Abstract
Background
Cardiovascular Magnetic Resonance (CMR) enables non-invasive quantification of cardiac output (CO) and thereby cardiac index (CI, CO indexed to body surface area). The aim of this study was to establish if CI decreases with age and compare the values to CI for athletes and for patients with congestive heart failure (CHF).
Methods
CI was measured in 144 healthy volunteers (39 ± 16 years, range 21–81 years, 68 females), in 60 athletes (29 ± 6 years, 30 females) and in 157 CHF patients with ejection fraction (EF) below 40% (60 ± 13 years, 33 females). CI was calculated using aortic flow by velocity-encoded CMR and is presented as mean ± SD. Flow was validated in vitro using a flow phantom and in 25 subjects with aorta and pulmonary flow measurements.
Results
There was a slight decrease of CI with age in healthy subjects (8 ml/min/m2 per year, r2 = 0.07, p = 0.001). CI in males (3.2 ± 0.5 l/min/m2) and females (3.1 ± 0.4 l/min/m2) did not differ (p = 0.64). The mean ± SD of CI in healthy subjects in the age range of 20–29 was 3.3 ± 0.4 l/min/m2, in 30–39 years 3.3 ± 0.5 l/min/m2, in 40–49 years 3.1 ± 0.5 l/min/m2, 50–59 years 3.0 ± 0.4 l/min/m2 and >60 years 3.0 ± 0.4 l/min/m2. There was no difference in CI between athletes and age-controlled healthy subjects but HR was lower and indexed SV higher in athletes. CI in CHF patients (2.3 ± 0.6 l/min/m2) was lower compared to the healthy population (p < 0.001). There was a weak correlation between CI and EF in CHF patients (r2 = 0.07, p < 0.001) but CI did not differ between patients with NYHA-classes I-II compared to III-IV (n = 97, p = 0.16) or patients with or without hospitalization in the previous year (n = 100, p = 0.72). In vitro phantom validation showed low bias (−0.8 ± 19.8 ml/s) and in vivo validation in 25 subjects also showed low bias (0.26 ± 0.61 l/min, QP/QS 1.04 ± 0.09) between pulmonary and aortic flow.
Conclusions
CI decreases in healthy subjects with age but does not differ between males and females. We found no difference in CI between athletes and healthy subjects at rest but CI was lower in patients with congestive heart failure. The presented values can be used as reference values for flow velocity mapping CMR.
Similar content being viewed by others
Background
Cardiac output (CO) is one of the most important physiological parameters as it directly and proportionally reflects the metabolism of the entire organism. CO is the sum of the systemic flow per minute and calculated by the product of stroke volume and heart rate. The ability of the body to adapt to increased workload and hence metabolism is the result of the ability of the heart to increase heart rate and stroke volume [1]. CO indexed to body surface area (BSA) or cardiac index (CI), is an important clinical parameter used in the assessment of patients with heart disease as well as critically ill patients and patients under anesthesia. CI is also of interest in pharmacological studies [2] and the definition of forward failure in patients with heart failure is a decrease in CI. In clinical cardiology, cardiac output is determined when quantifying intracardiac shunts and during right heart catheterization to determine pulmonary resistance in cases of pulmonary hypertension.
Most available methods for quantifying CI are hampered by the invasive approach and/or the low accuracy and precision [2–6]. Invasive methods include Fick’s principle, thermodilution and dye dilution techniques and non-invasive techniques Doppler measurements and bio-impedance. Cardiovascular magnetic resonance (CMR) offers non-invasive measurements of flow with high accuracy and precision [6, 7] and CI can therefore be directly quantified using aortic flow quantification. Previous CI reference values for CMR are based on volumetry measurements but no reference values for CMR using phase contract (PC) flow velocity mapping has been presented.
Flow measurement is recommended in clinical CMR protocols of patients with congenital and/or valvular heart disease [8]. However, it is not known to what degree CI is reduced in patients with heart failure or if athletes show higher CI at rest compared to normal subjects. Furthermore, the relationship between age and CI in normal physiology is not fully established.
Therefore, the main aims of this study were to establish if CI decreases with age and to compare CI in healthy subjects to athletes and patients with heart failure. The presented CI values may also be used as reference values.
Methods
Patient population and study design
The study was approved by the local ethics committee. Written informed consent was either obtained or waived by the ethics committee. BSA was quantified according to the Mosteller formula [9].
