baseline lung function, exercise-induced bronchoconstriction, and asthma-like symptoms in elite women ice hockey players. Med Sci Sports Exerc. 2004, 36: 405-10. 10.1249/01.MSS.0000117118.77267.BF.
Mannix ET, Manfredi F, Farber MO: A comparison of two challenge tests for identifying exercise-induced bronchospasm in figure skaters. Chest. 1999, 115: 649-53. 10.1378/chest.115.3.649.
Mannix ET, Farber MO, Palange P, Galassetti P, Manfredi F: Exercise-induced asthma in figure skaters. Chest. 1996, 109: 312-5. 10.1378/chest.109.2.312.
Provost-Craig MA, Arbour KS, Sestili DC, Chabalko JJ, Ekinci E: The incidence of exercise-induced bronchospasm in competitive figure skaters. J Asthma. 1996, 33: 67-71. 10.3109/02770909609077764.
Holzer K, Anderson SD, Douglass J: Exercise in elite summer athletes: Challenges for diagnosis. J Allergy Clin Immunol. 2002, 110: 374-80. 10.1067/mai.2002.127784.
Weiler JM, Layton T, Hunt M: Asthma in United States Olympic athletes who participated in the 1996 Summer Games. J Allergy Clin Immunol. 1998, 102: 722-6. 10.1016/S0091-6749(98)70010-7.
Weiler JM, Metzger WJ, Donnelly AL, Crowley ET, Sharath MD: Prevalence of bronchial hyperresponsiveness in highly trained athletes. Chest. 1986, 90: 23-8. 10.1378/chest.90.1.23.
Parsons JP, Kaeding C, Phillips G, Jarjoura D, Wadley G, Mastronarde JG: Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med Sci Sports Exerc. 2007, 39: 1487-92. 10.1249/mss.0b013e3180986e45.
Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR: Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001, 33: 208-13.
Rundell KW, Spiering BA, Baumann JM, Evans TM: Effects of montelukast on airway narrowing from eucapnic voluntary hyperventilation and cold air exercise. Br J Sports Med. 2005, 39: 232-6. 10.1136/bjsm.2004.014282.
Hofstra WB, Neijens HJ, Duiverman EJ, Kouwenberg JM, Mulder PG, Kuethe MC: Dose-responses over time to inhaled fluticasone propionate treatment of exercise- and methacholine-induced bronchoconstriction in children with asthma. Pediatr Pulmonol. 2000, 29: 415-23. 10.1002/(SICI)1099-0496(200006)29:6<415::AID-PPUL1>3.0.CO;2-7.
Anderson SD, Sue-Chu M, Perry CP, Gratziou C, Kippelen P, McKenzie DC: Bronchial challenges in athletes applying to inhale a [beta]2-agonist at the 2004 Summer Olympics. Journal of Allergy and Clinical Immunology. 2006, 117: 767-73. 10.1016/j.jaci.2005.12.1355.
Fitch KD, Sue-Chu M, Anderson SD, Boulet LP, Hancox RJ, McKenzie DC: Asthma and the elite athlete: summary of the International Olympic Committee's consensus conference, Lausanne, Switzerland, January 22-24, 2008. J Allergy Clin Immunol. 2008, 122: 254-60. 10.1016/j.jaci.2008.07.003. 60 e1-7
Rundell KW, Jenkinson DM: Exercise-induced bronchospasm in the elite athlete. Sports Med. 2002, 32: 583-600. 10.2165/00007256-200232090-00004.
Weinberger M, Abu-Hasan M: Perceptions and pathophysiology of dyspnea and exercise intolerance. Pediatr Clin North Am. 2009, 56: 33-48. 10.1016/j.pcl.2008.10.015.
Weinberger M: Exercise induced dyspnoea: if not asthma, then what?. Arch Dis Child. 2006, 91: 543-4. 10.1136/adc.2006.095000.
