Abstract
Background
Betacellulin (BTC), a member of the epidermal growth factor (EGF) family, binds and activates ErbB1 and ErbB4 homodimers. BTC was expressed in tumors and involved in tumor growth progression. CXCL8 (interleukin-8) was involved in tumor cell proliferation via the transactivation of the epidermal growth factor receptor (EGFR).
Materials and methods
The present study was designed to investigate the possible interrelation between BTC and CXCL8 in human lung cancer cells (A549) and demonstrated the mechanisms of intracellular signals in the regulation of both functions. Bio-behaviors of A549 were assessed using Cell-IQ Alive Image Monitoring System.
Results
We found that BTC significantly increased the production of CXCL8 through the activation of the EGFR-PI3K/Akt-Erk signal pathway. BTC induced the resistance of human lung cancer cells to TNF-α/CHX-induced apoptosis. Treatments with PI3K inhibitors, Erk1/2 inhibitor, or Erlotinib significantly inhibited BTC-induced CXCL8 production and cell proliferation and movement.
Conclusion
Our data indicated that CXCL8 production from lung cancer cells could be initiated by an autocrine mechanism or external sources of BTC through the EGFR–PI3K–Akt–Erk pathway to the formation of inflammatory microenvironment. BTC may act as a potential target to monitor and improve the development of lung cancer inflammation.
Similar content being viewed by others
Background
The epidermal growth factor receptor (EGFR) consists of an extracellular ligand-binding domain, a transmembrane domain and an intracellular tail with an ATP-binding site, tyrosine kinase activity, and capability of autophosphorylation [1, 2]. EGFR has been found to contribute the lung development [3] and multiplicity of cancer-related signal transduction pathways like cellular proliferation, adhesion, migration, neoangiogenesis, and apoptosis inhibition [4]. EGFR is also responsible for the sensitivity of human non-small cell lung cancer (NSCLC) cells to therapies and prognosis of patients [5, 6]. There are numerous ligands to bind with EGFR, such as EGF, transforming growth factor-α (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), epiregulin (ER), amphiregulin (AR), neuregulin (NRG) subfamily and betacellulin (BTC) [7]. The activation of EGFR can initiate the downstream signaling cascades, e.g. the Ras/mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K)/Akt [8–11].
BTC is a member of EGF family and acts as a potent mitogen for cell types, with the higher affinity and specificity for ErbB1/EGFR and ErbB4. Homologous or heterologous dimers of ErbB family receptor are then formed to activate signal transduction pathways, such as PI3K/PDK1/Akt and RAS/RAF/MEK/Erk, leading to a series of biological effects [12, 13]. Abnormal phosphorylation of Akt and Erk1/2 was considered as an important factor in the prognosis of cancer [14] and constitutive activation of EGFR–Akt–mTOR was found in about 18% of NSCLCs [15].
Our previous study on disease-specific biomarkers of patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) by integrating inflammatory mediators with clinical informatics demonstrated that BTC played an important role in the occurrence of AECOPD and was associated with the disease severities [16]. We also found that EGFR–PI3K–Akt–Erk pathway was involved in the development of lung cancer inflammatory microenvironment by the hyper-production of CXCL8 [17], responsible for leukocyte recruitment, cancer proliferation, and angiogenesis [18]. The present study further aimed at understanding the potential association and interaction mechanisms between BTC and CXCL8 in the inflammatory microenvironment, exploring the expression and biological function of BTC gene and protein and its receptors in human lung cancer cells, and define the role of BTC in the regulation of CXCL8 expression and production in lung cancer. The present study furthermore investigated the involvement of EGFR–PI3K–Akt–Erk activation in CXCL8 production induced by BTC with consequences on lung cancer cell proliferation and movement.