Healthy subjects
144 volunteers aged 21 to 81 years (39 ± 16 years, 68 females) were prospectively included in the study. Age did not differ between females (38 ± 16 years) and males (40 ± 15 years, p = 0.37). All subjects had a normal electrocardiogram (ECG), no history of cardiovascular disease, no cardioactive medication and a blood pressure (BP) of ≤ 140/90 at the time of inclusion or at the time of the magnetic resonance (MR) examination. All healthy subjects had a BMI ≤ 30.
Athletes
60 professional athletes with high aerobic capacity (at least 10 hours aerobic training per week) were prospectively included. The athletes were active at a professional level in triathlon, swimming, handball or soccer. Female athletes (n = 30) and male athletes (n = 30) had the same age range and mean ± SD age,19-44 (29 ± 6) years. Age and gender matched controls (n = 60) were selected for comparison of CI between groups. The age and gender distribution of athletes were: up to 29 years 15 males and 25 females; 30–39 years 14 males and 5 females; 40–49 years 1 male.
Patients with heart failure (HF)
157 patients from the age of 24 to 86 years, (60 ± 13 years, 33 females) with left ventricular ejection fraction ≤ 40% were recruited retrospectively from patients who had undergone CMR. Patients with more than mild mitral regurgitation (>20% regurgitant fraction), aortic regurgitation or aortic peak velocity >3.0 m/s were not included. Available medical records were reviewed and data was available in 97 patients on New-York Heart Association (NYHA) classification [10] and in 100 patients on the frequency of hospitalization during the last year. The age and gender distribution of patients were: up to 29 years 3 males and 2 females; 30–39 years 4 males and 5 females; 40–49 years 12 male and 0 females, 50–59 years 42 males and 7 females; above 60 years 63 males and 19 females.
CMR
All subjects were imaged in the supine position with flow quantification of the ascending aorta during free breathing. Two CMR-scanners were used, a) 1.5 T Magnetom Vision (Siemens, Erlangen, Germany) and b) Philips Achieva (Philips, Best, the Netherlands). Blood flow was measured through a transversal plane at the height of the pulmonary bifurcation perpendicular to the ascending aorta with ECG-triggered phase-encoded velocity-mapping sequences. Typical imaging parameters for Siemens Vision were: echo time 5 ms, slice thickness 8 mm, velocity encoding factor 150 cm/s, time resolution typically 40 ms and gradient strength 25mT/m. Velocity information was acquired by prospective ECG-triggering. Typical imaging parameters for Philips Achieva were: echo time 6 ms, slices thickness 6 mm, velocity encoding 200 cm/s, time resolution typically 30 ms, acquired by retrospective ECG-triggering. The gradients for Philips Achieva was set to “regular mode” imposing limits on the maximal gradient strength and slew rate used. A detailed description of the velocity mapping technique has been published previously [11, 12]. Cine images of the left ventricle was performed in the short axis view for left ventricular function.
In vitro and in vivo validation
All in vitro and in vivo validation were performed at the Philips Achieva scanner as we previously have performed and published phantom and in vivo validation for the Siemens scanner [7]. A flow phantom composed of two plastic tubes, connected to give flow in both directions simultaneously, was used. The tubes were surrounded by static material. Water, doped with the clinically used contrast agent, was pumped through the tubes at four different flow rates and was assessed with the same flow quantification sequence as used for the in vivo data. Timer and beaker quantification of flow was used as reference. Aortic and pulmonary flows were measured for in vivo validation in 25 subjects and bias according to Bland-Altman were calculated.
Image analysis
The ascending aorta was outlined in the anatomic images for each time point with flow calculation performed in the corresponding velocity-encoded phase images (Figure 1). The average flow velocity (cm/s) was multiplied by the area of the vessel (cm2) to obtain flow (ml/s) at each time point. Flow throughout the cardiac cycle was integrated over one cardiac cycle to obtain the stroke volume (SV). Cardiac output (CO) was determined as SV multiplied by heart rate (SV·HR), and Cardiac index (CI), as CO divided by body surface area (BSA).
CMR quantification of cardiac output. The modulus image (A) is used for anatomical delineation of the aorta (black circle) and measurement is performed in the corresponding phase image (B). Cardiac output can be calculated by quantifying stroke volume as the integral of the resulting flow curve (C) and multiplying with heart rate.