Joyner BL, Fiorino EK, Matta-Arroyo E, Needleman JP: Cardiopulmonary exercise testing in children and adolescents with asthma who report symptoms of exercise-induced bronchoconstriction. J Asthma. 2006, 43: 675-8. 10.1080/02770900600925460.
De Baets F, Bodart E, Dramaix-Wilmet M, Van Daele S, de Bilderling G, Masset S: Exercise-induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr Pulmonol. 2005, 39: 301-5. 10.1002/ppul.20185.
Abu-Hasan M, Tannous B, Weinberger M: Exercise-induced dyspnea in children and adolescents: if not asthma then what?. Ann Allergy Asthma Immunol. 2005, 94: 366-71.
Hallstrand TS, Bates PW, Schoene RB: Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest. 2000, 118: 1460-9. 10.1378/chest.118.5.1460.
Matsumoto I, Araki H, Tsuda K, Odajima H, Nishima S, Higaki Y: Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax. 1999, 54: 196-201.
Haas F, Pasierski S, Levine N, Bishop M, Axen K, Pineda H: Effect of aerobic training on forced expiratory airflow in exercising asthmatic humans. J Appl Physiol. 1987, 63: 1230-5.
Gibson GJ: Obesity, respiratory function and breathlessness. Thorax. 2000, 55: S41-4. 10.1136/thorax.55.suppl_1.S41.
Sin DD, Jones RL, Man SFP: Obesity Is a Risk Factor for Dyspnea but Not for Airflow Obstruction. Arch Intern Med. 2002, 162: 1477-81. 10.1001/archinte.162.13.1477.
Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B: Dyspnea on Exertion in Obese Women: Association with an Increased Oxygen Cost of Breathing. Am J Respir Crit Care Med. 2008, 178: 116-23. 10.1164/rccm.200706-875OC.
Parameswaran K, Todd DC, Soth M: Altered respiratory physiology in obesity. Can Respir J. 2006, 13: 203-10.
Koenig SM: Pulmonary complications of obesity. Am J Med Sci. 2001, 321: 249-79. 10.1097/00000441-200104000-00006.
Bedell GN, Wilson WR, Seebohm PM: Pulmonary function in obese persons. J Clin Invest. 1958, 37: 1049-60. 10.1172/JCI103686.
Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K: Effects of obesity on respiratory function. Am Rev Respir Dis. 1983, 128: 501-6.
Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ: The Total Work of Breathing in Normal and Obese Men. J Clin Invest. 1964, 43: 728-39. 10.1172/JCI104957.
Jensen D, Webb KA, Davies GA, O'Donnell DE: Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: Implications for respiratory sensation. J Physiol. 2008, 586: 4735-50. 10.1113/jphysiol.2008.158154.
Salvadori A, Fanari P, Fontana M, Buontempi L, Saezza A, Baudo S: Oxygen uptake and cardiac performance in obese and normal subjects during exercise. Respiration. 1999, 66: 25-33. 10.1159/000029333.
Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG: Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes (Lond). 2008, 32: 502-9. 10.1038/sj.ijo.0803752.
Thomson CC, Clark S, Camargo CA: Body Mass Index and Asthma Severity Among Adults Presenting to the Emergency Department. Chest. 2003, 124: 795-802. 10.1378/chest.124.3.795.
Karason K, Lindroos AK, Stenlof K, Sjostrom L: Relief of Cardiorespiratory Symptoms and Increased Physical Activity After Surgically Induced Weight Loss: Results From the Swedish Obese Subjects Study. Arch Intern Med. 2000, 160: 1797-802. 10.1001/archinte.160.12.1797.
McFadden ER, Zawadski DK: Vocal cord dysfunction masquerading as exercise-induced asthma. a physiologic cause for "choking" during athletic activities. Am J Respir Crit Care Med. 1996, 153: 942-7.
Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A: Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol. 1993, 22: 175-82.
Rundell KW, Spiering BA: Inspiratory stridor in elite athletes. Chest. 2003, 123: 468-74. 10.1378/chest.123.2.468.
