Abstract
Background
Knowledge about age-specific normal values for left ventricular mass (LVM), end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV) and ejection fraction (EF) by cardiac magnetic resonance imaging (CMR) is of importance to differentiate between health and disease and to assess the severity of disease. The aims of the study were to determine age and gender specific normal reference values and to explore the normal physiological variation of these parameters from adolescence to late adulthood, in a cross sectional study.
Methods
Gradient echo CMR was performed at 1.5 T in 96 healthy volunteers (11–81 years, 50 male). Gender-specific analysis of parameters was undertaken in both absolute values and adjusted for body surface area (BSA).
Results
Age and gender specific normal ranges for LV volumes, mass and function are presented from the second through the eighth decade of life. LVM, ESV and EDV rose during adolescence and declined in adulthood. SV and EF decreased with age. Compared to adult females, adult males had higher BSA-adjusted values of EDV (p = 0.006) and ESV (p < 0.001), similar SV (p = 0.51) and lower EF (p = 0.014). No gender differences were seen in the youngest, 11–15 year, age range.
Conclusion
LV volumes, mass and function vary over a broad age range in healthy individuals. LV volumes and mass both rise in adolescence and decline with age. EF showed a rapid decline in adolescence compared to changes throughout adulthood. These findings demonstrate the need for age and gender specific normal ranges for clinical use.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Fundamental structural and functional properties of the left ventricle including left ventricular mass (LVM), volumes, and function are often assessed in the clinical setting using two dimensional echocardiography. The clinical use of cardiac magnetic resonance imaging (CMR) has increased lately and a consensus panel report has established clinical indications for cardiovascular magnetic resonance [1].
It is of great clinical importance to be able to differentiate between normal and abnormal findings, but this may often prove difficult if adequate normal values are unknown. The need for age and gender specific normal values with CMR is therefore growing. Several studies have defined CMR normal ranges of LV volumes and function in limited age ranges [2–8], and none of these have examined these parameters over a wide age range in healthy individuals. A recent study presented age and gender specific normal ranges for CMR at 1.5 T using a steady state free precession sequence [9]. However, at times, some centers still need to use gradient echo sequences in clinical assessment at 1.5 T.
The aim of the study was, therefore, to suggest clinically usable age and gender specific normal ranges for LV volumes and function using gradient echo CMR at 1.5 T for the second through the eighth decade of life. We also sought to explore, in a cross sectional study, the normal variation of LV volumes and function in strictly healthy subjects over a wide age range in order to examine the age variation of these parameters.
Methods
Study population and design
The study population consisted of 96 healthy volunteers prospectively recruited by advertisement from the local community (76 adults, age 21–81 and 20 children, age 11–15, all caucasian). No subjects were excluded because of poor image quality. All subjects had a normal electrocardiogram (ECG) and blood pressure (systolic blood pressure (SBP) ≤ 140 mmHg and diastolic blood pressure (DBP) ≤ 90 mmHg) [10] and had no history of systolic or diastolic hypertension. Subjects with previous or current cardiovascular, systemic, metabolic disease, body mass index ≥ 30, visually overt aortic or mitral valve regurgitation in long axis MR images, or treatment with medication (except oral contraceptives [n=5], hormone replacement therapy [n=5] or oral incontinence medication [n=1]) were excluded from the study. MR imaging was performed within four weeks after inclusion with image analysis undertaken by independent observers blinded to subject characteristics. The investigation protocol and procedures were approved by the Lund University research ethics committee (reference number LU 207-00). Written informed consent was obtained from all subjects prior to inclusion.
Blood pressure
SBP and DBP were obtained in the supine or seated position by auscultation using a brachial cuff.
MR imaging
All patients were imaged in the supine position using a 1.5 T system (Magnetom Vision; Siemens, Erlangen, Germany) with a 25 mT/m gradient system and a phased-array body coil. Standard scout images were used to locate the orthogonal planes of the heart. End-expiratory ECG triggered short-axis gradient echo cine loops were then acquired throughout the left ventricle from the base (atrioventricular valve plane) to the apex. Typical imaging parameters were TR = 100 ms, and echo sharing gave an effective phase interval of 50 ms, TE = 4.8 ms, slice thickness 10 mm, field of view 350–420 mm, matrix 126 × 256, flip angle 20°. The number of cardiac phases per acquisition was determined as the integer obtained from the RR interval divided by TR. Nine to twelve slices were required to completely cover the left ventricle, depending on heart size.