Materials and methods
Cell lines and reagents
Human lung cancer cell line A549 cells were cultured in RPMI 1640 supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% heat inactivated fetal bovine serum (FBS). Human recombinant BTC, Enzyme-Linked Immunosorbent Assay (ELISA) kits for CXCL8, anti-human BTC neutralizing antibody were purchased from R&D Systems (Shanghai, China). PI3K/mTOR inhibitors (BEZ235, GDC0941, SHBM1009) and Erk1/2 inhibitor (PD98059) were purchased from Biovision (California, USA). EGFR inhibitor (Erlotinib) was from Roche (Basel, Switzerland). EGFR, ErbB2, ErbB3 and ErbB4 antibodies for immunofluorescent staining were purchased from Abcam (Hong Kong, China). Cell-IQ live cell imaging platform was manufactured by Chipmantech (Tampere, Finland) and equipped in Center for Biomedical Research, Zhongshan Hospital, Fudan University, Shanghai, China.
Measurement of gene expression
Total RNA was isolated using a guanidinium isothiocyanate/chloroform based technique (TRIZOL, Invitrogen, USA) and measured with OD 260 nm. RNA was subsequently reversed and transcribed to cDNA with the SuperScript First-strand Synthesis System (Invitrogen, USA). Quantitative RT-PCR was carried out using an ABI 7000 PCR instrument (Eppendorf, Hamburg, Germany) with the two-stage program parameters, as follows: 1 min at 95°C, and then 40 cycles of 5 s at 95°C and 30s at 60°C. The sequences of the primer sets used for this analysis are as follows: BTC, 5′-TGAAACTAATGGCCTCCTCTGT -3′ (forward [F]) and 5′-CTTTTACGACGTTTCCGAAGAG -3′ (reverse [R]); CXCL8, 5′-TTGCCAAGGAGTGCTAAAGAA -3′ (F) and 5′-GCCCTCTTCAAAAACTTCTCC -3′ (R); and for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-CCACCCATGGCAAATTCCATGGCA-3′ (F) and 5′-TCTACACGGCAGGTCAGGTCCACC-3′ (R). Specificity of the produced amplification product was confirmed by examination of dissociation reaction plots. Each sample was tested in triplicate with quantitative RT-PCR, and each group had six wells.
Production of BTC and CXCL8
A549 cells were cultured in 24 well cell culture microplates at 1 × 105 cells/well for 24 h and then treated with lipopolysaccharide (LPS) (Escherichia coli, 055:B5, Sigma, St. Louis, MO) at concentrations of 0.01, 0.1, and 1 μg/ml for an additional 24 h, respectively, to study LPS-induced productions of BTC or CXCL8. Cells were pre-incubated with an anti-human BTC neutralizing antibody at concentrations of 1, 10, 100 ng/ml or IgG as non-specific control 2 h before LPS stimulation to study the role of BTC in LPS-induced CXCL8 production. Cells were treated with BTC at 0.1 μg/ml or vehicle and pretreated with BEZ235, GDC0941, SHBM1009, Erlotinib, or PD98059 at 0.1, 1, or 10 μM, respectively, for 24 h to investigate the involvement of various signal pathways. Each experiment was done in six replicate wells for each drug concentration and each time point. Levels of BTC and CXCL8 proteins in supernatant were measured by ELISA at the absorbanceof 450 nm.
Expression of receptors
A549 were fixed with 4% paraformaldehyde, washed thrice, permeabilized with 0.1% Triton X-100, and blocked with 10% goat serum. Cells were incubated overnight with mouse monoclonal antibodies against EGFR, ErbB2, ErbB3, or ErbB4, respectively, and then strained with FITC conjugated anti mouse IgG antibody (Abcam, HK, China, 1:100). The counterstaining was performed with DAPI (4, 6′-diamidino-2-phenylindole) and cells were examined under immunofluorescence microscope (Olympus/BX51, Tokyo, Japan).
Measurements of apoptosis
Apoptosis was analyzed by FACS as previously described [19]. Lung cancer cells A549 (1 × 106 cell/ml) were treated with TNF-α at 20 ng/ml/CHX at 2.5 μmol/l (Sigma, St. Louis, MO) for 24 h in the presence or absence of BTC at 0.01, 0.1, 1 μg/ml, respectively. Cells were then harvested and washed thrice, resuspended in pre-diluted binding buffer, and stained with Annexin V-FITC (Sigma, St. Louis, MO) for 20 min. Cell apoptosis was analyzed by flow cytometry (BD Bioscience, San Diego,CA,USA) using Cell Quest Software (Biomedika, Canada).