Flow was measured using Segment 1.4 (http://segment.heiberg.se) [13]. Two independent observers analyzed the flow data from 20 controls to calculate interobserver variability. Ejection fraction was calculated for all patients using manual outlining of the endocardial contours in the end-diastolic and end-systolic phases.
Statistical analysis
GraphPad Prism 5.02 (GraphPad Software Inc.) was used for statistical calculations. Continuous variables are presented as mean ± SD. Limits of agreement defined as the mean ± 2SD for CI was calculated within each age group. The Mann–Whitney test was used to differentiate between CI, HR and SV in healthy subjects and patients with HF and athletes as well as between male and females and CI at different age groups. Two-way ANOVA was used to analyze variations in CI due to age and gender. Linear regression analysis was performed for CI, HR and SV vs. age and CI vs. ejection fraction as well as for the in vitro experiments. CI in patients with different NYHA class (I-II vs. III-IV) and the need for hospitalization the previous year was compared using the Mann–Whitney test. Interobserver variability, in vivo and in vitro validation were calculated according to Bland-Altman analysis as the mean difference between the measurements ± SD.
Results
Cardiac index in healthy subjects
Cardiac index in healthy subjects did not differ between males (3.2 ± 0.5 l/min/m2) and females (3.1 ± 0.4 l/min/m2, p = 0.64) but there was a slight decrease of CI with age (8 ml/min/m2 per year, r2 = 0.07, CI = −0.008xage + 3.511, p = 0.001), Figure 2. Similarly, age (p = 0.01) but not gender (p = 0.57) was a significant variable for CI in the two-way ANOVA analysis. The slight decrease of CI with age was explained by a decrease in SV with age (r2 = 0.06, SV = −0.27xage + 106.4, p = 0.004). Heart rate did not change with age (p = 0.87). The mean ± SD of CI and limits of agreement for healthy subjects according to age and gender is listed in Table 1. The minimum CI found in an individual was 2.3 l/min/m2. The interobserver variability of cardiac output measurements was 0.2 ± 0.2 l/min or 3 ± 4%.
Cardiac index in healthy subjects declined with age similarily in males (open squares) and females (filled circles). The linear regression lines are shown in solid lines and the 95% confidence interval with broken lines. The relationship between CI and age was similar in males (y = −0.0095age + 3.275, r2 = 0.08, p = 0.02) and females (y = −0.0072age + 3.423, r2 = 0.08, p = 0.02).
Cardiac index in athletes vs. healthy subjects
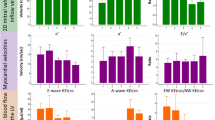
Cardiac index in female (3.4 ± 0.6 l/min/m2) and male athletes (3.7 ± 0.6 l/min/m2) did not differ significantly (p = 0.09, Figure 3). There was no difference in CI between athletes and age-matched healthy subjects in either females (p = 0.20) or males (p = 0.07). Athletes had higher stroke volume index quantified on PC-CMR (66 ± 7 ml/m2 for males and 60 ± 9 ml/m2 for females) compared to age and gender matched healthy subjects (54 ± 6 ml/m2 for males and 50 ± 7 ml/m2 for females, p < 0.001 for both). On the other hand, heart rates were lower in athletes (males 56 ± 7/min and females 58 ± 9/min) compared to controls (males 64 ± 10/min and females 64 ± 12/min, p = 0.003 and p = 0.01, respectively).
Cardiac index in patients vs. healthy subjects
CI in patients with congestive heart failure (2.3 ± 0.6 l/min/m2) was lower compared to the healthy population (3.2 ± 0.5 l/min/m2, p < 0.001), although there was a large overlap (Figure 4) with 49% of patients´ CI within mean ± 2SD of healthy subjects´ CI. Patients had a lower effective SV index (32 ± 9 ml/m2) compared to healthy subjects (51 ± 7 ml/m2, p < 0.001) and higher heart rate (73 ± 15/min and 64 ± 9/min respectively, p < 0.001). The subset of patients with normal CI had lower SV index (36 ± 7 ml/m2, p < 0.001) and higher heart rate (76 ± 15, p < 0.001) compared to healthy subjects but higher SV index and heart rate compared to patients with decreased CI (28 ± 8 ml/m2, p < 0.001 and 70 ± 15/min, p = 0.006, respectively). In patients, CI had a weak relationship with decreasing EF (r2 = 0.07, p < 0.001, equation CI = 0.02xEF + 1.7), Figure 5. There was no significant correlation with age in the patient population (r2 = 0.004, p = 0.56). Cardiac index did not differ between NYHA-classes I-II (2.3 ± 0.5 l/min/m2) and NYHA classes III-IV (2.2 ± 0.6 l/min/m2, p = 0.16), Figure 6. Neither did CI differ in patients with hospitalizations the previous year (2.2 ± 0.5 l/min/m2) vs. patients without hospitalizations (2.3 ± 0.6 l/min/m2, p = 0.72).