Heatley DG, Swift E: Paradoxical vocal cord dysfunction in an infant with stridor and gastroesophageal reflux. Int J Pediatr Otorhinolaryngol. 1996, 34: 149-51. 10.1016/0165-5876(95)01230-3.
Newman KB, Mason UG, Schmaling KB: Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995, 152: 1382-6.
Christopher KL, Wood RP, Eckert RC, Blager FB, Raney RA, Souhrada JF: Vocal-cord dysfunction presenting as asthma. N Engl J Med. 1983, 308: 1566-70.
Newsham KR, Klaben BK, Miller VJ, Saunders JE: Paradoxical Vocal-Cord Dysfunction: Management in Athletes. J Athl Train. 2002, 37: 325-8.
Blager FB, Gay ML, Wood RP: Voice therapy techniques adapted to treatment of habit cough: a pilot study. J Commun Disord. 1988, 21: 393-400. 10.1016/0021-9924(88)90024-X.
Fahey JT, Bryant NJ, Karas D, Goldberg B, Destefano R, Gracco LC: Exercise-induced stridor due to abnormal movement of the arytenoid area: videoendoscopic diagnosis and characterization of the "at risk" group. Pediatr Pulmonol. 2005, 39: 51-5. 10.1002/ppul.20076.
Mandell DL, Arjmand EM: Laryngomalacia induced by exercise in a pediatric patient. Int J Pediatr Otorhinolaryngol. 2003, 67: 999-1003. 10.1016/S0165-5876(03)00178-2.
Smith RJ, Bauman NM, Bent JP, Kramer M, Smits WL, Ahrens RC: Exercise-induced laryngomalacia. Ann Otol Rhinol Laryngol. 1995, 104: 537-41.
Bent JP, Miller DA, Kim JW, Bauman NM, Wilson JS, Smith RJ: Pediatric exercise-induced laryngomalacia. Ann Otol Rhinol Laryngol. 1996, 105: 169-75.
Hammo AH, Weinberger MM: Exercise-induced hyperventilation: a pseudoasthma syndrome. Ann Allergy Asthma Immunol. 1999, 82: 574-8.
Morgan WP: Hyperventilation syndrome: a review. Am Ind Hyg Assoc J. 1983, 44: 685-9.
Bass C: Hyperventilation syndrome: a chimera?. J Psychosom Res. 1997, 42: 421-6. 10.1016/S0022-3999(96)00365-0.
Salkovskis PM, Clark DM: Affective responses to hyperventilation: a test of the cognitive model of panic. Behav Res Ther. 1990, 28: 51-61. 10.1016/0005-7967(90)90054-M.
Salkovskis PM, Jones DR, Clark DM: Respiratory control in the treatment of panic attacks: replication and extension with concurrent measurement of behaviour and pCO2. Br J Psychiatry. 1986, 148: 526-32. 10.1192/bjp.148.5.526.
Rietveld S, Houtveen JH: Acquired sensitivity to relevant physiological activity in patients with chronic health problems. Behav Res Ther. 2004, 42: 137-53. 10.1016/S0005-7967(03)00104-9.
Wientjes CJ, Grossman P: Overreactivity of the psyche or the soma? Interindividual associations between psychosomatic symptoms, anxiety, heart rate, and end-tidal partial carbon dioxide pressure. Psychosom Med. 1994, 56: 533-40.
Rietveld S, van Beest I: Rollercoaster asthma: when positive emotional stress interferes with dyspnea perception. Behav Res Ther. 2007, 45: 977-87. 10.1016/j.brat.2006.07.009.
Rietveld S, Creer TL: Psychiatric factors in asthma: implications for diagnosis and therapy. Am J Respir Med. 2003, 2: 1-10.
Rietveld S, Brosschot JF: Current perspectives on symptom perception in asthma: a biomedical and psychological review. Int J Behav Med. 1999, 6: 120-34. 10.1207/s15327558ijbm0602_2.
De Pascalis V, Chiaradia C, Carotenuto E: The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002, 96: 393-402. 10.1016/S0304-3959(01)00485-7.