Measurements of cell bio-behaviors
The cell bio-behaviors including total cell number, cell differentiation, and cell movement were measured by the real-time cell monitoring system, using a Cell-IQ cell culturing platform (Chip-Man Technologies, Tampere, Finland), equipped with a phase-contrast microscope (Nikon CFI Achromat phase contrast objective with 10x magnification) and a camera (Nikon, Japan). The equipment was controlled by Imagen software (Chip-Man Technologies). Images were captured at 5 min intervals for 72 h. Analysis was carried out with a freely distributed Image software (Cell-IQ Imagen v2.9.5c, McMaster Biophotonics Facility, Hamilton, ON), using the Manual Tracking plug-in created by Fabrice Cordelieres (Institut Curie, Orsay, France). Cell-IQ system uses machine vision technology to monitor and record time-lapse data, and it can also analyze and quantify cell functions and morphological parameters. The movement of each individual cell was measured in the image field by metering the distance of cell movement.
Statistical analysis
Data were represented as mean ± SEM. Statistical significance was compared between groups by the Student’s t-test, after ANOVA analyses. Increased rates of total cell number and differentiation were calculated as the following: Rate (%) = (value at each time point-value of primary seeding cells)/value of primary seeding cells × 100. Cell movement was calculated as the mean of the distance of every cell moving between two images (5 min interval). p-Values less than 0.05 was considered to be significant.
Results
The mRNA expression and protein production of BTC in A549 cells significantly increased at the stimulation of LPS at 1 μg/ml, while those of CXCL8 significantly increased at both 0.1 and 1 μg/ml with a dose-dependent parttern, as shown in Figure 1 (p < 0.05 or 0.01, respectively). A positive correlation of BTC and CXCL8 expression in lung cancer was observed. Cells were pretreated with anti-human BTC neutralizing antibody to investigate the potential role of endogenous BTC in LPS-induced over-expression and over-production of CXCL8 mRNA and proteins. Pretreatment with BTC neutralizing antibodies at concentrations of 10 and 100 ng/ml could significantly prevent from LPS-induced over-expression of CXCL8 mRNA and over-production of CXCL8 proteins, as compared with those pretreated with vehicle and challenged with LPS (p < 0.05 or 0.01, respectively, in Figure 2A and B). The stimulation of exogenous BTC proteins from the dose of 0.01 μg/ml and on significantly increased the expression and production of CXCL8 mRNA and proteins (p < 0.05 or 0.01 in Figure 2C and D), as compared with those stimulated with vehicle.
LPS induces increased production of BTC and IL-8 in human lung cancer cells A549. A549 cells grown in complete medium were left untreated (control) or treated with LPS (0.01, 0.1 and 1 μg/ml) for 4 h. Total RNA was extracted and subjected to reverse transcription followed by qPCR to detect BTC (A) and IL-8 (C) mRNA, as described in Material and Methods. Data were normalized to control. A549 cells (1 × 105/ml) were stimulated with LPS for 24 h, then the cytokines including BTC (B) and IL-8 (D) in cell-free supernatants were assayed using sandwich ELISA. Each data point represents mean ± SEM of three experiments. * and ** stand for P -values less than 0.05 and 0.01, as compared to control.
LPS-induced IL-8 production is BTC dependent in human lung cancer cells A549. A549 cells were pretreated with anti-BTC neutralizing antibody (Neu-Ab) for 2 h. Subsequently, cells were stimulated with LPS (1 μg/ml) for 4 h. Total RNA was extracted and subjected to reverse transcription followed by qPCR to detect IL-8 mRNA (A). IL-8 in cell-free supernatants after 24 h stimulated by LPS were assayed using sandwich ELISA (B). A549 cells were then stimulated with BTC (0.01, 0.1 and 1 μg/ml) for 4 h to detect IL-8 mRNA (C) or 24 h to detect its secretion of IL-8 in supernatants (D). Each data point represents mean ± SEM of three experiments. * and ** stand for P- values less than 0.05 and 0.01, in comparison with untreated control cells, and + and ++ stand for P - values less than 0.05 and 0.01, as compared to LPS (1 μg/ml) and DMSO, respectively.