MR image analysis
i) Tracing of endocardial and epicardial contours
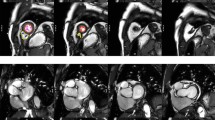
All measurements were undertaken manually without the aid of automated image analysis software. End-diastolic and end-systolic frames were identified according to ventricular blood pool area. Measurements of the left ventricular endocardial and epicardial areas (Scion Image Beta 4.0.2, Scion Imaging Corporation) in each image frame were performed in the short-axis view. At the base of the left ventricle, the aortic outflow tract below the valve was included in volume measurements. The free papillary muscles were included for LVM assessment, and excluded for left ventricular volume assessment [11]. In the basal region of the heart where the left atrium was seen, only the portion of the slice that could be identified as left ventricle was included for measurement (Figure 1).
Delineation of the left ventricular borders in the short axis plane in end-diastole and end-systole. Both the endocardial and epicardial borders were outlined manually for both ventricular volume and mass measurement (solid lines). Papillary muscle measurements were only included for mass measurements (dashed lines).
ii) Left ventricular mass and dimensions
The difference in area between the endocardial and epicardial contours multiplied by the slice thickness (10 mm) represented myocardial volume for a given slice. Total myocardial mass was obtained by calculating the sum of all myocardial slice volumes and multiplying by the myocardial specific gravity (1.05 g/cm3). End-diastolic volume (EDV) and end-systolic volume (ESV) were calculated as the endocardial volume at end-diastole and end-systole respectively. Stroke volume (SV) was calculated as the difference between EDV and ESV. Ejection fraction (EF) was calculated as SV divided by EDV. All parameters were divided by body surface area (BSA) to achieve values adjusted for body size (e.g. LVM/BSA for left ventricular mass adjusted for BSA). In addition, a subset of 20 subjects were analyzed by 2 readers blinded to each others results in order to examine interobserver variability. Intraobserver variability was assessed by one observer who performed repeated blinded measurements in the same 20 subjects one month later.
Statistical analysis
SPSS (version 16) was used for statistical calculations. A p value less than 0.05 was considered statistically significant. Values are expressed as mean ± SD. Unpaired Student's t test or ANOVA were used to test for significance between groups since both visual inspection and the Kolmogorov-Smirnov test showed that all measures were adequately normally distributed. Intra- and interobserver variability were assessed as the mean difference of measurements ± SD and by the intraclass correlation coeffecient (ICC) employing a two-way mixed model [12]. The coefficient of variation (CV) was calculated as the SD of the difference between two measurements expressed as percent of their mean. The coeffiecient of repeatability (CR) was calculated as two times the SD of the difference in two measurements. Pearson's correlation coefficient was used to assess the correlation between two variables, and expressed as its square (R2). BSA was calculated using a previously described technique [13]. Curve estimation and the 95% prediction intervals of LVM, dimensions, and function were defined using commercial software (Matlab curve fitting toolbox, Matlab version R12, Mathworks). The most appropriate curve fitting algorithm for LVM, dimensions and functional measures was identified as the rational polynomial of the form P(x)/Q(x) where both numerator and denominator were at most of the second degree and which had the highest adjusted R2 and lowest root mean square (RMS) of the error [14]. The predicted lower, mean and upper limits for normal values of LV parameters in each decade were calculated as the average of the mean and 95% prediction interval of the predicted values for each whole year as given by the curve estimation model.
Results
Population description
Data from 94 of the 96 subjects have, in part, previously been published in a study of LVM and wall stress [14]. Table 1 displays the baseline characteristics for the current study population. Blood pressure was similar between genders and well within accepted normal limits [10].
Normal variation of left ventricular measures with age
Table 2 lists suggested age-specific reference values for LV mass, EDV, ESV, SV and EF as absolute values and adjusted for BSA for males and females, respectively.
i) Left ventricular mass
Information on the age dependence of LV mass and wall stress in the majority of this population have been published and discussed previously [14]. The current study, however, provides tabular values in order to ease the use of this information as reference values. Figure 2 and Figure 3 describe the changes in left ventricular mass with age.
The normal age variation in left ventricular parameters in males (left) and females (right). Solid lines represent rational polynomial curve fit and dashed lines the 95% prediction intervals of this fit. Reference values are listed in Table 2. LVM = left ventricular mass, BSA = body surface area, EDV = end-diastolic volume.