Anbar RD: Self-hypnosis for management of chronic dyspnea in pediatric patients. Pediatrics. 2001, 107: E21-10.1542/peds.107.2.e21.
Ross RM: ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003, 167: 1451-author reply
Reindl I, Kleber FX: Exertional hyperpnea in patients with chronic heart failure is a reversible cause of exercise intolerance. Basic Res Cardiol. 1996, 91 (Suppl 1): 37-43.
D'Alonzo GE, Gianotti LA, Pohil RL, Reagle RR, DuRee SL, Fuentes F: Comparison of progressive exercise performance of normal subjects and patients with primary pulmonary hypertension. Chest. 1987, 92: 57-62. 10.1378/chest.92.1.57.
Rastogi D, Ngai P, Barst RJ, Koumbourlis AC: Lower airway obstruction, bronchial hyperresponsiveness, and primary pulmonary hypertension in children. Pediatr Pulmonol. 2004, 37: 50-5. 10.1002/ppul.10363.
Meyer FJ, Ewert R, Hoeper MM, Olschewski H, Behr J, Winkler J: Peripheral airway obstruction in primary pulmonary hypertension. Thorax. 2002, 57: 473-6. 10.1136/thorax.57.6.473.
Achouh L, Montani D, Garcia G, Jais X, Hamid AM, Mercier O: Pulmonary arterial hypertension masquerading as severe refractory asthma. Eur Respir J. 2008, 32: 513-6. 10.1183/09031936.00005408.
Efthimiadis GK, Giannakoulas G, Parcharidou DG, Ziakas AG, Papadopoulos CE, Karoulas T: Subaortic and midventricular obstructive hypertrophic cardiomyopathy with extreme segmental hypertrophy. 2007, Cardiovasc Ultrasound, 5: 12-
Maron BJ: Hypertrophic cardiomyopathy: a systematic review. Jama. 2002, 287: 1308-20. 10.1001/jama.287.10.1308.
Frank MJ, Abdulla AM, Watkins LO, Prisant L, Stefadouros MA: Long-term medical management of hypertrophic cardiomyopathy: usefulness of propranolol. Eur Heart J. 1983, 4 (Suppl F): 155-64.
Nishimura RA, Holmes DR: Hypertrophic Obstructive Cardiomyopathy. N Engl J Med. 2004, 350: 1320-7. 10.1056/NEJMcp030779.
Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C: Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005, 46: 690-6. 10.1016/j.jacc.2005.04.052.
Chatillon J: [Dyspnea on exercise, early symptom of Mobitz type II atrio-ventricular block (author's transl)]. Schweiz Rundsch Med Prax. 1978, 67: 185-90.
Besley DC, McWilliams GJ, Moodie DS, Castle LW: Long-term follow-up of young adults following permanent pacemaker placement for complete heart block. Am Heart J. 1982, 103: 332-7. 10.1016/0002-8703(82)90270-8.
Schofield PM, Bowes RJ, Brooks N, Bennett DH: Exercise capacity and spontaneous heart rhythm after transvenous fulguration of atrioventricular conduction. Br Heart J. 1986, 56: 358-65. 10.1136/hrt.56.4.358.
Lelovas P, Dontas I, Bassiakou E, Xanthos T: Cardiac implications of Lyme disease, diagnosis and therapeutic approach. Int J Cardiol. 2008, 129: 15-21. 10.1016/j.ijcard.2008.01.044.
Parker JM, Cary-Freitas B, Berg BW: Symptomatic vascular rings in adulthood: an uncommon mimic of asthma. J Asthma. 2000, 37: 275-80. 10.3109/02770900009055450.
Grathwohl KW, Afifi AY, Dillard TA, Olson JP, Heric BR: Vascular rings of the thoracic aorta in adults. Am Surg. 1999, 65: 1077-83.
Gossage JR, Kanj G: Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998, 158: 643-61.
Shovlin CL, Letarte M: Rare diseases bullet 4: Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax. 1999, 54: 714-29.