Figure 3 demonstrated the expression of ErbB1/EGFR, ErbB2, ErbB3, or ErbB4 on A549 cells evaluated by immunofluorescence staining. We found that A549 cells constitutively expressed EGFR (Figure 3A) and ErbB2 (Figure 3B), rather than ErbB3 (Figure 3C) and ErbB4 (Figure 3D), of which the expression of EGFR increased 24 hours after the stimulation of exogenous BTC. Our pilot study demonstrated that BTC at 0.1 μg/ml could significantly increase production of CXCL8 A549 cells from 12 h and on, which maintained till at 48 h. Treatments with PI3K inhibitors (BEZ235, GDC0941 and SHBM1009) at 1 μM, 10 μM and Erk1/2 inhibitor (PD98059) at 10 μM significantly inhibited BTC-induced CXCL8 production, as compared to cells treated with vehicle (P < 0.05 or 0.01, respectively, Figure 4A-E). Treatment with Erlotinib (TKI) at all concentrations significantly prevented BTC-stimulated over-production of CXCL8 (P < 0.01, Figure 4D).
Expression of ErbB family receptors in A549 human lung tumor cell line. A549 cell line is positive for EGFR (red labeling) (A), and ErbB2 (green) (B) and negative for ErbB3 (C) and ErbB4 (D) (FITC staining, green), respectively, in immunofluorescence analysis. DAPI staining (blue) indicates the localization of nuclei. One representative image from three independent experiments is presented.
Treatment with PI3K inhibitors, Erk1/2 inhibitor or Erlotinib significantly inhibited BTC-induced IL-8 production in A549 lung cancer cells. IL-8 production from cells was measured 24 h after the culture with DMSO alone, BTC at 0.1ug/ml plus DMSO, BEZ235 (BEZ; A), GDC0941 (GDC; B), SHBM1009 (SHBM; C) or Erlotinib (TKI; D), PD98059 (PD; E) at doses of 0.1, 1.0 or 10 μM. * and ** stand for P- values less than 0.05 and 0.01 as compared to cells only with DMSO, and + and ++ stand for P-values less than 0.05 and 0.01, as compared to BTC and DMSO, respectively. Data is presented as mean ± SEM and each group has six measurements.
The percentage of total cell number significantly increased after the stimulation of BTC at 0.1 μg/ml, as compared to those stimulated with vehicle ( P < 0.05 or 0.01, respectively, Figure 5), which was significantly inhibited by BEZ235 (Figure 5A), GDC0941 (Figure 5B), SHBM1009 (Figure 5C), Erlotinib (Figure 5D) or PD98059 (Figure 5E) at various concentrations. Of them inhibitory effects of PI3K inhibitors (BEZ235, GDC0941 and SHBM1009) showed a dose-dependent pattern. Inhibitors also significantly inhibited BTC-increased percentages of differentiated cells, as shown in Figure 6A–E. Figure 7A–E demonstrated similar inhibitory effects of inhibitors on the BTC-increased cell movements, as compared to BTC stimulation alone (P < 0.05 or 0.01, respectively). The number of apoptotic cells significantly increased in cells pretreated with vehicle and stimulated with TNF-α/CHX for 24 h, as compared with those pretreated with vehicle or BTC without the stimulation (p < 0.01, respectively, Figure 8). Cells pretreated with different doses of exogenous BTC developed into apoptosis less than those pretreated with vehicle after the stimulation with TNF-α/CHX.