The normal age variation in left ventricular parameters in males (left) and females (right). Solid lines represent rational polynomial curve fit and dashed lines the 95% prediction intervals of this fit. Reference values are listed in Table 2. BSA = body surface area, ESV = end-systolic volume, SV = stroke volume, EF = ejection fraction.
ii) End-diastolic and End-systolic volume
Ventricular volumes varied markedly between males and females (Figure 2 and Figure 3). Notably, there was a rise in EDV and ESV during adolescence and early adulthood in males, with a decline thereafter, possibly reflecting the concurrent change of LVM with age. This was absent in females with a trend towards a decrease of EDV and EDV/BSA with age and increase of ESV and ESV/BSA with age. Ventricular volumes in the adolescent group were similar between males and females (EDV: 120 ± 29 ml vs. 114 ± 23 ml, p = 0.66; EDV/BSA: 76 ± 11 ml/m2 vs. 77 ± 11 ml/m2, p = 0.95; ESV: 35 ± 8 ml vs. 32 ± 11 ml, p = 0.48; ESV/BSA: 22 ± 4 ml/m2 vs. 21 ± 7 ml/m2, p = 0.65). However, differences between genders became apparent for all ventricular volumes in the adult group with higher values in the male group (EDV: 153 ± 30 ml vs. 118 ± 19 ml, p < 0.001; EDV/BSA: 76 ± 13 ml/m2 vs. 69 ± 9 ml/m2, p = 0.006; ESV: 63 ± 17 ml vs. 43 ± 11 ml, p < 0.001; ESV/BSA: 31 ± 8 ml/m2 vs. 25 ± 6 ml/m2, p < 0.001).
iii) Left ventricular stroke volume and ejection fraction
In general, both SV and EF demonstrated a decline with age (Figure 3). Although differences in SV and EF between males and females were present in the adult group (SV: 91 ± 19 ml vs. 75 ± 15 ml, p < 0.001; SV/BSA: 45 ± 9 ml/m2 vs. 44 ± 9 ml/m2, p = 0.51; EF: 0.59 ± 0.07 vs. 0.64 ± 0.08, p = 0.014), SV and EF were similar between genders in the adolescent group (SV: 85 ± 22 ml vs. 83 ± 16 ml, p = 0.82; SV/BSA: 54 ± 9 ml/m2 vs. 55 ± 7 ml/m2, p = 0.70; EF: 0.71 ± 0.04 vs. 0.73 ± 0.06, p = 0.36). Notably, left ventricular EF in the adolescent group was higher (Figure 3) compared to adult subjects (0.72 ± 0.05 vs. 0.61 ± 0.07, p < 0.001) with a decline in both males and females from ~70% to ~60% with age.
Normal ranges for left ventricular mass, volumes and function
All variables varied according to age group (ANOVA, p < 0.05) except for EDV/BSA (p = 0.13) and ESV/BSA (p = 0.06) in males, and EDV (p = 0.39), EDV/BSA (p = 0.15), ESV/BSA (p = 0.28), SV (p = 0.14) and LVM/BSA (p = 0.24) in females.
Intra- and interobserver variability
Data on intra- and interobserver variability are presented in Table 3.
Discussion
This study suggests age and gender specific normal values for LV mass, volumes and function measured in a healthy population over a large age range using gradient echo CMR at 1.5 T.
One of the most important tasks in patient examination is to distinguish normal findings from those indicative of disease. Normal ranges for a given parameter encompassing 95% of the population constitute the mainstay of this procedure. With increasing use of cardiac magnetic resonance imaging, the need for age-specific ranges of LV mass, volumes and function for this modality is obvious. This is of specific importance in order to correctly exclude disease.
A) Left ventricular mass
The findings regarding left ventricular mass in the present study and its relation to earlier studies has been discussed earlier [14]. Other non-invasive techniques including three dimensional echocardiography [15, 16] and computerized tomography [17] have overcome many of the shortcomings of two dimensional echocardiography although no reference values for normal patients have been established in a large population.
B) Left ventricular volumes, stroke volume and ejection fractions
Six CMR studies [3–5, 7–9] have described overall values of EDV, ESV, SV, and EF, which are consistent with findings from other imaging modalities and are, in the corresponding age ranges, broadly consistent with the findings in this study, with one exception. Sandstede et al used a gradient echo sequence and reported as much as 20–30% lower values for LV mass and volumes. Those authors chose to only measure LV volumes and mass in short axis slices which contained more than 50% of the circumference of the LV wall in both end-diastole and end-systole. This approach does not take into account the long axis movement of the basal parts of the left ventricle [11], which in part may explain the lower values obtained in that study. Hudsmith et al, showed differences between subjects above and below the age of 35 [8], but continuous age-specific values of these parameters, however, have not been described using gradient echo CMR in populations that extend over the breadth of age as in the present study. We show that adult females had a slight progressive decrease in ESV with age, with a resulting slight progressive increase in EF, whereas Maceira et al [9], showed the opposite. BMI and blood pressure were similar for both populations, hence it is likely some other factor which contributes to the difference between the studies. The discrepancy is difficult to interpret, and one can only speculate as to what difference between the populations may explain this discrepancy.