Haitjema T, Disch F, Overtoom TT, Westermann CJ, Lammers JW: Screening family members of patients with hereditary hemorrhagic telangiectasia. Am J Med. 1995, 99: 519-24. 10.1016/S0002-9343(99)80229-0.
Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H: Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008, 63: 259-66. 10.1136/thx.2007.087452.
Robin ED, Laman D, Horn BR, Theodore J: Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med. 1976, 294: 941-3.
Dutton JA, Jackson JE, Hughes JM, Whyte MK, Peters AM, Ussov W: Pulmonary arteriovenous malformations: results of treatment with coil embolization in 53 patients. AJR Am J Roentgenol. 1995, 165: 1119-25.
Terry PB, White RI, Barth KH, Kaufman SL, Mitchell SE: Pulmonary arteriovenous malformations. Physiologic observations and results of therapeutic balloon embolization. N Engl J Med. 1983, 308: 1197-200.
Pennington DW, Gold WM, Gordon RL, Steiger D, Ring EJ, Golden JA: Treatment of pulmonary arteriovenous malformations by therapeutic embolization. Rest and exercise physiology in eight patients. Am Rev Respir Dis. 1992, 145: 1047-51.
Gupta P, Mordin C, Curtis J, Hughes JM, Shovlin CL, Jackson JE: Pulmonary arteriovenous malformations: effect of embolization on right-to-left shunt, hypoxemia, and exercise tolerance in 66 patients. AJR Am J Roentgenol. 2002, 179: 347-55.
Remy J, Remy-Jardin M, Giraud F, Wattinne L: Angioarchitecture of pulmonary arteriovenous malformations: clinical utility of three-dimensional helical CT. Radiology. 1994, 191: 657-64.
Remy J, Remy-Jardin M, Wattinne L, Deffontaines C: Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology. 1992, 182: 809-16.
Thompson RD, Jackson J, Peters AM, Dore CJ, Hughes JM: Sensitivity and specificity of radioisotope right-left shunt measurements and pulse oximetry for the early detection of pulmonary arteriovenous malformations. Chest. 1999, 115: 109-13. 10.1378/chest.115.1.109.
Dickinson JW, Whyte GP, McConnell AK, Nevill AM, Harries MG: Mid-expiratory flow versus FEV1 measurements in the diagnosis of exercise induced asthma in elite athletes. Thorax. 2006, 61: 111-4. 10.1136/thx.2005.046615.
Cottin V, Plauchu H, Bayle J-Y, Barthelet M, Revel D, Cordier J-F: Pulmonary Arteriovenous Malformations in Patients with Hereditary Hemorrhagic Telangiectasia. Am J Respir Crit Care Med. 2004, 169: 994-1000. 10.1164/rccm.200310-1441OC.
Chuang ML, Chang HC, Lim KE, Vintch JR: Gas exchange detection of right-to-left shunt in dyspneic patients: report of three cases. Int J Cardiol. 2006, 108: 117-9. 10.1016/j.ijcard.2005.02.022.
Schneider G, Uder M, Koehler M, Kirchin MA, Massmann A, Buecker A: MR Angiography for Detection of Pulmonary Arteriovenous Malformations in Patients with Hereditary Hemorrhagic Telangiectasia. Am J Roentgenol. 2008, 190: 892-901. 10.2214/AJR.07.2966.
Zukotynski K, Chan RP, Chow C-M, Cohen JH, Faughnan ME: Contrast Echocardiography Grading Predicts Pulmonary Arteriovenous Malformations on CT. Chest. 2007, 132: 18-23. 10.1378/chest.06-2356.
Fonkalsrud EW, Dunn JC, Atkinson JB: Repair of pectus excavatum deformities: 30 years of experience with 375 patients. Ann Surg. 2000, 231: 443-8. 10.1097/00000658-200003000-00019.
Jaroszewski DE, Fonkalsrud EW: Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg. 2007, 84: 429-33. 10.1016/j.athoracsur.2007.03.077.