The increased percentage of the total number of lung cancer cells (A549 cells) was assessed using Cell-IQ Alive Image Monitoring System, at 72 h. The percentage of total number of cells significantly increased (∎) after the stimulation with BTC, compared to the average of total cells values treated with vehicle (DMSO) alone (●), while cells treated with BEZ235 (A), GDC0941 (B), SHBM1009 (C), Erlotinib (D) or PD98059 (E) showed a lower increase in the percentage of total number of cells, at doses of 0.1, 1 or 10 μM, respectively. Data were presented as mean ± SEM and each group has 6–12 measurements.
The percentage of the differentiated lung cancer cells (A549 cells) was assessed using Cell-IQ Alive Image Monitoring System, at 72 h. The percentage of differentiated cells significantly increased (∎) after the stimulation with BTC, compared to the average of total cells values treated with vehicle (DMSO) alone (●), while cells treated with BEZ235 (A), GDC0941 (B), SHBM1009 (C), Erlotinib (D) or PD98059 (E) showed a lower increase, or even a decrease in the percentage of differentiated cells, at doses of 0.1, 1 or 10 μM, respectively. Data were presented as mean ± SEM and each group has 6–12 measurements.
The percentage of lung cancer cells (A549 cells) movement was assessed using Cell-IQ Alive Image Monitoring System, at 72 h. The percentage of A549 cells movement significantly increased (∎) after the stimulation with BTC, compared to the average of total cells values treated with vehicle (DMSO) alone (●), while cells treated with BEZ235 (A), GDC0941 (B), SHBM1009 (C), Erlotinib (D) or PD98059 (E) showed a lower increase, or even a decrease in the percentage of A549 cells movement, at doses of 0.1, 1 or 10 μM, respectively. Data were presented as mean ± SEM and each group has 6–12 measurements.
BTC pretreatment induces resistance of human lung cancer cells to apoptosis induction by TNF-α/CHX. A549 cells (1 × 106/ml) were pretreated with BTC for 4 h, then stimulated with 20 ng/ml TNF-α/CHX for 24 h. The cells were stained with annexin V and PI and subjected to FACS assay of cellular apoptosis. ** stand for P- value less than 0.01 as compared to control (DMSO only), and ++ stand for P-value less than 0.01, as compared to BTC + DMSO. Data were presented as mean ± SEM of three independent experiments.
Discussion
BTC is expressed in bronchial mucosa and lung tissue cells, e.g. the alveolar and airway epitheliums, fibroblasts, and macrophages [8, 20]. The evidence from our previous studies and others suggested that BTC play a critical role in the development of lung inflammation through the regulation of the cytokine secretion pattern and tumor cell progression through EGFR ligation, possibly associated with the over-production of CXCL8 [21–25]. The activation of the EGFR pathway could contribute to the over-expression of CXCL8 in human bronchial epithelial cells by multi-stimuli, e.g. HB-EGF [26], MMP-12 [27]. We found that EGF was involved in the development of the lung cancer inflammatory microenvironment through the over-production of CXCL8 associated with the activation of EGFR pathway [17]. The present study provided the further evidence that both BTC and CXCL8 could be over-produced directly by lung cancer cell per se in the inflammatory condition and/or stimuli like LPS.
Our data indicated that lung cancer cells per se may act as a primary receptor to be stimulated and challenged by inflammatory factors and as the secondary reactor to produce the mediators and accelerate the development of the local inflammatory microenvironment. The present study also evidenced that the potential mechanism by which lung cancer cells are regulated to produce chemoattractive factors could be that BTC produced by a lung cancer cell per se or by other neighbor cells might regulate the over-production of secondary inflammatory factors like CXCL8 through EGFR–PI3K–Akt–Erk pathway.
Many regulatory factors may contribute to the molecular mechanism by which LPS can stimulate lung cancer cells to produce inflammatory mediators. Results from the present study demonstrated that both endogenous and exogenous BTC could induce the over-production of CXCL8. The finding that levels of CXCL8 in cells blocked with anti-BTC neutralizing antibody and challenged with LPS were still significantly higher than those without LPS indicates the existence of biological efforts from other factors, like EGF [17]. We found that the signal pathway of BTC-EGFR-PI3K axis may play the crucial and dependent role in the mechanism of CXCL8 production of lung cancer cells, evidenced by the finding that the over-production of CXCL8 by BTC was fully prevented by PI3K and EGFR inhibitors. It implies that the BTC-EGFR-PI3K-CXCL8 chain can be the potential of new anti-inflammatory therapeutic target in lung cancer or chronic lung diseases.