The inotropy of the left ventricle has been shown to be related to growth hormone/IGF levels [18]. Although our study has not measured growth hormone levels, one might speculate that the higher EDV, SV and EF in younger subjects may possibly, in part, reflect the higher growth hormone levels within this age group. Alternatively, these higher values may be due to physical activity rather than hormones. However, we have previously published that self-reported physical activity increased with age in our population [14]. Thus, it is unclear exactly why these measures are larger in younger subjects.
Reproducibility of measurement
The reproducibility of CMR LV measures obtained in our study was consistent with previously reported studies of intra- and interobserver variability [19–21].
Limitations
Regurgitation in the aortic or mitral valve was only assessed visually in long axis CMR images, and this is a limitation. However, it is likely that this was sufficient to serve the purposes of this study in this population with otherwise unremarkable ECG, medical history and physical examination. The number of included subjects in each gender and decade is less than the minimally suggested n = 10 [21] for the size of a group needed to determine reference values. However, the suggestion of n = 10 is based on the use of the mean +/- 2SD of those subjects' measurements as a basis for calculating reference values. In contrast, we used a curve estimation model and its 95% prediction interval to determine our reference values. Using this method, the robustness of the reference values is based on data from the entire population and less susceptible to small numbers of subjects in individual decade groups. The normal values provided in this study are appropriate for studies undertaken using similar gradient echo sequences at 1.5 T. It has been shown that steady-state free precession based sequences may result in slightly lower LVM and greater LV volumes [7, 22, 23]. These differences are likely to be systematic in nature and do not alter the physiological significance of the age trends reported in the current study. Furthermore, the use of steady-state free precession based sequences at a field strength of 3 T is currently hampered by artifacts [24]. The presented normal values for gradient echo sequences at 1.5 T may thereby also be of value for assessing results from gradient echo cardiac imaging at 3 T.
Conclusion
This study suggests normal reference values for left ventricular mass, dimensions, and function in healthy humans from early adolescence to the eighth decade according to gender using gradient echo CMR at 1.5 T. LV volumes, mass and function vary over a broad age range in healthy individuals. LV volumes and mass both rise in adolescence and decline with age. EF showed a rapid decline in adolescence compared to changes throughout adulthood. These findings further demonstrate the need for age and gender specific normal ranges for clinical cardiac MR examinations.
Abbreviations
- (BSA):
-
body surface area
- (CMR):
-
cardiac magnetic resonance
- (DBP):
-
diastolic blood pressure
- (ECG):
-
electrocardiogram
- (EDV):
-
end-diastolic volume
- (EF):
-
ejection fraction
- (ESV):
-
end-systolic volume
- (ICC):
-
intraclass correlation coefficient
- (LV):
-
left ventricle
- (LVM):
-
left ventricular mass
- (SBP):
-
systolic blood pressure
- (SV):
-
stroke volume
- (T):
-
Tesla
- (TE):
-
time to echo
- (TR):
-
time to repetition.
References
Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK: Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. J Cardiovasc Magn Reson. 2004, 6 (4): 727-765. 10.1081/JCMR-200038581.
Hees PS, Fleg JL, Lakatta EG, Shapiro EP: Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002, 90 (11): 1231-1236. 10.1016/S0002-9149(02)02840-0.
Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP: Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999, 1 (1): 7-21. 10.3109/10976649909080829.
Marcus JT, DeWaal LK, Gotte MJ, Geest van der RJ, Heethaar RM, Van Rossum AC: MRI-derived left ventricular function parameters and mass in healthy young adults: relation with gender and body size. Int J Card Imaging. 1999, 15 (5): 411-419. 10.1023/A:1006268405585.
Sandstede J, Lipke C, Beer M, Hofmann S, Pabst T, Kenn W, Neubauer S, Hahn D: Age- and gender-specific differences in left and right ventricular cardiac function and mass determined by cine magnetic resonance imaging. Eur Radiol. 2000, 10 (3): 438-442. 10.1007/s003300050072.
Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ: Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002, 39 (6): 1055-1060. 10.1016/S0735-1097(02)01712-6.
Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU: Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003, 17 (3): 323-329. 10.1002/jmri.10262.
Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S: Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005, 7 (5): 775-782. 10.1080/10976640500295516.