In vitro and in vivo validation
Phantom measurements showed an excellent correlation (r2 = 1.00, y = 1.08x + 0.79, p < 0.001) and Bland-Altman analysis showed a low bias (−0.8 ± 19.8 ml/s) between flow as measured by timer and beaker and by MR, Figure 7. The bias according to Bland-Altman analysis between pulmonary and aortic flow was 0.26 ± 0.61 l/min or 4 ± 8%. This results in a QP/QS of 1.04 ± 0.09.
Discussion
This study has confirmed that CI decreases with age, probably caused by a decreased level of metabolism. There were no differences between CI in males and females in healthy subjects and athletes had similar CI values to healthy subjects. CI was found to be lower in patients with heart failure compared to healthy subjects, although 49% of the heart failure patients fall within the normal ranges of CI. Finally, there was a weak correlation of CI and EF but no differences were found in CI between patients with NYHA-class I-II and III-IV or the absence/presence of hospitalization during the last year.
Normal CI values and variation with age
This study showed a decrease of CI with age (8 ml/min/m2) which is in line with lower stroke volumes in older subjects found in reference values of cine CMR [14, 15]. Metabolism decreases with age, especially in sedentary individuals [16, 17] and this is the most likely explanation to the decrease for CI with age. We did not find any gender difference in CI which is in line with our previously published normal values for gradient-echo cine CMR images [15] where no gender differences were seen in SV adjusted for BSA. Maceira et al., however, did find higher SV corrected for BSA in males compared to females [14]. The difference between our studies are not clear and further studies in other cohorts comparing SV and CI between gender would therefore be of interest. The mean CI values in this study, 3.1 l/min/m2 for women and 3.2 l/min/m2 for men, are the same mean values previously described by our group [7].
Cardiac index in athletes
CI at rest was found to be similar in athletes and subjects with regular exercise levels already in the 1960´s using invasive techniques [1] but this is the first study to show that the same reference values can be used for CI in athletes as for other healthy subjects. The lack of a difference in CI between normals and elite athletes underscores that cardiac CI is primarily dependent on the basal metabolism. The higher SV and lower HR in athletes compared to controls reiterates the higher reserve capacity for increasing CI through HR increase during stress in athletes. Even if there are no differences in CI between healthy volunteers and elite athletes at rest, the differences become clear during exercise [1]. The main difference induced by training is that stroke volume at peak exertion increases [18]. This is in line with the findings from our previous CMR study of 60 healthy volunteers and 71 elite athletes demonstrating that VO2max is proportional to total heart volume [19]. The larger total heart volumes in athletes results in a possibility of generating larger cardiac output due to higher stroke volume at similar heart rate.
Cardiac index in heart failure
Half of the patients had CI within normal limits and this was obtained through a higher heart rate although stroke volume was decreased compared to healthy subjects. The finding that 49% of the patients with heart failure were within normal limits of CI at rest shows that CI on its own is not useful as an indicator of heart failure in patients that fall within normal limits. An earlier study has suggested a method to differentiate these patients from healthy subjects by relating VO2max to total heart volume (THV) [20]. In that study, patients with increased THV fell below normal subjects in VO2max capacity.
Quantification of CI is primarily performed in the clinical management of heart failure when assessing pulmonary vascular resistance and shunt lesions. Medication of heart failure often aims to increase the CI and CMR could play an important role when assessing new therapies for heart failure. The high accuracy with CMR would lower the number needed for detection of therapeutic effect.