The EGFR-dominated signal pathway, e.g. PI3K, Erk1/2 and STAT, are related to CXCL8 expression in airway epithelium cells [28, 29]. We provided direct evidence that A549 cells constitutively expressed EGFR and ErbB2, while the expression of EGFR increased after BTC stimulation in human lung cancer cells. PI3K inhibitors (BEZ235, GDC0941 and SHBM1009) and Erk1/2 inhibitor PD98059 could inhibit over-production of CXCL8 initiated by the over activation of BTC-EGFR pathway.
The PI3K activation has been recently found to play the important role in the development of acute and chronic lung inflammation and injury [30]. PI3K/Akt and Erk1/2 pathways could play the decisive role in lung cancer development and proliferation [30, 31], while the inhibition of PI3K/Akt pathway could reduce the migration and invasion of NSCLC cells [32]. We found that BTC could increase the proliferation, differentiation and movement of lung cancer cells, which could be down-regulated by PI3K, Erk, and EGFR inhibitors. The pretreatment with BTC could increase the resistance of lung cancer cells against TNF-α/CHX-induced apoptosis in a dose-associated pattern.
Conclusions
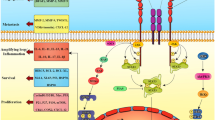
In summary, the present study demonstrated that LPS increased the over-production of BTC and CXCL8 from human lung cancer cells, which could be blocked by anti-BTC neutralizing antibody. Both endogenous and exogenous BTC could increase the over-production CXCL8 through EGFR–PI3K–Akt–Erk pathway activation. Of EGFRs, EGFR expression increased after the stimulation of BTC. BTC also increased the proliferation, differentiation, and movement of lung cancer cells and increased cell resistance against apoptosis. It indicates that lung cancer cells per se contribute to the development of the local inflammatory microenvironment, probably leading to the recruitment of inflammatory cells in the cancer tissue and the formation of inflammatory microenvironment (Figure 9). Thus, our data indicate that the signal pathway of BTC-EGFR–PI3K–Akt–Erk-CXCL8 plays an important role in the inflammatory microenvironment in lung cancer, as a novel therapeutic approach to lung cancer.
Proposed mechanism of BTC-stimulated IL-8 production and bio-behaviors of lung cancer cells. BTC could directly stimulate IL-8 production and bio-behaviors of lung cancer cells through the activation of EGFR, PI3K/Akt, and Erk signal pathway, then lead to the recruitment of inflammatory cells in the cancer tissue and the formation of inflammatory microenvironment. This has been highlighted as an important factor responsible for the sensitivity of human lung cancer to therapies and prognosis of patients.
Abbreviations
- AR:
-
Amphiregulin
- AECOPD:
-
Acute exacerbations of chronic obstructive pulmonary disease
- BTC:
-
Betacellulin
- CXCL8:
-
Interleukin-8
- ER:
-
Epiregulin
- EGFR:
-
Epidermal growth factor receptor
- ELISA:
-
Enzyme-linked immunosorbent assay
- HB-EGF:
-
Heparin-binding EGF-like growth factor
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen activated protein kinase
- NRG:
-
Neuregulin
- NSCLC:
-
Non-small cell lung cancer
- PI3K:
-
Phosphoinositide-3 kinase
- TGF-α:
-
Transforming growth factor-α.
References
Li J, Li Y, Feng ZQ, Chen XG: Anti-tumor activity of a novel EGFR tyrosine kinase inhibitor against human NSCLC in vitro and in vivo. Cancer Lett. 2009, 279: 213-220. 10.1016/j.canlet.2009.01.042.
Lin CC, Lin WN, Cheng SE, Tung WH, Wang HH, Yang CM: Transactivation of EGFR/PI3K/Akt involved in ATP-induced inflammatory protein expression and cell motility. J Cell Physiol. 2012, 227: 1628-1638. 10.1002/jcp.22880.