Fonkalsrud EW, DeUgarte D, Choi E: Repair of pectus excavatum and carinatum deformities in 116 adults. Ann Surg. 2002, 236: 304-12. 10.1097/00000658-200209000-00007.
Smyth RJ, Chapman KR, Wright TA, Crawford JS, Rebuck AS: Ventilatory patterns during hypoxia, hypercapnia, and exercise in adolescents with mild scoliosis. Pediatrics. 1986, 77: 692-7.
Garfinkel SK, Kesten S, Chapman KR, Rebuck AS: Physiologic and nonphysiologic determinants of aerobic fitness in mild to moderate asthma. Am Rev Respir Dis. 1992, 145: 741-5.
Finder JD: Primary bronchomalacia in infants and children. J Pediatr. 1997, 130: 59-66. 10.1016/S0022-3476(97)70311-1.
Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ: Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005, 128: 3391-7. 10.1378/chest.128.5.3391.
Marciniuk DD, Sridhar G, Clemens RE, Zintel TA, Gallagher CG: Lung volumes and expiratory flow limitation during exercise in interstitial lung disease. J Appl Physiol. 1994, 77: 963-73.
Harris-Eze AO, Sridhar G, Clemens RE, Zintel TA, Gallagher CG, Marciniuk DD: Role of hypoxemia and pulmonary mechanics in exercise limitation in interstitial lung disease. Am J Respir Crit Care Med. 1996, 154: 994-1001.
O'Donnell DE, Chau LK, Webb KA: Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol. 1998, 84: 2000-9.
Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE: Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008, 177: 622-9. 10.1164/rccm.200707-1064OC.
O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC: Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007, 4: 145-68. 10.1513/pats.200611-159CC.
Nery LE, Wasserman K, Andrews JD, Huntsman DJ, Hansen JE, Whipp BJ: Ventilatory and gas exchange kinetics during exercise in chronic airways obstruction. J Appl Physiol. 1982, 53: 1594-602.
Carter R, Nicotra B, Blevins W, Holiday D: Altered exercise gas exchange and cardiac function in patients with mild chronic obstructive pulmonary disease. Chest. 1993, 103: 745-50. 10.1378/chest.103.3.745.
van Adel BA, Tarnopolsky MA: Metabolic myopathies: update 2009. J Clin Neuromuscul Dis. 2009, 10: 97-121. 10.1097/CND.0b013e3181903126.
Hooper RG, Thomas AR, Kearl RA: Mitochondrial enzyme deficiency causing exercise limitation in normal-appearing adults. Chest. 1995, 107: 317-22. 10.1378/chest.107.2.317.
Haller RG, Lewis SF, Estabrook RW, DiMauro S, Servidei S, Foster DW: Exercise intolerance, lactic acidosis, and abnormal cardiopulmonary regulation in exercise associated with adult skeletal muscle cytochrome c oxidase deficiency. J Clin Invest. 1989, 84: 155-61. 10.1172/JCI114135.
Flaherty KR, Wald J, Weisman IM, Zeballos RJ, Schork MA, Blaivas M: Unexplained exertional limitation: characterization of patients with a mitochondrial myopathy. Am J Respir Crit Care Med. 2001, 164: 425-32.
Rundell KW, Slee JB: Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008, 122: 238-46. 10.1016/j.jaci.2008.06.014.
Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA: Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000, 161: 2086-91.
Henriksen AH, Tveit KH, Holmen TL, Sue-Chu M, Bjermer L: A study of the association between exercise-induced wheeze and exercise versus methacholine-induced bronchoconstriction in adolescents. Pediatr Allergy Immunol. 2002, 13: 203-8. 10.1034/j.1399-3038.2002.01034.x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Both authors have made substantive contributions to drafting and revising the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Weiss, P., Rundell, K.W. Imitators of exercise-induced bronchoconstriction. All Asth Clin Immun 5, 7 (2009). https://doi.org/10.1186/1710-1492-5-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1710-1492-5-7