Measurements of CI with CMR
Flow quantification with PC-CMR has been extensively validated and found to have high accuracy in large [7, 21–23] and small [24, 25] vessels. Studies with segmented sequences in modern scanners with shorter bore have shown problems with phase offsets thay may cause errors in CI measurements PC-CMR [26, 27]. The baseline shifts caused by phase offsets can be corrected for by dedicated post-processing or by using phantoms [28]. The transversal image plane for aortic flow used in our study have lower phase offset compared to oblique image planes more proximal to the aortic valve [27]. The CI quantification in this study was done using a non-segmented sequence during free breathing and with the use of a lower gradient mode. Restricting the gradient strength can help to decrease the phase-shift errors (personal communication Dr. M Kouwenhoven, Philips Healthcare, Best, the Netherlands) and the flow results from the validations in this study in phantoms and in vivo showed low bias within the physiological range. Of note, the in vivo validation found a mean QP/QS of 1.04 which is the expected value taking into account a coronary artery flow of 3-5% of cardiac output.
Limitations
This study was performed in a single center using two different scanners from different vendors. Full medical records could not be obtained in all patients and therefore not all patients were included in the analysis for a possible relationship between CI and NYHA class and presence of hospitalization. Examinations in a CMR scanner may cause psychological stress due to the confined space and examination in a hospital setting. This may have resulted in a higher CI values compared to the CI at complete rest at home.
Conclusions
CI over a wide age range in healthy subjects are presented and can be used as reference values for CMR. CI decreases in healthy subjects with age but does not differ between males and females. We found no difference in CI between athletes and healthy subjects but CI was lower in patients with congestive heart failure.
References
Åstrand PO, Rodahl K: Textbook or work physiology. 1970, McGraw Hill
Geerts BF, Aarts LP, Jansen JR: Methods in pharmacology: measurement of cardiac output. Br J Clin Pharmacol. 2011, 71 (3): 316-330. 10.1111/j.1365-2125.2010.03798.x.
Hillis LD, Firth BG, Winniford MD: Analysis of factors affecting the variability of Fick versus indicator dilution measurements of cardiac output. Am J Cardiol. 1985, 56 (12): 764-768. 10.1016/0002-9149(85)91132-4.
Cigarroa RG, Lange RA, Hillis LD: Oximetric quantitation of intracardiac left-to-right shunting: limitations of the Qp/Qs ratio. Am J Cardiol. 1989, 64 (3): 246-247. 10.1016/0002-9149(89)90471-2.
Critchley LA, Critchley JA: A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999, 15 (2): 85-91. 10.1023/A:1009982611386.
Muthurangu V, Taylor A, Andriantsimiavona R, Hegde S, Miquel ME, Tulloh R, Baker E, Hill DL, Razavi RS: Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase-contrast magnetic resonance flow. Circulation. 2004, 110 (7): 826-834. 10.1161/01.CIR.0000138741.72946.84.
Arheden H, Holmqvist C, Thilen U, Hanseus K, Bjorkhem G, Pahlm O, Laurin S, Stahlberg F: Left-to-right cardiac shunts: comparison of measurements obtained with MR velocity mapping and with radionuclide angiography. Radiology. 1999, 211 (2): 453-458.
Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E: Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008, 10: 35-10.1186/1532-429X-10-35.
Mosteller RD: Simplified calculation of body-surface area. N Engl J Med. 1987, 317 (17): 1098-
Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA: 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009, 119 (14): 1977-2016.
Carlsson M, Cain P, Holmqvist C, Stahlberg F, Lundback S, Arheden H: Total heart volume variation throughout the cardiac cycle in humans. Am J Physiol Heart Circ Physiol. 2004, 287 (1): H243-H250. 10.1152/ajpheart.01125.2003.
Carlsson M, Ugander M, Heiberg E, Arheden H: The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am J Physiol Heart Circ Physiol. 2007, 293 (1): H636-H644. 10.1152/ajpheart.01376.2006.
Heiberg E, Wigström L, Carlsson M, Bolger AF, Karlsson M: Time Resolved Three-dimensional Automated Segmentation of the Left Ventricle. Proc IEEE Comput Cardiol. 2005, 32: 599-602.
Maceira AM, Prasad SM, Khan M, Pennell DJ: Normalized Left Ventricular Systolic and Diastolic Function by Steady State Free Precession Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson. 2006, 8: 417-426. 10.1080/10976640600572889.
Cain PA, Ahl R, Hedstrom E, Ugander M, Allansdotter-Johnsson A, Friberg P, Arheden H: Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imag. 2009, 9: 2-10.1186/1471-2342-9-2.
Poehlman ET, Melby CL, Badylak SF: Relation of age and physical exercise status on metabolic rate in younger and older healthy men. J Gerontol. 1991, 46 (2): B54-B58.