Li HJ, Liu Y, Hao HS, Du WH, Zhao XM, Wang D, Qin T, Ma YJ, Zhu HB: [Relationship of epidermal growth factor receptor in lung development]. Yi Chuan. 2012, 34: 27-32. 10.3724/SP.J.1005.2012.00027.
Roskoski R: The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2013, 79C: 34-74.
Li H, Schmid-Bindert G, Wang D, Zhao Y, Yang X, Su B, Zhou C: Blocking the PI3K/AKT and MEK/ERK signaling pathways can overcome gefitinib-resistance in non-small cell lung cancer cell lines. Adv Med Sci. 2011, 56: 275-284.
Steins MB, Reinmuth N, Bischoff H, Kindermann M, Thomas M: Targeting the epidermal growth factor receptor in non-small cell lung cancer. Onkologie. 2010, 33: 704-709. 10.1159/000322214.
Schneider MR, Wolf E: The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009, 218: 460-466. 10.1002/jcp.21635.
Hardie WD, Hagood JS, Dave V, Perl AK, Whitsett JA, Korfhagen TR, Glasser S: Signaling pathways in the epithelial origins of pulmonary fibrosis. Cell Cycle. 2010, 9: 2769-2776.
Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J: The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med. 2013, 18: 218-230.
Yang J, Li Q, Zhou XD, Kolosov VP, Perelman JM: Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-kappaB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol Cell Biochem. 2011, 351: 29-40. 10.1007/s11010-010-0708-y.
Yi YW, Hong W, Kang HJ, Kim HJ, Zhao W, Wang A, Seong YS, Bae I: Inhibition of the PI3K/AKT pathway potentiates cytotoxicity of EGFR kinase inhibitors in triple-negative breast cancer cells. J Cell Mol Med. 2013, 17: 648-656. 10.1111/jcmm.12046.
Miura K, Doura H, Aizawa T, Tada H, Seno M, Yamada H, Kawano K: Solution structure of betacellulin, a new member of EGF-family ligands. Biochem Biophys Res Commun. 2002, 294: 1040-1046. 10.1016/S0006-291X(02)00585-5.
Saito T, Okada S, Ohshima K, Yamada E, Sato M, Uehara Y, Shimizu H, Pessin JE, Mori M: Differential activation of epidermal growth factor (EGF) receptor downstream signaling pathways by betacellulin and EGF. Endocrinology. 2004, 145: 4232-4243. 10.1210/en.2004-0401.
Tanaka Y, Terai Y, Tanabe A, Sasaki H, Sekijima T, Fujiwara S, Yamashita Y, Kanemura M, Ueda M, Sugita M, Franklin WA, Ohmichi M: Prognostic effect of epidermal growth factor receptor gene mutations and the aberrant phosphorylation of Akt and ERK in ovarian cancer. Cancer Biol Ther. 2011, 11: 50-57. 10.4161/cbt.11.1.13877.
Dobashi Y, Suzuki S, Kimura M, Matsubara H, Tsubochi H, Imoto I, Ooi A: Paradigm of kinase-driven pathway downstream of epidermal growth factor receptor/Akt in human lung carcinomas. Hum Pathol. 2011, 42: 214-226. 10.1016/j.humpath.2010.05.025.
Chen H, Song Z, Qian M, Bai C, Wang X: Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: a preliminary study. J Cell Mol Med. 2012, 16: 1286-1297. 10.1111/j.1582-4934.2011.01416.x.
Zhang Y, Wang L, Zhang M, Jin M, Bai C, Wang X: Potential mechanism of interleukin-8 production from lung cancer cells: an involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J Cell Physiol. 2012, 227: 35-43. 10.1002/jcp.22722.
Campbell LM, Maxwell PJ, Waugh DJ: Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer. Pharmaceuticals (Basel). 2013, 6: 929-959. 10.3390/ph6080929.