Maceira AM, Prasad SK, Khan M, Pennell DJ: Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006, 8 (3): 417-426. 10.1080/10976640600572889.
1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999, 17 (2): 151-183.
Pennell DJ: Ventricular volume and mass by CMR. J Cardiovasc Magn Reson. 2002, 4 (4): 507-513. 10.1081/JCMR-120016389.
Shrout P, Fleiss JL: Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979, 420-428. 10.1037/0033-2909.86.2.420. 2
Mosteller RD: Simplified calculation of body-surface area. N Engl J Med. 1987, 317 (17): 1098-
Cain PA, Ahl R, Hedstrom E, Ugander M, Allansdotter-Johnsson A, Friberg P, Marild S, Arheden H: Physiological determinants of the variation in left ventricular mass from early adolescence to late adulthood in healthy subjects. Clin Physiol Funct Imaging. 2007, 27 (4): 255-262. 10.1111/j.1475-097X.2007.00735.x.
Gopal AS, Keller AM, Shen Z, Sapin PM, Schroeder KM, King DL, King DL: Three-dimensional echocardiography: in vitro and in vivo validation of left ventricular mass and comparison with conventional echocardiographic methods. J Am Coll Cardiol. 1994, 24 (2): 504-513.
Gopal AS, Schnellbaecher MJ, Shen Z, Boxt LM, Katz J, King DL: Freehand three-dimensional echocardiography for determination of left ventricular volume and mass in patients with abnormal ventricles: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 1997, 10 (8): 853-861. 10.1016/S0894-7317(97)70045-2.
Wachspress JD, Clark NR, Untereker WJ, Kraushaar BT, Kurnik PB: Systolic and diastolic performance in normal human subjects as measured by ultrafast computed tomography. Cathet Cardiovasc Diagn. 1988, 15 (4): 277-283. 10.1002/ccd.1810150414.
Bisi G, Podio V, Valetto MR, Broglio F, Bertuccio G, G DER, Boghen MF, Berti F, Muller EE, Ghigo E: Radionuclide angiocardiographic evaluation of the cardiovascular effects of recombinant human IGF-I in normal adults. Eur J Endocrinol. 1999, 140 (4): 322-327. 10.1530/eje.0.1400322.
Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS: Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995, 8 (3): 221-228. 10.1016/0895-7061(94)00178-E.
Germain P, Roul G, Kastler B, Mossard JM, Bareiss P, Sacrez A: Inter-study variability in left ventricular mass measurement. Comparison between M-mode echography and MRI. Eur Heart J. 1992, 13 (8): 1011-1019.
Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ: Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000, 2 (4): 271-278. 10.3109/10976640009148691.
Plein S, Bloomer TN, Ridgway JP, Jones TR, Bainbridge GJ, Sivananthan MU: Steady-state free precession magnetic resonance imaging of the heart: comparison with segmented k-space gradient-echo imaging. J Magn Reson Imaging. 2001, 14 (3): 230-236. 10.1002/jmri.1178.
Thiele H, Nagel E, Paetsch I, Schnackenburg B, Bornstedt A, Kouwenhoven M, Wahl A, Schuler G, Fleck E: Functional cardiac MR imaging with steady-state free precession (SSFP) significantly improves endocardial border delineation without contrast agents. J Magn Reson Imaging. 2001, 14 (4): 362-367. 10.1002/jmri.1195.
Michaely HJ, Nael K, Schoenberg SO, Laub G, Reiser MF, Finn JP, Ruehm SG: Analysis of cardiac function–comparison between 1.5 Tesla and 3.0 Tesla cardiac cine magnetic resonance imaging: preliminary experience. Invest Radiol. 2006, 41 (2): 133-140. 10.1097/01.rli.0000192023.96494.af.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2342/9/2/prepub
Acknowledgements
This work was supported in part by research funding from the Swedish Research Council, Swedish Heart and Lung Foundation, and Lund University Faculty of Medicine and the Region of Scania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PC performed data acquisition, data analysis, statistical analysis and drafted the manuscript. RA and AAJ performed data acquisition and data analysis, and participated in critically revising the manuscript for important intellectual content. EH performed data acquisition, data analysis, statistical analysis and helped to draft the manuscript. MU performed data acquisition, data analysis, statistical analysis and helped to draft the manuscript. PF conceived of the study, participated in its design and coordination, and participated in critically revising the manuscript for important intellectual content. HA conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors' read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cain, P.A., Ahl, R., Hedstrom, E. et al. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging 9, 2 (2009). https://doi.org/10.1186/1471-2342-9-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2342-9-2