Van Pelt RE, Jones PP, Davy KP, Desouza CA, Tanaka H, Davy BM, Seals DR: Regular exercise and the age-related decline in resting metabolic rate in women. J Clin Endocrinol Metab. 1997, 82 (10): 3208-3212. 10.1210/jc.82.10.3208.
Rerych SK, Scholz PM, Sabiston DC, Jones RH: Effects of exercise training on left ventricular function in normal subjects: a longitudinal study by radionuclide angiography. Am J Cardiol. 1980, 45 (2): 244-252. 10.1016/0002-9149(80)90642-6.
Steding K, Engblom H, Buhre T, Carlsson M, Mosen H, Wohlfart B, Arheden H: Relation between cardiac dimensions and peak oxygen uptake. J Cardiovasc Magn Reson. 2010, 12 (1): 8-10.1186/1532-429X-12-8.
Engblom H, Steding K, Carlsson M, Mosen H, Heden B, Buhre T, Ekmehag B, Arheden H: Peak oxygen uptake in relation to total heart volume discriminates heart failure patients from healthy volunteers and athletes. J Cardiovasc Magn Reson. 2010, 12: 74-10.1186/1532-429X-12-74.
Firmin DN, Nayler GL, Klipstein RH, Underwood SR, Rees RS, Longmore DB: In vivo validation of MR velocity imaging. J Comput Assist Tomogr. 1987, 11 (5): 751-756. 10.1097/00004728-198709000-00001.
Powell AJ, Maier SE, Chung T, Geva T: Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: in vitro and in vivo validation. Pediatr Cardiol. 2000, 21 (2): 104-110. 10.1007/s002469910014.
Carlsson M, Toger J, Kanski M, Markenroth Bloch K, Stahlberg F, Heiberg E, Arheden H: Quantification and visualization of cardiovascular 4D velocity mapping accelerated with parallel imaging or k-t BLAST: Head to head comparison and validation at 1.5T and 3T. J Cardiovasc Magn Reson. 2011, 13 (1): 55-10.1186/1532-429X-13-55.
Arheden H, Saeed M, Tornqvist E, Lund G, Wendland MF, Higgins CB, Stahlberg F: Accuracy of segmented MR velocity mapping to measure small vessel pulsatile flow in a phantom simulating cardiac motion. J Magn Reson Imaging. 2001, 13 (5): 722-728. 10.1002/jmri.1100.
Bloch KM, Carlsson M, Arheden H, Stahlberg F: Quantifying coronary sinus flow and global LV perfusion at 3 T. BMC Med Imag. 2009, 9: 9-10.1186/1471-2342-9-9.
Gatehouse PD, Rolf MP, Graves MJ, Hofman MB, Totman J, Werner B, Quest RA, Liu Y, von Spiczak J, Dieringer M: Flow measurement by cardiovascular magnetic resonance: a multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J Cardiovasc Magn Reson. 2010, 12: 5-10.1186/1532-429X-12-5.
Rolf MP, Hofman MB, Gatehouse PD, Markenroth Bloch K, Heymans MW, Ebbers T, Graves MJ, Totman JJ, Werner B, Rossum AC: Sequence optimization to reduce velocity offsets in cardiovascular magnetic resonance volume flow quantification - A multi-vendor study. J Cardiovasc Magn Reson. 2011, 13 (1): 18-10.1186/1532-429X-13-18.
Holland BJ, Printz BF, Lai WW: Baseline correction of phase-contrast images in congenital cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010, 12: 11-10.1186/1532-429X-12-11.
Acknowledgements
This study was supported by grants from the Region of Scania, the Swedish Heart Lung foundation and the Swedish Research Council (2008–2461, 2008–2949).
The research technicians Ann-Helen Arvidsson and Christel Carlander are gratefully acknowledged for their help with data acquisition and analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
KMB is an employee of Philips Healthcare.
Authors’ contributions
MC and HA conceived and designed the study and drafted the manuscript. KMB and FS carried out the phantom data collection and analysis. KSE and HM participated in subject enrollment and data collection. RA and BE reviewed the patients charts for clinical data. MC performed the statistical analysis. All authors revised the manuscript during its preparation and have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Carlsson, M., Andersson, R., Markenroth Bloch, K. et al. Cardiac output and cardiac index measured with cardiovascular magnetic resonance in healthy subjects, elite athletes and patients with congestive heart failure. J Cardiovasc Magn Reson 14, 51 (2012). https://doi.org/10.1186/1532-429X-14-51
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1532-429X-14-51