Liu Q, Chen T, Chen H, Zhang M, Li N, Lu Z, Ma P, Cao X: Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun. 2004, 319: 980-986. 10.1016/j.bbrc.2004.04.201.
de Boer WI, Hau CM, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS: Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006, 125: 184-192. 10.1309/W1AXKGT7UA37X257.
Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y: Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998, 157: 1907-1912. 10.1164/ajrccm.157.6.9609040.
Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, Komaki R, Varella-Garcia M, Hong WK, Aldape KD, Wistuba II: HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res. 2009, 15: 4829-4837. 10.1158/1078-0432.CCR-08-2921.
Schneider MR, Dahlhoff M, Herbach N, Renner-Mueller I, Dalke C, Puk O, Graw J, Wanke R, Wolf E: Betacellulin overexpression in transgenic mice causes disproportionate growth, pulmonary hemorrhage syndrome, and complex eye pathology. Endocrinology. 2005, 146: 5237-5246. 10.1210/en.2005-0418.
Fernandes AM, Hamburger AW, Gerwin BI: Production of epidermal growth factor related ligands in tumorigenic and benign human lung epithelial cells. Cancer Lett. 1999, 142: 55-63. 10.1016/S0304-3835(99)00166-4.
Normanno N, Bianco C, Damiano V, de Angelis E, Selvam MP, Grassi M, Magliulo G, Tortora G, Bianco AR, Mendelsohn J, Salomon DS, Ciardiello F: Growth inhibition of human colon carcinoma cells by combinations of anti-epidermal growth factor-related growth factor antisense oligonucleotides. Clin Cancer Res. 1996, 2: 601-609.
McGovern T, Risse PA, Tsuchiya K, Hassan M, Frigola G, Martin JG: LTD(4) induces HB-EGF-dependent CXCL8 release through EGFR activation in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010, 299: L808-815. 10.1152/ajplung.00438.2009.
Le Quement C, Guenon I, Gillon JY, Lagente V, Boichot E: MMP-12 induces IL-8/CXCL8 secretion through EGFR and ERK1/2 activation in epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008, 294: L1076-1084. 10.1152/ajplung.00489.2007.
Liu K, Gualano RC, Hibbs ML, Anderson GP, Bozinovski S: Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J Biol Chem. 2008, 283: 9977-9985. 10.1074/jbc.M710257200.
Ganesan S, Unger BL, Comstock AT, Angel KA, Mancuso P, Martinez FJ, Sajjan US: Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax. 2013, 68: 131-141. 10.1136/thoraxjnl-2012-201719.
Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu G, Szabo E, Brody J, Massion PP, Lenburg ME, Lam S, Bild AH, Spira A: Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010, 2: 26ra25-
Lu ZJ, Zhou Y, Song Q, Qin Z, Zhang H, Zhou YJ, Gou LT, Yang JL, Luo F: Periplocin inhibits growth of lung cancer in vitro and in vivo by blocking AKT/ERK signaling pathways. Cell Physiol Biochem. 2010, 26: 609-618. 10.1159/000322328.
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA, Chen JH: Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol. 2010, 632: 23-32. 10.1016/j.ejphar.2010.01.009.
Acknowledgements
The work was supported by Shanghai Leading Academic Discipline Project (B115), Zhongshan Distinguished Professor Grant (XDW), The National Nature Science Foundation of China (91230204, 81270099, 81320108001, 81270131), The Shanghai Committee of Science and Technology (12JC1402200, 12431900207, 11410708600), Zhejiang Provincial Natural Science Foundation (Z2080988), Zhejiang Provincial Science Technology Department Foundation (2010C14011), and Ministry of Education, Academic Special Science and Research Foundation for PhD Education (20130071110043).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing of interests.
Authors’ contributions
Conceived and designed the study: CC, XW and QW; Performed the biological experiments: LS, LW and BW; Statistical analysis: LS. Wrote the paper: LS and SMC. All authors read and proofed the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shi, L., Wang, L., Wang, B. et al. Regulatory mechanisms of betacellulin in CXCL8 production from lung cancer cells. J Transl Med 12, 70 (2014). https://doi.org/10.1186/1479-5876-12-70
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-12-70